Review Article Open Access
Alternative Options to Manage Menopausal Symptoms with a Focus on Melatonin and Osteoporosis
| Holly Lassila1, Nutjaree Pratheepawanit Johns2, Christine K O’Neil1, Jeffrey R Johns3, Judith L Balk4 and Paula A Witt-Enderby5* | |
| 1Division of Clinical, Social and Administrative Sciences, School of Pharmacy, Duquesne University, Pittsburgh, PA, USA | |
| 2Department of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, 123 Khon Kaen University, Naimaung, Muang Khon Kaen Thailand | |
| 3Department of Social and Administrative Pharmacy, Faculty of Pharmaceutical Sciences, 123 Khon Kaen University, Naimaung, Muang Khon Kaen Thailand | |
| 4Department of Obstetrics and Gynecology, Temple University School of Medicine, USA | |
| 5Division of Pharmaceutical Sciences, School of Pharmacy, Duquesne University, Pittsburgh, PA, USA | |
| Corresponding Author : | Paula A Witt-Enderby School of Pharmacy, Duquesne University Pittsburgh, 421 Mellon Hall, PA 15282, USA Tel: 412-396-4346 Fax: 412-396-5599 E-mail: wittp@duq.edu |
| Received December 13, 2013; Accepted February 10, 2014; Published February 12, 2014 | |
| Citation: Lassila H, Johns NP, O'Neil CK, Johns JR, Balk JL, et al. (2014) Alternative Options to Manage Menopausal Symptoms with a Focus on Melatonin and Osteoporosis. Clin Pharmacol Biopharm 3:115. doi:10.4172/2167-065X.1000115 | |
| Copyright: © 2014 Lassila H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Many menopausal women are seeking symptomatic relief from hot flushes, irritability, sleep disturbances, anxiety and depression, and to prevent bone loss. Instead of pharmaceutical approaches, many women are opting for alternative modalities such as yoga, meditation and natural products. Melatonin is a molecule released from the pineal gland in response to darkness and is commonly used as a sleep aid due to its soporific effects and/or due to its ability to reentrain circadian rhythms out of synchrony with the light dark cycle. The focus of this mini-review is to highlight the novel use of melatonin on managing menopausal symptoms and menopausal bone loss and describe food sources that are rich in melatonin.
| Keywords |
| Melatonin; Complementary and alternative medicine; Menopause; Osteoporosis; CAM; Peri-menopause; Integrative medicine |
| Introduction |
| As a woman transitions through life, changes in the hormone profiles of estrogen, progesterone, cortisol and testosterone occur [1]. Paralleling these changes is a decline in nocturnal melatonin levels, likely contributing to the sleep disturbances commonly associated with menopause. In addition to the sleep disturbances, some women note a decline in their health status and quality of life (QOL) and, as such, seek medical attention. The costs associated with treating menopause-related health issues are high and include physician visits, follow-up visits and telephone calls for the management of medication-related side effects and changes in medication, over-the counter remedies, laboratory tests; lost productivity at work; and others. Many menopausal women are seeking symptomatic relief from hot flushes, irritability, sleep disturbances, anxiety and depression, and to prevent bone loss. Many women are using alternative modalities such as yoga, meditation and natural products instead of pharmaceutical approaches. The focus of this mini-review is to highlight the novel use of melatonin on managing menopausal symptoms and menopausal bone loss and describe food sources that are rich in melatonin. |
| Menopause, Symptoms and Treatment Options |
| Menopause is defined as the final menstrual period, and post menopause is confirmed when a woman has been amenorrheic for 12 consecutive months. Menopause is associated with reduced functioning of the ovaries due to aging, resulting in lower levels of estrogen and other hormones. It marks the permanent end of fertility. Menopause occurs, on average, at age 51 [2]. The most common symptoms are vasomotor symptoms such as hot flushes and night sweats, sleep disruption, mood changes including depression and anxiety, and vaginal dryness [3,4]. During the menopause transition, which is defined as the period between pre-menopause and menopause, the main ovarian hormones, estrogen and progesterone, become dysregulated and no longer follow a regular pattern. The menopause transition is characterized by hypoestrogenism and hypoprogesteronism [5,6]. The exact cause of vasomotor symptoms is not known, but it is thought to be due to rapid declines in estrogen concentrations. Once a woman has completed menopause, vasomotor symptoms tend to resolve. Women most commonly seek medical treatment for vasomotor symptoms. Night sweats often lead to sleep disruption, but even in the absence of night sweats, sleep disruption is common. Women also seek medical treatment for vaginal dryness, because it can lead to dyspareunia and even inability to participate in sexual activity. Women also may seek treatment for mood problems such as anxiety, depression, and irritability. |
| Of the pharmaceutical options, hormone therapy is most efficacious. Other pharmaceutical options used include selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), gabapentin, and clonidine [7]. Hormone therapy’s risks and benefits are controversial; the Women’s Health Initiative (WHI) found increased risks of breast cancer, stroke, myocardial infarction, and thromboembolic disorders with oral combined hormone therapy [8]. However, the WHI may not be generalizable to all women and to other forms of hormone therapy. The other pharmaceutical options are also limited by side effects including sexual dysfunction and dizziness. Also, many women are not comfortable with the perceived stigma of taking antidepressants. Women are seeking alternatives like Complementary and Alternative Medicine (CAM) to pharmaceutical options for management of menopausal symptoms. |
| Use of CAM to Manage Menopause-Related Symptoms and Disease |
| The results of both cross-sectional and longitudinal cohort studies have reported the prevalence of CAM use in menopausal aged women ranging from 45.2%-91% depending on the population surveyed [9-12]. Many women are seeking alternative methods like yoga, meditation, and herbal therapies for relief of menopausal symptoms because, for many women, current drug therapy is not desirable, or not an option due to drug-associated side effects or their medical risk factors. In fact, the number of menopausal women using CAM therapies for relief of menopausal symptoms is great with some studies reporting numbers as high as 91% [10]. For most women, their use of CAM is for stress management or to manage menopause symptoms [9], specifically hot flashes and to promote long-term health and well-being [13]. |
| There has been very little well designed research on assessing the efficacy of CAM therapies for relief of menopausal symptoms [14]. There have been a number of review articles published that have generally concluded that individual trials may suggest a benefit for certain CAM therapies; however long term well designed larger studies are still needed [15-17]. The majority of these studies have included phytoestrogens and other biologically based agents which have shown mixed results related to menopausal vasomotor symptoms, lipid profiles and bone mineral density [16,17]. Other alternatives, like melatonin, should be tested because, unlike many of the previously tested therapies, melatonin may provide relief from a multitude of symptoms rather than one. |
| Use of Melatonin to Manage Menopausal Symptoms |
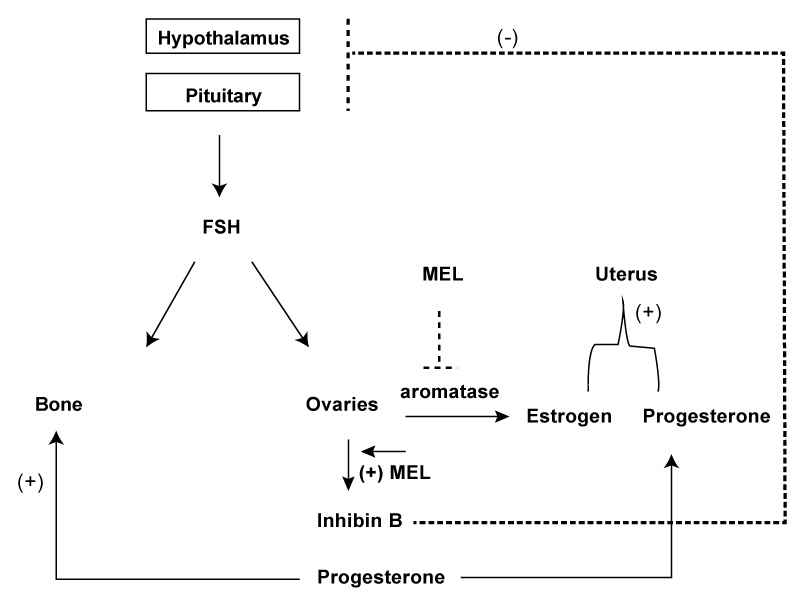
| Melatonin is known most for its beneficial effects on sleep through its resynchronization of circadian rhythms to align more with the light/dark cycle in middle aged to elderly patients without harmful side effects [18-20], making it a safe alternative for use in an aging population [21-25]. Besides having positive effects on sleep, melatonin has also been shown to be closely linked with reproductive functioning in women [26-29], which has led some to speculate that melatonin may play a role in the menopausal transition [22,30,31]. Melatonin levels decrease with age, particularly during the peri-menopausal period [32,33] coincident with the appearance of menstrual irregularities, sleep disturbances, bone loss, elevated estrogen and reduced progesterone levels. Melatonin is inversely correlated with FSH [34] and estrogen [35-38] and positively correlated with progesterone [39-43] so the loss of melatonin that occurs with age may be contributing to menopauserelated symptoms due to dysregulated FSH, estrogen and progesterone levels. Melatonin may be acting to rebalance disrupted hormonal rhythms through its actions on the ovary to lower FSH and estrogen levels and raise progesterone levels improving menopausal symptoms and reducing the risk of menopausal bone loss (Figure 1). Previous studies have shown that women aged 43-49 yr taking melatonin have a restoration of their pituitary functions back to juvenile (premenopausal) patterns of regulation [30,44], improvement in the physical aspects of menopause (i.e., flatulence or gas pains, aching in muscles and joints, feeling tired or worn out, difficulty sleeping, aches in back of neck or head, decrease in physical strength, decrease in stamina, lack of energy, dry skin, weight gain, increased facial hair, changes in appearance, texture or tone of skin, feeling bloated, low backache, frequent urination, involuntary urination when laughing or coughing, breast tenderness, vaginal bleeding or spotting, and leg pains or cramps) [28], decreases in the number of menstrual cycles [28] and a normalization of bone markers to resemble the premenopausal state [28]. |
| Menopause and Osteoporosis |
| Throughout the menopause transition, women may experience menstrual irregularities, vasomotor symptoms, and sleep disturbances; however, what most women do not know is that they will also begin experiencing bone loss [45-47]. In the US, adults over the age of 50 have either osteopenia (48 million) or osteoporosis (9 million), which increases their risk for developing bone fracture. If the prevalence of bone disease in the US population is not reduced, it is predicted that by 2030, 64.3 million adults will have osteopenia and 11.9 million will have osteoporosis [48]. A greater prevalence of bone loss and related fractures occurs in postmenopausal women and this is attributed, in part, to hormonal imbalances that occur during the menopausal transition [49]. Thirty percent of all post menopausal women have osteoporosis and of these women, 40% of them are anticipated to have one or more fractures during their lifetime [50], which could lead to morbidity and mortality [51]. According to the North American Menopause Society (NAMS), pharmacotherapy is recommended for all post menopausal women who have had an osteoporotic vertebral fracture, postmenopausal women who have BMD values consistent with osteoporosis, and all postmenopausal women with BMD T-scores from -2 to -2.5 and at least one risk factor (i.e., thinness, low body mass index, history of fragility fractures since menopause, or history of hip fracture in a parent) [52]. |
| Life Style Approaches to Managing Bone Loss |
| As stated previously, women are using CAM during menopause, in part, to promote long-term health and well-being [13]. As it relates to preventing bone loss, women are making lifestyle adjustments to prevent bone loss like increasing weight-bearing exercise (i.e., walking, running, and step aerobics), and by increasing calcium/vitamin D consumption. These lifestyle adjustments may provide some benefit on bone mass as a meta-analysis found that spinal bone mineral density (BMD) increased 2% in women who exercise [53] and, through its positive effects on muscle mass, strength and balance, exercise has been associated with a reduction in fall-related injuries and fractures. Research has also been done on the effects of passive stress (highfrequency vibration systems) on bone mass with promising results [54]. |
| Vitamin D is essential for calcium absorption and also has been found to improve muscle strength, balance and reduce the risk of falling. While randomized controlled trials of calcium with vitamin D in doses of 400 units and 800 units have not been shown to significantly reduce fracture risk, a meta-analysis of these trials in postmenopausal women revealed that 700 to 800 units of vitamin D was associated with a reduced risk of hip and non-vertebral fractures [55]. The NAMS currently recommends 700 to 800 units of vitamin D daily in women at risk for deficiency due to inadequate sun exposure [52]. |
| The primary management goals for patients with osteoporosis are to prevent fracture, decrease further bone loss, decrease pain, and maintain function. The current therapeutic approaches are listed in Table 1. Even with treatment, morbidity and mortality are still on the rise [51,56]. A woman’s health condition, her risk factors and the adverse effects of the drug therapy may preclude her from using the current therapies to manage her bone loss. So, many women are seeking alternative therapies to manage their health including bone loss [9-12] that include phytoestrogens and other biologically based agents [16,17]. Most studies to date are treatment-focused attempting to reverse the bone loss that has already occurred rather than preventing the bone loss from occurring at all. Melatonin should be another therapy to consider for preventing bone loss starting at peri-menopause and continuing throughout life. |
| Melatonin Effects on Hormone Rhythms and Bone |
| Melatonin levels decrease with age, particularly during the perimenopausal period [32,33] coincident with the appearance of bone loss. An increase risk of hip and wrist fracture occurs in shift workers who have worked for greater than 20 years [57]; a decrease in BMD also occurs in those who work shifts other than the daytime shift [58]; shift workers experience light exposure at night suppressing the nocturnal surge in melatonin, thus, removing melatonin’s known bone-protective actions [59-66]. Melatonin is inversely correlated with FSH [34] and estrogen [35-38] and positively correlated with progesterone [39-43] so the loss of melatonin that occurs with age may be contributing to menopause-related bone loss because FSH is involved in bone resorption [67] and progesterone is involved in bone formation [47]. In support of this, peri-menopausal women taking 3 mg melatonin nightly for 6 months show improvement in markers of bone turnover (decreased bone resorption, increased bone formation) resembling the more balanced bone marker turnover (1:1 bone resorption: bone absorption) seen in pre-menopausal women [28]. It is hypothesized that melatonin may be resynchronizing these disrupted hormonal rhythms through its actions on the ovary decreasing FSH mediated bone resorption while increasing progesterone-mediated bone formation (Figure 1). |
| Alternative Sources of Melatonin |
| As stated previously, use of herbal medicines for management of one’s health has dramatically increased [68,69] perhaps because many find natural products to be more desirable than synthetic versions, for personal reasons. Melatonin, a highly evolutionary conserved molecule, is present in nearly all forms of life including: unicellular algae, Gonyaulax polyedra [70,71], invertebrates [72,73], and vertebrates. It is considered to have a functional role in plants, protecting them against internal and environmental oxidative stressors, and a means of coping with harsh environments [74]. Because of this, the wide variety of plants and plant products rich in melatonin can be expected to provide therapeutic efficacy even though levels have been shown to vary widely in concentration and sub-cellular localization [75]. Another reason for this variation in melatonin content in plants and plant products could be due to the method by which melatonin is extracted and analyzed. Thus, the levels reported in the literature for the same plant or plant product may be different dependent on the solvents and conditions used for extraction, maceration, sonication and purification as well as on the type of analysis used [e.g. high-performance liquid chromatography (HPLC), gas chromatography mass spectrometry (GC-MS) and immunoassay [76]. Variations are also likely to be due to species varieties, ripeness, and part of the plant analyzed. For this review, selected plant materials frequently consumed and/or that contain substantial amounts of melatonin were chosen for analysis and discussion. For a more comprehensive review of the literature, the reader is referred to other excellent reviews [75,77]. |
| Food Sources Rich in Melatonin |
| Primary sources of dietary melatonin are likely to come from herbs, seeds and grains, vegetables, and fruits. However, secondary sources, rich in melatonin, can be derived from plants like coffee, wine, oil, and beer. For a listing of these food sources and their melatonin levels, please refer to Table 2. Many herbs are consumed for relief from inflammation, sleep disturbances and dementia most likely due to their anti-oxidant properties and their high levels of melatonin. High levels of melatonin were identified in fever few fresh green leaves (Tanacetum parthenium), St. Johns Wort flowers (Hypericum perforatum), and Huang-qin (Scutellaria blacalensis) [78]. Melatonin was also investigated in more than 100 Chinese medicinal herbs as dried powders derived from flowers, seeds, leaves, roots or stems [79] and, of the 100 tested, 64 contained melatonin in excess of 10 ng/g dry mass and 10 contained more than 1,000 ng/g dry mass. Traditionally, it is those herbs containing the highest levels of melatonin that are used to treat ailments related to oxidative stress. In Thai traditional medicine and for use as a sleep aid, a remedy using leaves of seven edible herbs showed significant levels of melatonin [black pepper (Piper nigrum, 1,093 ng/g of dry sample weight), Burmese grape (Baccaurea ramiflora, 43.2 ng/g of dry sample weight), humming bird tree/scarlet wisteria (Sesbania glandiflora, 26.3 ng/g of dry sample weight), bitter gourd (Moringa charantia, 21.4 ng/g of dry sample weight), Sesbania tora (10.5 ng/g of dry sample weight) and Sesbania sesban (8.7 ng/g of dry sample weight)] [80]. Likewise, other Thai herbs used for inflammation, antibacterial and glucose control were screened and showed melatonin content ranging from 0.5 to 146 ng/g dry weight [81]. |
| Seeds are thought to contain high levels of melatonin to protect themselves against UV-radiation and oxidative stress during germination and early growth [74]. High levels of melatonin (2-200 ng/g) were detected in the seeds of 15 edible plants, with the highest concentrations observed in white (Brassica hirta) and black mustard (Brassica nigra) seeds [82]. None of these plants are primary foodstuffs consumed in appreciable quantities. However, levels of approximately 1 ng/g have been reported in oat, sweet corn, and rice [83] and a variety of Chinese corn seeds contained amounts of melatonin ranging from 0-2, 034 ng/g and Chinese rice seeds 0-264 ng/g dry weight [84]. |
| Melatonin has also been detected in vegetables, leaves and fruit where the concentration of melatonin varies with the plant’s degree of maturation [85]. Unlike dry herbs or seeds, the majority of fresh vegetables and fruits contain high water content so when levels of melatonin are reported, they are reported as “per wet weight”. This reporting makes their melatonin values much lower compared to dried products. Modest amounts of melatonin (10-113 pg/g wet tissue) were quantified by HPLC in cabbage, onion, cucumber, asparagus, Indian spinach and carrot. Highest amounts of melatonin are found in the Japanese radish (Bassica campestris, 657 pg/g) and ginger (Zingiber officinale, 0.6 ng/g) [83] and these levels were confirmed using GC-MS [86]. The melatonin content in tomato (Lycopersicon esculentum) has been widely studied and levels vary greatly (2.2 pg/g to 114.5 ng/g fresh weight) dependent on the variety of tomato and the year of harvest, [83,86-90]. |
| A large number of edible fruits (i.e., strawberry, kiwi-fruit, pineapple, and apple) have melatonin levels between 12-48 pg/g wet tissue. Levels of melatonin in strawberries range between 1.4-11.3 ng/g melatonin fresh weight dependent on the variety [83,88]. Melatonin has been detected in fresh-frozen Montmorency (13.5 ng/g), and Balaton tart cherries (2.1 ng/g, Prunus cerasus) and in both the leaves and whole fruit of figs (Ficus carica, 12.9 ng/g for leaves and 4 ng/g whole fruit) [91,92]. Melatonin levels within fresh cherries varied based on variety (tart vs sweet) with sweet cherries containing much lower levels of melatonin (0-0.22 ng/g fresh fruit) than tart, but not on the orchard of origin nor the time of harvest [93]. |
| Food products derived from plants show substantial amounts of melatonin and the food production process can either increase or decrease melatonin content in the product. Coffee (Coffea canphora and Coffea arabica), for example, has been shown to contain significant amounts of melatonin (5,800 to 12,500 ng/g dry weight in green beans and 8,000 to 9,600 ng/g in roasted beans) [94], however, brewing the coffee reduces the melatonin to about half these levels. |
| Grape (Vitis vinifera) cultivars, Nebbiolo, Croatina, Sangiovese, Merlot, Marzemino, Cabernet Franc, Cabernet Sauvignon and Barbera, were found to contain between 2.4 to 428 pg/ml (0.005-0.97 ng/g) melatonin in grape skin extracts with varieties of Nebbiolo and Croatina containing the highest amounts [95,96]. It is also shown that the melatonin content in wines increases following indoleamine synthesis by yeasts during the fermentation process [97]. Rodriguez- Naranjo and co-workers [98] analyzed eight red wines (Jaen Tinto, Merlot, Palomino Negro, Petit Verdot, Syrah, Cabernet Sauvignon, Prieto Picudo and Tempranillo) and found between 140 and 277 pg/ mL melatonin. Additionally, although melatonin was not detected in pomegranate fruit, levels up to 5.50 ng/mL were detected in pomegranate wine [99]. |
| A wide variety of beers has also been shown to contain melatonin, with levels ranging from 51.8 to 169.7 pg/mL [100] where no correlation existed between melatonin content, the type of beer (lager, bitter or stout) or with the barley or hop source; however, there was a correlation with alcohol content (r = 0.56, p = 0.045), perhaps due to the yeast fermentation process similar to that observed for wine. |
| The melatonin contents of the leaves of seven edible herbs used in a Thai traditional sleeping formula based on Ayurveda traditional medicine have also been determined by HPLC and ELISA. The melatonin contents of the extracts of Baccaurea ramiflora (L.), Sesbania glandiflora (L.), Momordica charantia (L.), Senna tora (L.) and Sesbania sesban (L.) Merr. Was 43.2, 26.3, 21.4, 10.5 and 8.7 ng/g dry sample weight, respectively. The highest melatonin content was from P. nigrum extract (1092.7 ng/g dry sample weight) suggesting that it may be very effective as a traditional sleeping medicine especially when used in combination with other herbs with sedative properties [101]. |
| Bioavailability of Melatonin in Food Sources |
| It has always been questioned whether or not food sources can provide enough melatonin to derive any therapeutic benefit from them. Similar to taking a melatonin supplement, melatonin, when consumed in food, will be absorbed from the gastrointestinal tract and go through first-pass metabolism in the liver with the remainder being distributed into different parts of the body before being excreted mainly as a soluble metabolite (6-sulfatoxymelatonin, aMT6s) in urine. Of the few bioavailability studies performed in animals, most have shown that foods rich in melatonin increase significantly blood levels of melatonin. In the first animal study, comparing plasma melatonin levels in 2-week old chickens fed a diet high in melatonin content (i.e, 3.5 ng melatonin/g diet) to those fed a diet low in melatonin (less than 100 pg/g diet), it is shown that chickens fed the high melatonin diet had significantly higher levels of plasma melatonin ranging from 20 pg/mL to 34 pg/mL after 1.5 hrs (p<0.01) compared to the 8 pg/mL observed in chickens fed the low melatonin diet [83]. Similarly, in another study comparing serum melatonin concentrations in rats fed walnuts (Juglans regia L.; melatonin content 3.5 ± 1.0 ng/g) to those fed regular chow, it is shown that rats fed the walnuts had significantly higher serum melatonin concentrations than chow fed rats (38.0 ± 4.3 pg/mL vs 11.5 ± 1.9 pg/mL; p<0.01). More importantly, these higher levels of melatonin correlated with increases in ‘total antioxidant power’ of the serum measured by trolox equivalent antioxidant capacity (TEAC) and ferric-reducing antioxidant power (FRAP) methods [102]. |
| In addition to the animal studies, studies performed in humans show that consumption of melatonin-rich foods results in significant levels of melatonin in the blood and correlate with higher antioxidative capacity. For example, Japanese women consuming the highest vegetable intake had higher levels of aMT6s in their first-void morning urine compared to those who did not have the highest intake of vegetables; however, because of incomplete data for melatonin in the plants, it was impossible to estimate dietary melatonin intake from vegetables and/or from the entire diet [103]. In another study, healthy Japanese women, 24–55 years of age, who consumed 350 g/day of six selected vegetables (intake 1,288 ng melatonin/day) for 65 days, had slightly elevated urinary aMT6s from baseline (from 48.1 to 49.6 ng/ mg creatinine). This is in contrast to the decrease in aMT6s levels from baseline (from 55.5 to 50.8 ng/mg creatinine) observed in women who were asked to avoid these same six vegetables during the same period (intake 5.3 ng melatonin/day; p = 0.03) [104]. In another crossover study examining levels of urinary aMT6s following consumption of 200 g of whole cherries (Prunus avium L., 200 g 2x/day for 3 days as lunch and dinner desserts) in 6 middle-aged and 6 elderly people, it was shown that aMT6s levels increased by 125-220% compared to baseline. Even more, the rise in aMT6s levels were accompanied by significant rises in total antioxidant capacity and improved sleep (i.e., sleep time, total nocturnal activity, assumed sleep, and immobility) [105]. Likewise, in another crossover study assessing urinary aMT6s levels in 30 young healthy volunteers following consumption of 6 tropical fruits sequentially per week (with a one-week washout period between fruit), it was shown that the largest increases in urine aMT6s concentration occurred in those who consumed pineapple (266% of baseline, p =0.004), followed by consumption of bananas (180% of baseline, p = 0.001), and then oranges (47% of baseline, p = 0.007); however, lipid peroxidation (8-isoPGF2α marker) was not significantly decreased after 6 weeks of the study though a trend was observed (p=0.07) [106]. |
| Although it has been shown that levels of aMT6s correlate with melatonin levels in the blood [107], it is not a direct measure of circulating melatonin in the body and in tissues. Levels of melatonin fluctuate in response to the light: dark cycle resulting in serum melatonin levels ranging between 120-200 pg/mL at night and 10-20 pg/mL during the day [108]. Melatonin can also accumulate in tissues achieving 2-3 orders of magnitude to that in serum [109,110]. It is shown that 3 mg nocturnal consumption of melatonin produced significant and positive effects on menopausal symptoms in women [28] and even though the source of melatonin was via capsules in this study, consumption of foods rich in melatonin is expected to provide the same benefit. The first evidence showing that ingestion of foods rich in melatonin increases serum melatonin levels was recently reported in young, healthy volunteers who sequentially consumed juice extracted from one kilogram of either oranges, pineapple or two whole bananas following a light breakfast over one week with a one week wash-out period between fruits [111]. The highest serum melatonin concentration occurred 120 minutes following consumption of all fruits with the greatest increase occurring following consumption of pineapple juice, and then followed by orange juice and then bananas (Table 3). Paralleling these increases in melatonin were increases in serum antioxidant capacity as measured by FRAP and oxygen radical antioxidant capacity (ORAC; Table3). Overall, these data show that tropical fruit consumption can elevate serum melatonin up to nocturnal levels and can raise the anti-oxidant capacity in serum of young healthy volunteers. |
| Conclusions and Future Perspectives |
| Much of the research studying the efficacy of alternative therapies to prevent or treat disease is focused on a reductionistic approach trying to find “the” component within the plant or plant product responsible for the health-promoting effect. However, given the fact that plants and plant products contain multiple phytochemicals, research in the area of natural products should concentrate more on the interactions between these phytochemicals within the plant rather than one or two and determine how these interactions result in positive health outcomes. Melatonin found in plants and plant products is most likely producing its beneficial effects on health through its interactions with other phytochemicals like anthocyanins, vitamin C and E, polyphenols, Resveratrol (grape), licopene (tomato), harmanine (passion flower) to name a few. Also, another area that should be focused upon is the extra pineal production of melatonin, for example, in the GI tract. This is an emerging and important area of research because the GI tract contains the enzymes responsible for the biosynthesis of melatonin (i.e., serotonin-N-acetyltranferase (NAT) and acetyl serotonin methyltransferase (ASMT) [76]. Seeing that previous studies report increases in serum melatonin concentration after tryptophan product ingestion as a result of gastrointestinal melatonin synthesis [112-115] and that many of the plants investigated contain appreciable amounts of tryptophan and serotonin, then ingestion of these melatonin-rich plants may achieve even greater levels of melatonin and anti-oxidative capacity than that measured in serum. |
| Overall, because the trends are showing that more women are turning to alternative therapies for managing their menopausal symptoms as well as to maintain good health, more research in this area is essential. Melatonin should be another therapy investigated for its efficacy to manage menopausal symptoms and to prevent menopauseassociated disease like osteoporosis since research is showing beneficial effects in this population [28,30,44]. Research should not be limited to females as middle-aged and elderly men (45-50 years and older) experience age- or drug-related bone loss and osteoporosis; supplementation of their diets with melatonin starting around age 45- 50 and continuing throughout life is expected to provide protection from bone loss as well as other diseases like prostate cancer [116]. Use of melatonin supplements or consumption of foods naturally rich in melatonin or genetically engineered to produce higher levels of melatonin [117,118] alone or in combination with other therapies to enhance endogenous nocturnal levels is expected to attenuate many disorders associated with reduced melatonin levels caused by light exposure at night, menopause and aging [23,29,116,119,120]. More research in this area is warranted. |
| References |
References
- Smith PW (2009)What you must know about women's hormones: your guide to natural hormone treatment for PMS, menopause, osteoporosis, PCOS, and more. Garden City Park: Square One.
- The North Ameerican Menopause Society.
- Freeman EW, Grisso JA, Berlin J, Sammel M, Garcia-Espana B, et al. (2001) Symptom reports from a cohort of African American and white women in the late reproductive years. Menopause 8: 33-42.
- Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, et al. (2010) Postmenopausal hormone therapy: an Endocrine Society scientific statement. J ClinEndocrinolMetab 95: s1-1s66.
- Santoro N, Brown JR, Adel T, Skurnick JH (1996) Characterization of reproductive hormonal dynamics in the perimenopause. J ClinEndocrinolMetab 81: 1495-1501.
- Prior JC (2005) Ovarian aging and the perimenopausal transition: the paradox of endogenous ovarian hyperstimulation. Endocrine 26: 297-300.
- Cheema D, Coomarasamy A, El-Toukhy T (2007) Non-hormonal therapy of post-menopausal vasomotor symptoms: a structured evidence-based review. Arch GynecolObstet 276: 463-469.
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, et al. (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA 288: 321-333.
- Newton KM, Buist DS, Keenan NL, Anderson LA, LaCroix AZ (2002) Use of alternative therapies for menopause symptoms: results of a population-based survey. ObstetGynecol 100: 18-25.
- Lunny CA, Fraser SN (2010) The use of complementary and alternative medicines among a sample of Canadian menopausal-aged women. J Midwifery Womens Health 55: 335-343.
- Brett KM, Keenan NL (2007) Complementary and alternative medicine use among midlife women for reasons including menopause in the United States: 2002. Menopause 14: 300-307.
- Bair YA, Gold EB, Zhang G, Rasor N, Utts J, et al. (2008) Use of complementary and alternative medicine during the menopause transition: longitudinal results from the Study of Women's Health Across the Nation. Menopause 15: 32-43.
- Gollschewski S, Kitto S, Anderson D, Lyons-Wall P (2008) Women's perceptions and beliefs about the use of complementary and alternative medicines during menopause. Complement Ther Med 16: 163-168.
- The National Center for Complementary and Alternative Medicine.
- Nedrow A, Miller J, Walker M, Nygren P, Huffman LH, et al. (2006) Complementary and alternative therapies for the management of menopause-related symptoms: a systematic evidence review. Arch Intern Med 166: 1453-1465.
- Kang HJ, Ansbacher R, Hammoud MM (2002) Use of alternative and complementary medicine in menopause. Int J GynaecolObstet 79: 195-207.
- Kronenberg F, Fugh-Berman A (2002) Complementary and alternative medicine for menopausal symptoms: a review of randomized, controlled trials. Ann Intern Med 137: 805-813.
- Arendt J (1997) Safety of melatonin in long-term use (?) J Biol Rhythms 12: 673-681.
- Markantonis SL, Tsakalozou E, Paraskeva A, Staikou C, Fassoulaki A (2008) Melatonin pharmacokinetics in premenopausal and postmenopausal healthy female volunteers. J ClinPharmacol 48: 240-245.
- Wade A, Downie S (2008) Prolonged-release melatonin for the treatment of insomnia in patients over 55 years. Expert OpinInvestig Drugs 17: 1567-1572.
- Zhdanova IV, Wurtman RJ, Lynch HJ, Ives JR, Dollins AB, et al. (1995) Sleep-inducing effects of low doses of melatonin ingested in the evening. ClinPharmacolTher 57: 552-558.
- Brzezinski A (1998) "Melatonin replacement therapy" for postmenopausal women: is it justified? Menopause 5: 60-64.
- Reiter RJ, Tan DX, Manchester LC, PilarTerron M, Flores LJ, et al. (2007) Medical implications of melatonin: receptor-mediated and receptor-independent actions. Adv Med Sci 52: 11-28.
- Laakso ML, Lindblom N, Leinonen L, Kaski M (2007) Endogenous melatonin predicts efficacy of exogenous melatonin in consolidation of fragmented wrist-activity rhythm of adult patients with developmental brain disorders: a double-blind, placebo-controlled, crossover study. Sleep Med 8: 222-239.
- Brzezinski A, Vangel MG, Wurtman RJ, Norrie G, Zhdanova I, et al. (2005) Effects of exogenous melatonin on sleep: a meta-analysis. Sleep Med Rev 9: 41-50.
- Reiter RJ (1980) The pineal and its hormones in the control of reproduction in mammals. Endocr Rev 1: 109-131.
- Srinivasan V, Spence WD, Pandi-Perumal SR, Zakharia R, Bhatnagar KP, et al. (2009) Melatonin and human reproduction: shedding light on the darkness hormone. GynecolEndocrinol 25: 779-785.
- Kotlarczyk MP, Lassila HC, O'Neil CK, D'Amico F, Enderby LT,, et al. (2012) Melatonin osteoporosis prevention study (MOPS): a randomized, double-blind, placebo-controlled study examining the effects of melatonin on bone health and quality of life in perimenopausal women. J Pineal Res 52: 414-426.
- Reiter RJ, Tan DX, Manchester LC, Paredes SD, Mayo JC, et al. (2009) Melatonin and reproduction revisited. BiolReprod 81: 445-456.
- Bellipanni G, Bianchi P, Pierpaoli W, Bulian D, Ilyia E (2001) Effects of melatonin in perimenopausal and menopausal women: a randomized and placebo controlled study. ExpGerontol 36: 297-310.
- Diaz BL, Llaneza PC (2008) Endocrine regulation of the course of menopause by oral melatonin: first case report. Menopause 15: 388-392.
- Iguichi H, Kato KI, Ibayashi H (1982) Age-dependent reduction in serum melatonin concentrations in healthy human subjects. J ClinEndocrinolMetab 55: 27-29.
- Zhou JN, Liu RY, van Heerikhuize J, Hofman MA, Swaab DF (2003) Alterations in the circadian rhythm of salivary melatonin begin during middle-age. J Pineal Res 34: 11-16.
- Vakkuri O, Kivelä A, Leppäluoto J, Valtonen M, Kauppila A (1996) Decrease in melatonin precedes follicle-stimulating hormone increase during perimenopause. Eur J Endocrinol 135: 188-192.
- Okatani Y, Watanabe K, Morioka N, Hayashi K, Sagara Y (1997) Nocturnal changes in pineal melatonin synthesis during puberty: relation to estrogen and progesterone levels in female rats. J Pineal Res 22: 33-41.
- Okatani Y, Morioka N, Hayashi K (1999) Changes in nocturnal pineal melatonin synthesis during the perimenopausal period: relation to estrogen levels in female rats. J Pineal Res 27: 65-72.
- Okatani Y, Morioka N, Wakatsuki A (2000) Changes in nocturnal melatonin secretion in perimenopausal women: correlation with endogenous estrogen concentrations. J Pineal Res 28: 111-118.
- Sánchez-Barceló EJ, Cos S, Mediavilla D, Martínez-Campa C, González A, et al. (2005) Melatonin-estrogen interactions in breast cancer. J Pineal Res 38: 217-222.
- MacPhee AA, Cole FE, Rice BF (1975) The effect of melatonin on steroidogenesis by the human ovary in vitro. J ClinEndocrinolMetab 40: 688-696.
- Webley GE, Luck MR (1986) Melatonin directly stimulates the secretion of progesterone by human and bovine granulosa cells in vitro. J ReprodFertil 78: 711-717.
- Webley GE, Leidenberger F (1986) The circadian pattern of melatonin and its positive relationship with progesterone in women. J ClinEndocrinolMetab 63: 323-328.
- Woo MM, Tai CJ, Kang SK, Nathwani PS, Pang SF, et al. (2001) Direct action of melatonin in human granulosa-luteal cells. J ClinEndocrinolMetab 86: 4789-4797.
- Taketani T, Tamura H, Takasaki A, Lee L, Kizuka F, et al. (2011) Protective role of melatonin in progesterone production by human luteal cells. J Pineal Res 51: 207-213.
- Bellipanni G, DI Marzo F, Blasi F, Di Marzo A (2005) Effects of melatonin in perimenopausal and menopausal women: our personal experience. Ann N Y AcadSci 1057: 393-402.
- Recker R, Lappe J, Davies K, Heaney R (2000) Characterization of perimenopausal bone loss: a prospective study. J Bone Miner Res 15: 1965-1973.
- Abrahamsen B, Nissen N, Hermann AP, Hansen B, Bärenholdt O, et al. (2002) When should densitometry be repeated in healthy peri- and postmenopausal women: the Danish osteoporosis prevention study. J Bone Miner Res 17: 2061-2067.
- Seifert-Klauss V, Prior JC (2010) Progesterone and bone: actions promoting bone health in women. J Osteoporos 2010: 845180.
- National Osteoporosis Foundation (2013) NOF Encourages Women to Break Free from Osteoporosis in Honor of National Women's Health Week.
- Avis NE, Colvin A, Bromberger JT, Hess R, Matthews KA, et al. (2009) Change in health-related quality of life over the menopausal transition in a multiethnic cohort of middle-aged women: Study of Women's Health Across the Nation. Menopause 16: 860-869.
- Reginster JY, Burlet N (2006) Osteoporosis: a still increasing prevalence. Bone 38: S4-9.
- Wolinsky FD, Fitzgerald JF, Stump TE (1997) The effect of hip fracture on mortality, hospitalization, and functional status: a prospective study. Am J Public Health 87: 398-403.
- [No authors listed] (2010) Management of osteoporosis in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause 17: 25-54.
- Kelley GA, Kelley KS, Tran ZV (2002) Exercise and lumbar spine bone mineral density in postmenopausal women: a meta-analysis of individual patient data. J Gerontol A BiolSci Med Sci 57: M599-604.
- Rubin C, Recker R, Cullen D, Ryaby J, McCabe J, et al. (2004) Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res 19: 343-351.
- Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, et al. (2005) Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA 293: 2257-2264.
- Abrahamsen B, Eiken P, Eastell R (2009) Subtrochanteric and diaphyseal femur fractures in patients treated with alendronate: a register-based national cohort study. J Bone Miner Res 24: 1095-1102.
- Feskanich D, Hankinson SE, Schernhammer ES (2009) Nightshift work and fracture risk: the Nurses' Health Study. OsteoporosInt 20: 537-542.
- Kim BK, Choi YJ, Chung YS (2013) Other than daytime working is associated with lower bone mineral density: the Korea national health and nutrition examination survey 2009. Calcif Tissue Int 93: 495-501.
- Ladizesky MG, Cutrera RA, Boggio V, Somoza J, Centrella JM, et al. (2001) Effect of melatonin on bone metabolism in ovariectomized rats. Life Sci 70: 557-565.
- Koyama H, Nakade O, Takada Y, Kaku T, Lau KH (2002) Melatonin at pharmacologic doses increases bone mass by suppressing resorption through down-regulation of the RANKL-mediated osteoclast formation and activation. J Bone Miner Res 17: 1219-1229.
- Ostrowska Z, Kos-Kudla B, Marek B, Kajdaniuk D (2003) Influence of lighting conditions on daily rhythm of bone metabolism in rats and possible involvement of melatonin and other hormones in this process. EndocrRegul 37: 163-174.
- Ostrowska Z, Kos-Kudla B, Nowak M, Swietochowska E, Marek B, et al. (2003) The relationship between bone metabolism, melatonin and other hormones in sham-operated and pinealectomized rats. EndocrRegul 37: 211-224.
- Moreau A, Wang DS, Forget S, Azeddine B, Angeloni D, et al. (2004) Melatonin signaling dysfunction in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 29: 1772-1781.
- Radio NM, Doctor JS, Witt-Enderby PA (2006) Melatonin enhances alkaline phosphatase activity in differentiating human adult mesenchymal stem cells grown in osteogenic medium via MT2 melatonin receptors and the MEK/ERK (1/2) signaling cascade. J Pineal Res 40: 332-342.
- Sethi S, Radio NM, Kotlarczyk MP, Chen CT, Wei YH, et al. (2010) Determination of the minimal melatonin exposure required to induce osteoblast differentiation from human mesenchymal stem cells and these effects on downstream signaling pathways. J Pineal Res 49: 222-238.
- Uslu S, Uysal A, Oktem G, Yurtseven M, Tanyalçin T, et al. (2007) Constructive effect of exogenous melatonin against osteoporosis after ovariectomy in rats. Anal Quant CytolHistol 29: 317-325.
- Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, et al. (2006) FSH directly regulates bone mass. Cell 125: 247-260.
- Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, et al. (1998) Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA 280: 1569-1575.
- Tindle HA, Davis RB, Phillips RS, Eisenberg DM (2005) Trends in use of complementary and alternative medicine by US adults: 1997-2002. AlternTher Health Med 11: 42-49.
- Poeggeler B, Balzer I, Hardeland R, Lerchl A (1991) Pineal hormone melatonin oscillates also in the dinoflagellateGonyaulaxpolyedra. Naturwissenschaften 78:268-269.
- Hardeland R, Burkhardt S, Antolín I, Fuhrberg B, Coto-Montes A (1999) Melatonin and 5-methoxytryptamine in the bioluminescent dinoflagellateGonyaulaxpolyedra. Restoration of the circadian glow peak after suppression of indoleamine biosynthesis or oxidative stress. AdvExp Med Biol 460: 387-390.
- Viven-Roels B, Pevet P (1993) Melatonin: presence and formation in invertebrates. Experimetia 49:642-647.
- Hardeland R, Poeggeler B (2003) Non-vertebrate melatonin. J Pineal Res 34: 233-241.
- Tan DX, Hardeland R, Manchester LC, Korkmaz A, Ma S, et al. (2012) Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J Exp Bot 63: 577-597.
- Paredes SD, Korkmaz A, Manchester LC, Tan DX, Reiter RJ (2009) Phytomelatonin: a review. J Exp Bot 60: 57-69.
- Garcia-Parrilla MC, Cantos E, Troncoso A (2009) Analysis of melatonin in foods. Journal of Food Composition and Analysis 22:177-183.
- Reiter RJ, Tan DX, Manchester LC, Simopoulos AP, Maldonado MD, et al. (2007) Melatonin in edible plants (phytomelatonin): Identification, concentrations, bioavailability and proposed functions. World Rev Nutr Diet 97: 211-230.
- Murch SJ, Simmons CB, Saxena PK (1997) Melatonin in feverfew and other medicinal plants. Lancet 350: 1598-1599.
- Chen G, Huo Y, Tan DX, Liang Z, Zhang W, et al. (2003) Melatonin in Chinese medicinal herbs. Life Sci 73: 19-26.
- Padumanoda J, Johns A, Sangkasat A, Tiyaworanund S (2013). Rapid analysis of melatonin content in Thai herbs used as sleeping aids. DARU. (in press)
- Johns J, S B-o, Pratheepawanit-Johns N (2011) Screening of Thai medicinal herbs, fruit and vegetables for dietary melatonin. FASEB Summer Research Conference: Melatonin Receptors: Actions and Therapeutics, Colorado.
- Manchester LC, Tan DX, Reiter RJ, Park W, Monis K, et al. (2000) High levels of melatonin in the seeds of edible plants: possible function in germ tissue protection. Life Sci 67: 3023-3029.
- Hattori A, Migitaka H, Iigo M, Itoh M, Yamamoto K, et al. (1995) Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. BiochemMolBiolInt 35: 627-634.
- Jinying W, Chuan J, Shuke L, Jingui Z (2009) Study on analysis method of melatonin and melatonin content in corn and rice seeds. Chinese Agricultural Science Bulletin 25:20-24.
- Okazaki M, Ezura H (2009) Profiling of melatonin in the model tomato (Solanumlycopersicum L.) cultivar Micro-Tom. J Pineal Res 46: 338-343.
- Badria FA (2002) Melatonin, serotonin, and tryptamine in some egyptian food and medicinal plants. J Med Food 5: 153-157.
- Dubbels R, Reiter RJ, Klenke E, Goebel A, Schnakenberg E, et al. (1995) Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J Pineal Res 18: 28-31.
- Sturtza M, Cerezoa A, Cantos-Villarb E, Garcia-Parrilla M (2011) Determination of the melatonin content of different varieties of tomatoes (Lycopersiconesculentum) and strawberries (Fragariaananassa). Food Chemistry 127:1329-1334.
- Van Tassel DL, Roberts N, Lewy A, O'Neill SD (2001) Melatonin in plant organs. J Pineal Res 31: 8-15.
- Pape C, Lüning K (2006) Quantification of melatonin in phototrophic organisms. J Pineal Res 41: 157-165.
- Burkhardt S, Tan DX, Manchester LC, Hardeland R, Reiter RJ (2001) Detection and quantification of the antioxidant melatonin in Montmorency and Balaton tart cherries (Prunuscerasus). J Agric Food Chem 49: 4898-4902.
- Zohara R, Izhakib I, Koplovichb A, Ben-Shlomoa R (2011) Phytomelatonin in the leaves and fruits of wild perennial plants. PhytochemLett 4:222-226.
- Gonzalez-Gomez D, Lozano M, Fernandez-Leon M, Ayuso M, Bernalte M, Rodriguez AB (2009) Detection and quantification of melatonin and serotonin in eight Sweet Cherry cultivars (Prunusaviium L.). Eur Food Res Technol 229:223-229.
- Ramakrishna A, Giridhar P, Sankar KU, Ravishankar GA (2012) Melatonin and serotonin profiles in beans of Coffea species. J Pineal Res 52: 470-476.
- Iriti M, Faoro F (2006) Grape phytochemicals: a bouquet of old and new nutraceuticals for human health. Med Hypotheses 67: 833-838.
- Iriti M, Rossoni M, Faoro F (2006) Melatonin content in grape: myth or panacea? J Sci Food Agriculture 86:1432-1438.
- Sprenger J, Hardeland R, Fuhrberg B, Han S-Z (1999) Melatonin and other 5-methoxylated indoles in yeast: presence in high concentration and dependence on tryptophan availability. Cytologia 64:209-213.
- Rodriguez-Naranjo M, Gil-Izquierdo A, Troncoso A, Cantos E, Garcia-Parrilla M (2011) Melatonin: a new bioactive compound in wine. J Food Comp and Analysis 24:603-608.
- Mena P, Gil-Izquierdo A, Moreno D, Marti N, Garcia-Viguera C (2012) Assessment of the melatonin production in pomegranate wines. LWT-Food Science and Technology 47:13-18.
- Maldonado MD, Moreno H, Calvo JR (2009) Melatonin present in beer contributes to increase the levels of melatonin and antioxidant capacity of the human serum. ClinNutr 28: 188-191.
- Padumanonda T, Johns J, Sangkasat A, Tiyaworanant S (2014) Determination of melatonin content in traditional Thai herbal remedies used as sleeping aids. Daru 22: 6.
- Reiter RJ, Manchester LC, Tan DX (2005) Melatonin in walnuts: influence on levels of melatonin and total antioxidant capacity of blood. Nutrition 21: 920-924.
- Nagata C, Nagao Y, Shibuya C, Kashiki Y, Shimizu H (2005) Association of vegetable intake with urinary 6-sulfatoxymelatonin level. Cancer Epidemiol Biomarkers Prev 14: 1333-1335.
- Oba S, Nakamura K, Sahashi Y, Hattori A, Nagata C (2008) Consumption of vegetables alters morning urinary 6-sulfatoxymelatonin concentration. J Pineal Res 45: 17-23.
- Garrido M, Paredes SD, Cubero J, Lozano M, Toribio-Delgado AF, et al. (2010) Jerte Valley cherry-enriched diets improve nocturnal rest and increase 6-sulfatoxymelatonin and total antioxidant capacity in the urine of middle-aged and elderly humans. J Gerontol A BiolSci Med Sci 65: 909-914.
- Johns NP, Johns J, Porasuphatana S, Plaimee P, Sae-Teaw M (2013) Dietary intake of melatonin from tropical fruit altered urinary excretion of 6-sulfatoxymelatonin in healthy volunteers. J Agric Food Chem 61: 913-919.
- Kovács J, Brodner W, Kirchlechner V, Arif T, Waldhauser F (2000) Measurement of urinary melatonin: a useful tool for monitoring serum melatonin after its oral administration. J ClinEndocrinolMetab 85: 666-670.
- Brzezinski A (1997) Melatonin in humans. N Engl J Med 336: 186-195.
- Tan D, Manchester LC, Reiter RJ, Qi W, Hanes MA, et al. (1999) High physiological levels of melatonin in the bile of mammals. Life Sci 65: 2523-2529.
- Tan DX, Manchester LC, Sanchez-Barcelo E, Mediavilla MD, Reiter RJ (2010) Significance of high levels of endogenous melatonin in Mammalian cerebrospinal fluid and in the central nervous system. CurrNeuropharmacol 8: 162-167.
- Sae-Teaw M, Johns J, Johns NP, Subongkot S (2013) Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers. J Pineal Res 55: 58-64.
- Huether G (1994) Melatonin synthesis in the gastrointestinal tract and the impact of nutritional factors on circulating melatonin. Ann N Y AcadSci 719: 146-158.
- Huether G, Poeggeler B, Reimer A, George A (1992) Effect of tryptophan administration on circulating melatonin levels in chicks and rats: evidence for stimulation of melatonin synthesis and release in the gastrointestinal tract. Life Sci 51: 945-953.
- Bubenik GA (2002) Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci 47: 2336-2348.
- Sanchez S, Sanchez C, Paredes SD (2008) The effect of tryptophan administration on the circadian rhythms of melatonin in plasma and the pineal gland of rats. J Appl Biomed 6:177-186.
- Reiter RJ, Tan DX, Korkmaz A, Erren TC, Piekarski C, et al. (2007) Light at night, chronodisruption, melatonin suppression, and cancer risk: a review. Crit Rev Oncog 13: 303-328.
- Okazaki M, Higuchi K, Hanawa Y, Shiraiwa Y, Ezura H (2009) Cloning and characterization of a ChlamydomonasreinhardtiicDNAarylalkylamine N-acetyltransferase and its use in the genetic engineering of melatonin content in the Micro-Tom tomato. J Pineal Res 46: 373-382.
- Kang K, Lee K, Park S, Kim YS, Back K (2010) Enhanced production of melatonin by ectopic overexpression of human serotonin N-acetyltransferase plays a role in cold resistance in transgenic rice seedlings. J Pineal Res 49: 176-182.
- Witt-Enderby P, Clafshenkel WP, Kotlarczyk M, Sethi S (2011) Melatonin in bone health. In: Watson RR, ed. Melatonin in the promotion of health. Boca Raton: CRC Press, 261-270.
- Davis VL, Dodda BR, Witt-Enderby PA (2012) Prevention and treatment of breast cancer using melatonin. In: Watson RR, ed. Melatonin in the promotion of health. Boca Raton: CRC Press, 271-285.
- McClung MR (2003) Bisphosphonates. EndocrinolMetabClin North Am 32: 253-271.
- Chesnut III CH, Skag A, Christiansen C, Recker R, Stakkestad JA, et al. (2004) Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 19: 1241-1249.
- Cranney A, Guyatt G, Krolicki N, Welch V, Griffith L, et al. (2001) A meta-analysis of etidronate for the treatment of postmenopausal osteoporosis. OsteoporosInt 12: 140-151.
- Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, et al. (2006) Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 296: 2927-2938.
- Mellström DD, Sörensen OH, Goemaere S, Roux C, Johnson TD, et al. (2004) Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int 75: 462-468.
- Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, et al. (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356: 1809-1822.
- Colón-Emeric CS, Caminis J, Suh TT, Pieper CF, Janning C, et al. (2004) The HORIZON Recurrent Fracture Trial: design of a clinical trial in the prevention of subsequent fractures after low trauma hip fracture repair. Curr Med Res Opin 20: 903-910.
- Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, et al. (1999) Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 282: 637-645.
- Martino S, Cauley JA, Barrett-Connor E,Powles TJ, Mershon J, et al. (2004) Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst 96:1751-1761.
- Chesnut CH 3rd, Silverman S, Andriano K,Genant H, Gimona A, et al. (2000) A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med 109:267-276.
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, et al. (2001) Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344: 1434-1441.
- Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, et al. (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361: 756-765.
- Reginster JY, Seeman E, De Vernejoul MC, Adami S, Compston J, et al. (2005) Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J ClinEndocrinolMetab 90: 2816-2822.
- Murch SJ, Saxena PK (2006) A melatonin-rich germplasm line of St John's wort (Hypericumperforatum L.). J Pineal Res 41: 284-287.
- Puerta C, Carrascosa-Salmoral M, Garcia-Luna P, et al. (2007) Melatonin is a phytochemical in olive oil. Food Chemistry 104:609-612.
- Cos S, González A, Martínez-Campa C, Mediavilla MD, Alonso-González C, et al. (2008) Melatonin as a selective estrogen enzyme modulator. Curr Cancer Drug Targets 8: 691-702.
- Deif M, Fawzy E, Gad H (2007) Effect of melatonin on some bone turnover markers in ovarectomized rats. Alexandria J Med 43.
- Prior JC (1998) Perimenopause: the complex endocrinology of the menopausal transition. Endocr Rev 19: 397-428.
- Tamura H, Nakamura Y, Korkmaz A, Manchester LC, Tan DX, et al. (2009) Melatonin and the ovary: physiological and pathophysiological implications. Fertil Steril 92: 328-343.
- Webley GE, Luck MR, Hearn JP (1988) Stimulation of progesterone secretion by cultured human granulosa cells with melatonin and catecholamines. J Reprod Fertil 84: 669-677.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 22086
- [From(publication date):
June-2014 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 17429
- PDF downloads : 4657
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
