Commentary Open Access
Alzheimer's disease: a novel hypothesis for the development and the subsequent role of beta amyloid
Herbert B Allen*, Diego Morales, Krister Jones and Suresh G Joshi
Drexel University College of Medicine, Philadelphia, USA
- *Corresponding Author:
- Herbert B Allen
Drexel University College of Medicine, Philadelphia, USA
Tel: 2157625550
Fax: 2157625570
E-mail: Herbert.Allen@drexelmed.edu
Received date: March 23, 2016; Accepted date: April 23, 2016; Published date: April 25, 2016
Citation: Allen HB, Morales D, Jones K, Joshi SG (2016) Alzheimers Disease: A Novel Hypothesis for the Development and the Subsequent Role of Beta Amyloid. J Neuroinfect Dis 7:211. doi:10.4172/2314-7326.1000211
Copyright: © 2016 Allen HB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Neuroinfectious Diseases
Abstract
Spirochetes, biofilms, innate immune system activity have all been recently found in the brains of Alzheimer's disease patients. The mechanism and actions of those entities in producing the disease were postulated in those studies. The production and role of beta amyloid were not included in the discussion; we hypothesize herein how the development of that molecule occurs as a result of the Toll-like receptor 2 activation leading not only to TNFα, but also NFκB which themselves have been previously shown to induce the secretases necessary to cleave the amyloid precursor protein. This leads directly to beta amyloid. The beta amyloid (Aβ) has been shown to be antimicrobial, and its presence on and around the hippocampal plaques (the pathological hallmark of Alzheimer's disease) has been demonstrated. It becomes apparent that the Aβ tries to kill the spirochetes but cannot penetrate the biofilm. Its buildup then interrupts and destroys the neurocircuitry of the brains.
Keywords
Spirochetes; Biofilm; Toll-like receptor 2; Secretases; TNFα; NFκB, Beta amyloid; Autoimmunity
Commentary
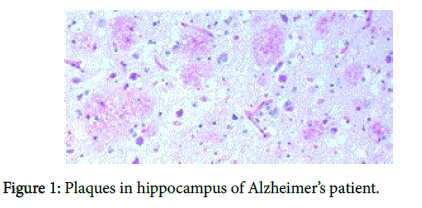
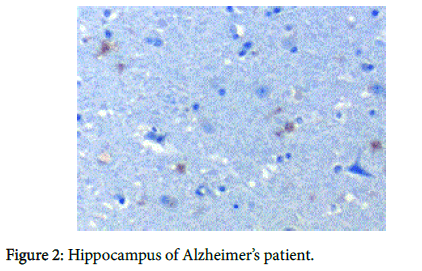
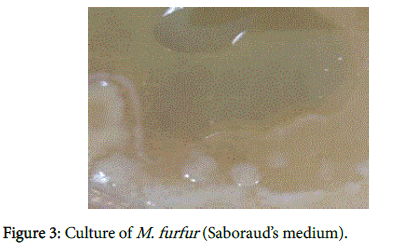
We recently have demonstrated pathologically that biofilms are present in the brains of Alzheimer’s disease (AD) patients [1] (Figure 1). The biofilms were undoubtedly created by the dental and Lyme spirochetes which have previously been shown to be present there [2-5]; consequently, these biofilms would represent a chronic infection [6]: We also provided immunopathological evidence that the innate immune system reactant, Toll-like receptor 2 (TLR 2), was upregulated in that same tissue (Figure 2). We postulated that TLR 2, while trying to destroy the spirochetes, could not penetrate the biofilm (Figure 3) and attacked the surrounding tissue instead [1]. We also alluded to the recent work showing how the adaptive immune system became involved after traumatic brain injury and very rapidly created much more devastating damage than the innate immune system [7].
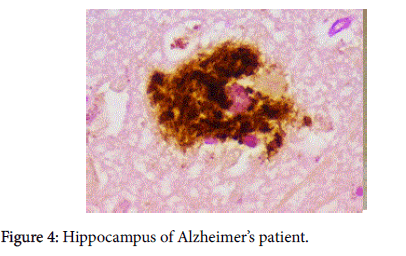
Our other observation, which combined pathology and immunopathology was demonstrating the co-localization of beta amyloid (Aβ) and biofilm [1] (Figure 4). The significance of the finding represented by that photomicrograph was not alluded to. We present herein observations and marshal the evidence that gives substantiation to the significance of that finding.
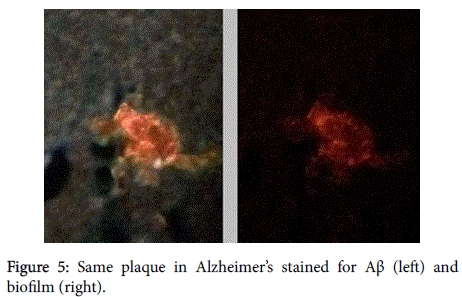
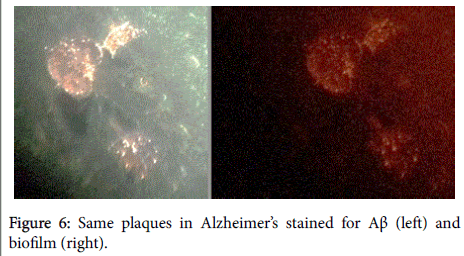
Aβ has been shown, in a 3-dimensional pathology (side-by-side) presentation, to lay on top of the biofilms [8] (Figures 5 and 6). This finding, plus the aforementioned co-localization, positions the Aβ in direct contiguity with the biofilms (which actually form the pathological plaques of AD).
An issue that remains is: how does the Aβ become positioned there, and what is its potential purpose?
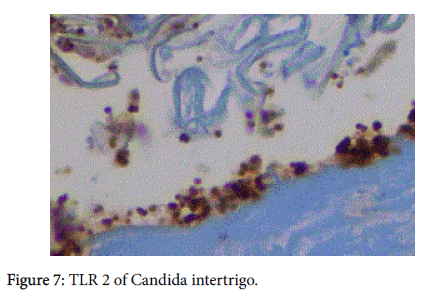
We believe it may be related to the presence of TLR 2. This innate immune system molecule kills by activating the MyD88 pathway which leads to NFκB and ultimately to TNFα [9]. It is the TNFα that is the “killing” agent for TLR 2. (Figure 7). It is capable of destroying planktonic gram positive organisms, yeasts, and spirochetes.
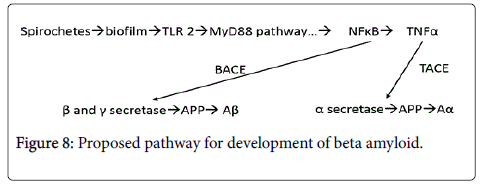
TNFα has been shown to be cleaved by TNFα converting enzyme (TACE) which has been shown to be dramatically upregulated in AD (Figure 8). TACE is localized in the neuronal membranes where it is in direct proximity to the spirochetal derived biofilms and where it acts to upregulate alpha secretase [10]. Beta secretase, and gamma secretase are upregulated by NFκB [11,12]. NFκB then links with BACE (beta amyloid converting enzyme) that cleaves the amyloid precursor protein (APP) that changes the precursor molecule to Aβ [12]. The γ- secretase has been linked to the genetic form of the disease [13].
Aβ has been shown to be antimicrobial [14]. It seems that is its purpose for which it is generated. However, and this is most important, it is not able to penetrate the biofilm either (just as TNFα cannot).
Thus, the body in trying to rid itself of the spirochetal parasites in one case (TNFα) most assuredly contributes to the disease. In the other case, while also trying to act anti-microbially, the innate immune system creates a substance (Aβ) that further damages the tissue and the neuronal circuits.
As has been said previously, it is most important to treat these microbes before they get to the brain or before the do damage (make biofilms) [15].
All that is necessary is an antibiotic that is bactericidal and crosses the blood brain barrier. If necessary, a biofilm dispersing agent that also crosses the blood-brain barrier, such as a furan, a pyrrole, a piperidine, or a thiophene or other [16-19] could also be added to the regimen (All these pharmaceuticals cross the blood brain barrier). The spirochetes, biofilm, immune system, and Aβ are capable of marked neuronal damage which is non-reversible. This makes treatment and potential prevention both urgent and compelling.
References
- Allen HB, Morales D, Jones K, Joshi S (2016) Alzheimer’s disease: A Novel Hypothesis Integrating Spirochetes, Biofilm and the Immune System. J Neuroinfect Dis 7:1
- Miklossy J (2011) Alzheimer’s disease - a neurospirochetosis. Analysis of the evidence following Koch's and Hill's criteria. J Neuroinflammation 8:90.
- Riviere GR, Riviere KH, Smith KS (2002) Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer’s disease. Oral Microbiol Immunol 17: 113-118.
- MacDonald AB (2006) Spirochetal cyst forms in neurodegenerative disorders,...hiding in plain sight. Med Hypotheses 67: 819-832.
- Kamer AR, Dasanayake AP, Craig RG, Glodzik-Sobanska L, Bry M, et al. (2008) Alzheimer's disease and peripheral infections: the possible contribution from periodontal infections, model and hypothesis. JAD 13: 437-449.
- Jacovides CL, Kreft R, Adeli B, Hozack B, Ehrlich GD, et al. (2012) Successful identification of pathogens by polymerase chain reaction (PCR)-based electron spray ionization time-of-flight mass spectrometry (ESI-TOF-MS) in culture-negative periprosthetic joint infection. J Bone Joint Surg Am 94:2247-2254.
- Doyle KP, Quach LN, Sole M, Axtell RC, Nguyen TV, et al. (2015) Beta-lymphocyte mediated delayed cognitive impairment following stroke. J Neurosci 35: 2133-2145.
- https://spirodementia.wordpress.com/featured-new-discovery-blood-borne-borrelia-biofilms-coated-with-beta-amyloid-7-oct-2105/
- Allen HB, Vaze ND, Choi C, Hailu T, Tulbert BH, et al. (2014) The presence and impact of biofilm-producing staphylococci in atopic dermatitis. JAMA Dermatol 150: 260-265.
- Skovronsky DM, Fath S, Lee VM-Y, Milla ME (2001) Neuronal localization of the TNFα converting enzyme (TACE) in brain tissue and its correlation to amyloid plaques. J Neurobiol 49:40-46.
- Chami L, Checler F (2012) BACE1 is at the crossroad of a toxic vicious cycle involving cellular stress and β-amyloid production in Alzheimer’s disease. Molecular Neurodegeneration 7: 52.
- O’Brien RJ, Wong PC (2011) Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci 34: 185-204.
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, et al. (1999) A pre-senilin-1 dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature 398: 518-522.
- Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, et al. (2010) The Alzheimer’s disease-associated β-protein is an anti-microbial peptide. PLoS ONE 5: e9505.
- Allen HB, Hannaway M, Joshi S (2015) Tertiary treponematosis. J Clin Exp Dermatol Res 6:4.
- Ghanwate NA (2012) Biofilm eradication studies on uropathogenic E. coli using ciprofloxacin and nitrofurantoin. Int J Pharm 3:127-131.
- Richards JJ, Reed CS, Melander C (2008) Effects of N-pyrrole substitution on the anti-biofilm activities of oroidin derivatives against Acinetobacter baumannii. Bioorg Med Chem Lett 18: 4325-4327.
- Kagan S, Jabbour A, Sianov E, Alquntar AA, Steinberg D, et al. (2013) Anti- Candida albicans biofilm effect of novel heterocyclic compounds. J AntimicrobChemother 69: 416-427.
- Liu H, Zhao Y, Shao D, Zhao D, Gong T, et al. (2015) Antibacterial and anti-biofilm activities of thiazolidione derivatives against clinical staphylococcus strains. Emerging microbes and infections.
Relevant Topics
- Bacteria Induced Neuropathies
- Blood-brain barrier
- Brain Infection
- Cerebral Spinal Fluid
- Encephalitis
- Fungal Infection
- Infectious Disease in Children
- Neuro-HIV and Bacterial Infection
- Neuro-Infections Induced Autoimmune Disorders
- Neurocystercercosis
- Neurocysticercosis
- Neuroepidemiology
- Neuroinfectious Agents
- Neuroinflammation
- Neurosyphilis
- Neurotropic viruses
- Neurovirology
- Rare Infectious Disease
- Toxoplasmosis
- Viral Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 11982
- [From(publication date):
June-2016 - Jul 11, 2025] - Breakdown by view type
- HTML page views : 10921
- PDF downloads : 1061