Case Report Open Access
Alzheimers Disease: A Novel Hypothesis Integrating Spirochetes, Biofilm, and the Immune System
| Herbert B. Allen*, Diego Morales, Krister Jones and Suresh Joshi | |
| Drexel University College of Medicine, Philadelphia, USA | |
| *Corresponding Author : | Herbert B. Allen Drexel University College of Medicine Philadelphia, USA Tel: 2157625550 Fax: 215 7625570 E-mail: Herbert.Allen@drexelmed.edu |
| Received December 01, 2015; Accepted December 30, 2015; Published January 02, 2016 | |
| Citation: Allen HB, Morales D, Jones K, Joshi S (2016) Alzheimer’s Disease: A Novel Hypothesis Integrating Spirochetes, Biofilm, and the Immune System. J Neuroinfect Dis 7:200. doi:10.4172/2314-7326.1000200 | |
| Copyright: © 2016 Allen HB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Journal of Neuroinfectious Diseases
Abstract
In the light of recent studies showing the presence of spirochetes in the brains of Alzheimer’s disease (AD) patients, we have studied (post mortem) the hippocampus region in the brains of similarly affected AD patients utilizing both pathology and immunohistochemistry. Our findings demonstrate that the plaques, which are characteristically found in AD brains, reveal the presence of biofilms. These biofilms are undoubtedly made by the spirochetes present there; further, we have also found that the biofilms co-localize with the β amyloid that is a signature finding in the disease. Also, we have shown activation of Toll-like receptor 2 in the same areas. We postulate this is related to the disease because this innate immune system molecule cannot penetrate the biofilm to destroy the spirochetes present there, so, inasmuch as it is activated, it destroys the surrounding tissue instead. We compare this destruction to that which is caused by activation of the adaptive immune system, which leads to much more severe devastation, much more rapidly.
| Keywords |
| Immune system; Hippocampus; Osteoarthritis; Biofilms |
| Introduction |
| Recently, spirochetes have been found in the brains of Alzheimer’s disease (AD) patients [1-3] and, even more recently, the pathology of general paresis of the insane (neurosyphilis) has been shown to be exactly similar to that of AD [4]. In addition, joints in osteoarthritis, another chronic disease, have recently been shown to contain biofilms despite the joints being culture negative [5]. In each of these disease states, dental spirochetes and Borrelia burgdorferi spirochetes have been implicated [6,7]. In a similar fashion, chronic otitis media has been shown to contain biofilms while cultures have been negative [8]. Most organisms have the capability of making biofilms, and, once made, the now “slime”-covered organisms are no longer able to be cultured routinely nor be treated by antibiotics. |
| We recently have found that normal skin flora staphylococci make biofilms, occlude eccrine sweat ducts, activate the innate immune system (Toll-like receptor 2 [TLR 2]) and thereby trigger pathways to create the signs and symptoms of atopic dermatitis, another chronic disease [9]. We have applied the same pathological and immunohistochemical procedures that were used in the atopic dermatitis study to the specimens from AD to determine whether biofilms were present and whether the innate immune system was involved. |
| Methods |
| Seven hippocampal specimens from patients who had previously been confirmed both clinically and pathologically (post mortem) to have Alzheimer’s disease were examined by five pathologists. Ten control hippocampal specimens, from age and sex matched patients who died of unrelated, non-cerebral diseases and/or causes were included for study. All specimens were stained with hematoxylin and eosin (H+E), periodic acid Schiff (PAS), Congo red routine stains; all specimens were also stained with treponema pallidum (TPI), β amyloid, CD 282 (TLR 2) and CD 284 (TLR 4) immunostains. The technique for these stains was as previously published [9]. PAS and β amyloid stains were applied sequentially to the specimens and were examined. Routine light microscopy was employed. The specimens were not “blinded” because by gross examination alone, the AD specimens could be distinguished from the controls. |
| Results |
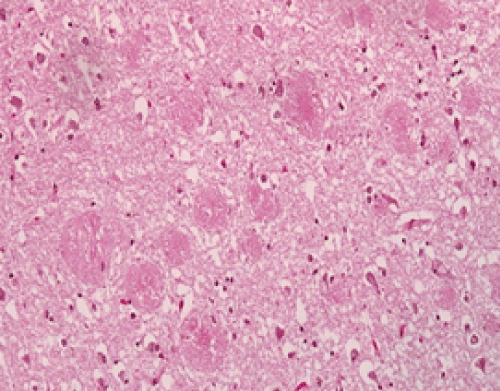
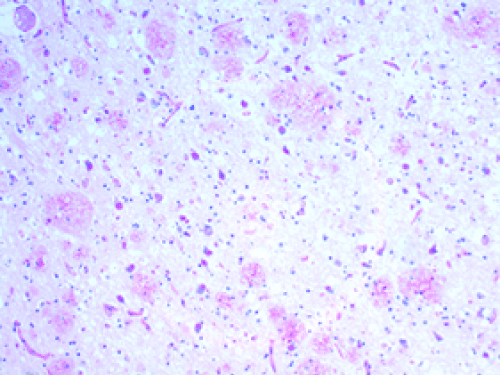
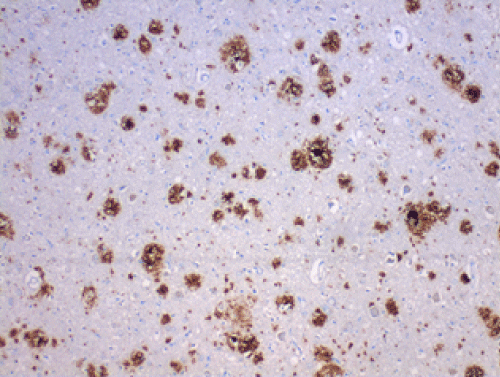
| The AD brains showed the expected histologic findings of plaques, tangles, curly fibers, Tau protein, and β amyloid on routine staining. (Figures 1-3) None of the controls demonstrated these. The PAS stained positively in the same locations as the plaques in AD patients; the controls showed no PAS positivity. The β amyloid/PAS preparations that were sequentially stained on the same specimens showed co-localization of the two stains. (Figure 4) CD 282, representing TLR 2, showed focal staining in the AD brains with nothing being evident in the controls. (Figures 4-6) CD 284 showed minimal reactivity. |
| Discussion |
| We believe the positive PAS and Congo red findings confirm the presence of biofilms in the AD brains. The PAS stains the extracellular polysaccharide material that makes up the bulk of the biofilms and the Congo red stains the amyloid that forms the infrastructure of the biofilm. The amyloid itself is not responsible for the PAS positivity as it is, at most, an oligosaccharide. The co-localization of the β amyloid and the PAS stain demonstrates that finding as well by positioning the biofilm and the β amyloid in the same place. The CD 282 positivity reveals activation of the innate immune system. There was a concern that the positive TLR 2 staining represented lipofuscin which is a marker of senescence. In some instances, it did; namely, the intracytoplasmic and the clumped material that was noted. Two controls also showed clumped material that stained positively. In the AD patients, the TLR 2 staining that was extracellular, and non-clumped, we considered actual TLR 2. |
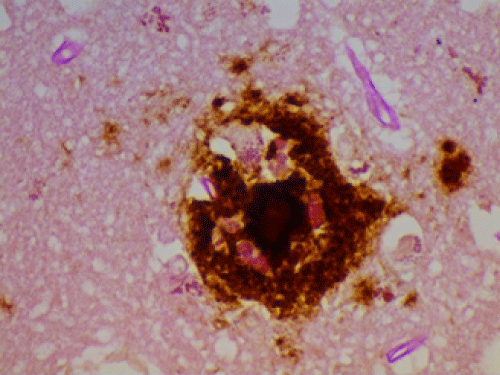
| The presence (by polymerase chain reaction) of spirochetes (75% dental and 25% Lyme Borrelia) has previously been reported, as has the total similarity of AD and general paresis (tertiary syphilis) as relates to pathology [1,4]. The two diseases therefore are similar save for being caused by different spirochetal organisms. Spirochetes have been shown to form biofilms, [10] and it appears that is occurring in the afflicted brains. Most likely, they are creating biofilms via the “quorum sensing” mode: when there are sufficient organisms present, they “sense” it and spin out biofilms to protect themselves. This renders them impenetrable not only to antibiotics, but to the immune system as well. (One can get a better sense of this from the photo of a representative biofilm as seen in Figure 7. Organisms have been shown to form biofilms when exposed to salt, water, alcohol, low dose antibiotics, etc. as well as via the quorum sensing mechanism [11]. |
| The spirochetes take considerable time to divide (90 days); [12] thus, the process to build a quorum is lengthy. This may account in part for the prolonged time for the disease to develop (up to 50 years for syphilitic dementia) [13]. The presence of TLR 2 may help explain the disease process. The organisms enter the brain during a bacteremic phase of a dental procedure or during dissemination of Lyme Borrelia. (The same process occurs in secondary syphilis with the organisms being systemic during that stage in the disease.) The dental spirochetes and the Lyme spirochetes divide slowly, as previously noted, accumulate a quorum, spin out biofilms, and cause the activation of TLR 2. TLR 2, incidentally, has previously been found, but not shown pathologically, in AD [14,15]. We are thus presenting the same findings in a different way. A mechanism by which TLR 2 is activated has recently been elucidated [16,17]. |
| TLR 2 ordinarily combats gram positive organisms by coating and killing them with TNF-α; however, TLR 2 has been shown to be upregulated by spirochetes even though the spirochetes themselves are weakly gram negative [18]. Once a biofilm is made, the TLR 2 cannot penetrate the slime coating and thus cannot destroy the organisms inside. The TLR 2 is “primed”, and the surrounding tissue, as an “innocent by stander” is conceivably destroyed. The cascade of events is pictured in (Table 1). |
| Contrasted with the innate immune system, it is of exceeding interest what occurs when the activated adaptive immune system is involved. This takes place in the aftermath of a cerebrovascular accident (CVA or stroke) [19] where the blood brain barrier is breached. B lymphocytes are then capable of entering the brain; IgG is noted in large areas of neural tissue, and T cells are recruited and enter the previously forbidden zone. The occurrence of Alzheimer’s disease after a stroke is rapid (1-3 years), compared to the 30-50 years ordinarily noted [13]. The adaptive immune system with complement, alternate complement, opsonization, killer T cells, etc. eradicates organisms with much greater efficiency likening it to a “machine gun vs a bow and arrow” when compared to the innate immune system. However, just as in the situation with the innate immune system, it cannot penetrate the biofilm either, thus it destroys greater amounts of the surrounding neural tissue much more rapidly. |
| Consequently, in all likelihood, it is not the presence of the organisms, or the biofilms, or the β amyloid that is the etiology of AD. It is rather the reaction of the immune system to these organisms coated with biofilms that leads to the disease with all its manifestations. As it has been previously discussed, killing these organisms before they arrive at the brain or before they do damage (create biofilms) is exceedingly important in this disease [20]. Moreover, it will likely lead to a “cure” of this woeful infirmity, just as has been the case in syphilitic dementia. |
References
- Miklossy J (2011) Alzheimer's disease-a neurospirochetosis. Analysis of the evidence following Koch's and Hill's criteria.J Neuroinflammation 8: 90.
- Riviere GR, Riviere KH, Smith KS (2002) Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer's disease. Oral MicrobiolImmunol 17: 113-118.
- MacDonald AB (2006) Spirochetal cyst forms in neurodegenerative disorders,hiding in plain sight.Med Hypotheses 67: 819-832.
- Miklossy J (2015) Historic evidence to support a causal relationship between spirochetal infections and Alzheimer's disease.Front Aging Neurosci 7: 46.
- Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, et al. (2012) Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am 94:2247-2254.
- Kamer AR, Dasanayake AP, Craig RG, Glodzik-Sobanska L, Bry M, et al. (2008) Alzheimer's disease and peripheral infections: the possible contribution from periodontal infections, model and hypothesis.J Alzheimers Dis 13: 437-449.
- Stanek G, Wormser GP, Gray J, Strle F (2012) Lyme borreliosis. Lancet 379: 461-473.
- Hall-Stoodley L, Hu FZ, Gieseke A, Nistico L, Nguyen D, et al. (2006) Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 296: 202-211.
- Allen HB, Vaze ND, Choi C, Hailu T,Tulbert BH, et al. (2014) The presence and impact of biofilm-producing staphylococci in atopic dermatitis. JAMA Dermatol 150: 260-265.
- Sapi E, Bastian SL, Mpoy CM, Scott S, Rattelle A, et al. (2012) Characterization of biofilm formation by Borrelia burgdorferi in vitro. PLoS One 7: e48277.
- Knobloch JK,Bartscht K, Sabottke A, Rohde H, Feucht HH, et al. (2001) Biofilm formation by Staphylococcus epidermidis depends on functional RsbU, an activator of the sigB operon: differential activation mechanisms due to ethanol and salt stress. J Bacteriol 2001;183:2624-2633.
- Rosen EJ, Vrabek JT, Quinn FB.Infections of the labyrinth. UTMB 2000; Grdrds. UTMB.edu
- GJESTLAND T (1955) The Oslo study of untreated syphilis; an epidemiologic investigation of the natural course of the syphilitic infection based upon a re-study of the Boeck-Bruusgaard material. Acta DermVenereolSuppl (Stockh) 35: 3-368.
- Jana M, Palencia CA, Pahan K (2008) Fibrillar amyloid-beta peptides activate microglia via TLR2: implications for Alzheimer's disease. J Immunol 181: 7254-7262.
- Singhal G, Jaehne EJ, Corrigan F, Toben C, Baune BT (2014) Inflammasomes in neuroinflammation and changes in brain function: a focused review. Front Neurosci 8: 315.
- Dziarski R, Wang Q, Miyake K, Kirschning CJ, Gupta D (2001) MD-2 enables Toll-like receptor 2 (TLR2)-mediated responses to lipopolysaccharide and enhances TLR2-mediated responses to Gram-positive and Gram-negative bacteria and their cell wall components. J Immunol 166: 1938-1944.
- Gallo PM, Rapsinski GJ, Wilson RP, Oppong GO, Sriram U, et al. (2015) Amyloid-DNA Composites of Bacterial Biofilms Stimulate Autoimmunity. Immunity 42: 1171-1184.
- Tükel C, Wilson RP, Nishimori JH, Pezeshki M, Chromy BA, et al. (2009) Responses to amyloids of microbial and host origin are mediated through toll-like receptor 2. Cell Host Microbe 6: 45-53.
- Doyle KP1, Quach LN2, Solé M3, Axtell RC2, Nguyen TV1, et al. (2015) B-lymphocyte-mediated delayed cognitive impairment following stroke. J Neurosci 35: 2133-2145.
- Allen HB, Hannaway M, Joshi S (2015) Tertiary treponematosis. J ClinExp Dermatol Res 6: 288-290.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
 |
 |
 |
|
| Figure 5 | Figure 6 | Figure 7 |
Relevant Topics
- Bacteria Induced Neuropathies
- Blood-brain barrier
- Brain Infection
- Cerebral Spinal Fluid
- Encephalitis
- Fungal Infection
- Infectious Disease in Children
- Neuro-HIV and Bacterial Infection
- Neuro-Infections Induced Autoimmune Disorders
- Neurocystercercosis
- Neurocysticercosis
- Neuroepidemiology
- Neuroinfectious Agents
- Neuroinflammation
- Neurosyphilis
- Neurotropic viruses
- Neurovirology
- Rare Infectious Disease
- Toxoplasmosis
- Viral Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 14110
- [From(publication date):
March-2016 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 12865
- PDF downloads : 1245
