Alternative Imaging Response Criteria with the Use of Axitinib in Head and Neck Cancer: An Exploratory Analysis Utilizing the Choi Criteria
Received: 12-Oct-2017 / Accepted Date: 23-Oct-2017 / Published Date: 30-Oct-2017 DOI: 10.4172/2161-119X.1000326
Abstract
Objective: The purpose of this study was to explore the utility of the Choi Criteria in judging response to axitinib therapy in unresectable recurrent or distant metastatic head and neck squamous cell carcinoma.
Methods: Radiologic and clinical data was evaluated in a retrospective fashion from a single-arm phase II clinical trial of axitinib for the treatment of unresectable recurrent or metastatic head and neck squamous cell carcinoma. Twenty-nine patients had imaging to which the Choi Criteria were applied in an exploratory fashion. Responses rates by Choi Criteria were compared to those identified by RECIST v1.0 and statistical analyses were performed to evaluate significance. Association of best response to survival was also examined for each criteria (RECIST v1.0 and Choi).
Results: Application of the Choi Criteria demonstrated that 65.5% of patients achieved a partial response versus 6.9% by RECIST v1.0. Disease control rate was identical by Choi Criteria and RECIST v1.0 at 72.4%. Response to therapy based on Choi Criteria correlated to significantly improved estimated overall survival at 12 months (63% vs. 20%, p=0.03), whereas response to therapy based on RECIST was not a significant predictor of survival.
Conclusion: The Choi Criteria appear to better identify patients responding to therapy with the anti-angiogenic tyrosine kinase inhibitor axitinib versus RECIST v1.0 in this exploratory analysis. Use of the Choi Criteria to guide treatment decisions in further studies utilizing axitinib in this population may better identify patients benefiting from therapy.
Keywords: Head and neck neoplasm; Metastatic head and neck cancer; Axitinib; Vascular endothelial growth factor receptor; Choi Criteria; Imaging response criteria; Tyrosine kinase inhibitor
Introduction
Five year overall survival rates for unresectable recurrent or metastatic (R/M) head and neck squamous cell carcinoma (R/M HNSCC) are poor for which there are few effective treatment options although platinum based chemotherapy remains the standard [1-3]. To date the best median overall survival is seen with combinations of a platinum agent, 5-flourouracil and cetuximab, but the toxicity of this treatment limits its use [4]. Once the disease is refractory to platinumbased therapies, survival is poor, generally less than 6 months [5]. The only approved targeted therapy for HNSCC is the anti-EGFR monoclonal antibody, cetuximab, which has demonstrated only a modest improvement in survival in the metastatic setting [4].
Correlative studies have documented numerous dysregulated pathways including VEGFR, EGFR and PDGFR [6-9]. Based on this data, a phase II clinical trial was performed utilizing axitinib, a potent oral multi-tyrosine kinase inhibitor (TKI) with activity against VEGFR 1, 2 and 3, PDGFR and c-kit in R/M HNSCC [10]. Evaluation of patient outcomes demonstrated an impressive median overall survival of 10.9 months. However, the study demonstrated paradoxically low rates of progression-free survival (3.7 months) and response (6.7%) as defined by RECIST v1.0. Upon further analysis, a disease control rate (SD+PR+CR) of 77% was observed and, interestingly, some lesions demonstrated cystic attenuation and a moderate increase in size shortly following treatment initiation. The role of RECIST as the most appropriate response criteria with antiangiogenic therapies in HNSCC has thus been questioned [11].
An alternate response criteria known as the Choi Criteria have previously been proposed as a tool for evaluating response to TKI therapy in place of RECIST [12,13]. In gastrointestinal stromal tumors (GISTs), treated with the TKI imatinib, the use of Choi Criteria was found to correlate with progression-free and disease-specific survival, whereas RECIST response did not [12]. Studies have also examined the use of Choi Criteria in other malignancies including renal cell carcinoma [14]. Based on this data, we performed an exploratory analysis to compare the utility of the Choi Criteria versus RECIST in predicting survival during axitinib therapy in R/M HNSCC.
Methods
This study was approved by our institutional review board and all medical records were reviewed under the compliance of the Health Insurance Portability and Accountability Act (HIPAA). This study reexamined radiologic and clinical data from a single-arm, phase II clinical trial of axitinib for the treatment of unresectable, recurrent or metastatic HNSCC from 01/2012-08/2014. The original study recruited 42 patients, of which 30 were evaluable for response. The study design, treatment plan and primary results have been previously published [11]. In brief, patients were initiated on axitinib (InlytaTM) with a planned dose escalation in the absence of toxicities. The treatment cycle length was 28 days with evaluation of response of radiographic target lesions performed during week 8 of treatment (corresponding with the completion of cycle 2). In the absence of progression by RECIST v1.0, treatment was continued with re-staging imaging performed every 8 weeks (2 cycles). Patients were taken off study if evidence of progression on imaging. Thirty evaluable patients were enrolled to evaluate the efficacy of axitinib in R/M HNSCC. One patient had only MRI imaging to which the CT Choi criteria could not be applied and is excluded from this analysis. The remaining 29 patients with contrast enhanced CT imaging of neck, chest and/or abdominal metastasis to which the Choi Criteria could be applied, are the subjects of this study [15].
The Choi Criteria were applied to all target lesions identified for measurement of RECIST criteria [13]. Target lesions comprised of 52 lung lesions, 10 head and neck, 8 liver, 4 mediastinal and 2 pleural based lesions (mean of 2.6, range 1-6 lesions per patient). Follow-up CTs were obtained a mean of 165 days following initial CTs (range 46-694). Attenuation measurements of metastatic lesion attenuation values were made by a single author (ED) on contrast-enhanced axial CT images by drawing a freehand region of interest (ROI) around the largest possible region which could be confidently identified as metastasis (i.e. excluding normal background parenchyma) on a clinical PACS station (McKesson Radiology). All follow up scans for a single patient were measured during a single session to ensure consistency in the identification of lesions and the drawing of ROIs. Non-contrast CT examinations were excluded from this study.
Partial response rate and disease control rates defined under the different criteria were compared with Fisher’s exact test. Overall survival was defined from date of initiation of experimental treatment to death, estimated using the Kaplan-Meier method and tested using a log-rank test. Statistical analyses were performed using SAS software, Version 13.1 of the SAS System for Windows.
Results
Twenty-nine patients were assessed by Choi Criteria of which the demographics are shown in Table 1. The number of metastatic lesions tracked by RECIST and Choi Criteria in each patient ranged from 1-6 (mean 2.7, SD ± 1.6). No patients had complete response (CR) to therapy by RECIST v 1.0 or by Choi Criteria (both of which require complete disappearance of all target lesions without detection of new lesions). By RECIST v1.0 criteria, 2/29 (6.9%) patients demonstrated partial response (PR) at any point versus the Choi Criteria in which 19/29 (65.5%) of patients achieved PR at any point (p-value for Fisher’s exact test <0.0001). The disease control rate (CR, PR or stable disease) at any point by RECIST v1.0 was 21/29 (72.4%) versus Choi Criteria with a disease control rate of 21/29 (72.4%) (Table 2).
| Patient Demographics (n=29) | ||
|---|---|---|
| Age | 62 years old (range: 40-84) | |
| Gender | Male: 22 (76%)/Female: 7 (23%) | |
| ECOG Performance Status | PS 0: 18/ PS 1: 7/Missing: 4 | |
| Location of Disease | Local Recurrence | 4 (14%) |
| Distant Metastases | 21 (72%) | |
| Local+Distant | 4 (14%) | |
| Tumor HPV Status | Positive: 14/Negative: 12/Missing: 3 | |
| Prior Chemotherapy | 0 lines | 3 (10%) |
| 1 line | 11 (38%) | |
| 2 lines | 8 (28%) | |
| 3+ lines | 7 (24%) | |
| Exposure to Platinum Therapy | No platinum therapy | 5 (17%) |
| First Line | 23 (79%) | |
| Second Line | 1 (3%) | |
Table 1: Patient demographics (n=29).
| RECIST v1.0 Criteria | Choi Criteria | |||
|---|---|---|---|---|
| PR | SD | PD | Total | |
| PR | 2 | 0 | 0 | 2 |
| SD | 17 | 2 | 0 | 19 |
| PD | 0 | 0 | 8 | 8 |
| Total | 19 | 2 | 8 | 29 |
Table 2: Best Overall Response based on Choi Criteria versus RECIST v1.0.
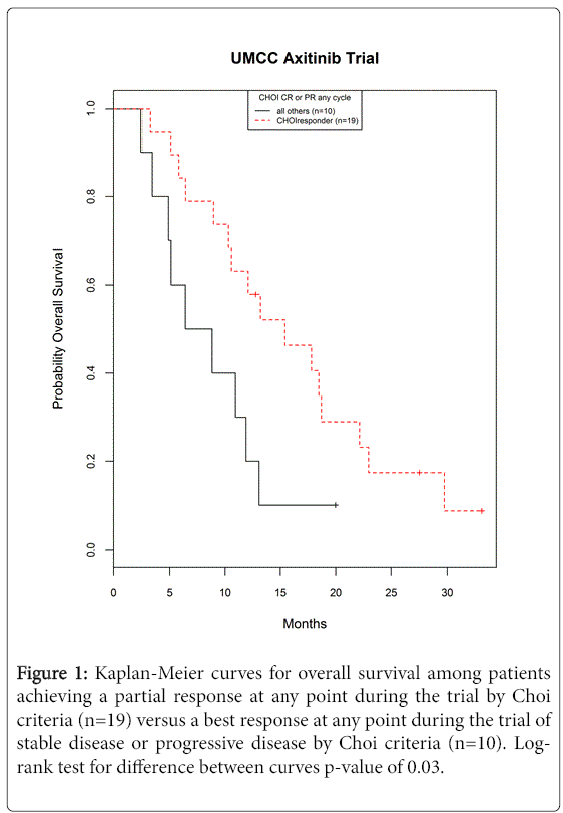
Patients who achieved a partial response at any point in their follow-up by the Choi Criteria (n=19) experienced significantly different overall survival relative to the patients who experienced stable disease or progression (n=10) (log-rank p-value of 0.03). The estimated probability of overall survival at 12 months was 63% (95% CI from 38-80%) for patients with partial response by Choi Criteria versus 20% (95% CI from 3-47%) for other patients not receiving a partial response (Figure 1). Comparing overall patient survival to the patient’s best response by RECIST was not predictive of overall survival in this study (log rank p-value of 0.21 for PR vs all others). It should be noted though that there were only 2 observed partial responses by RECIST criteria one of which was still alive with 33 months of follow-up, therefore the p-value in this case should be interpreted with caution.
Figure 1: Kaplan-Meier curves for overall survival among patients achieving a partial response at any point during the trial by Choi criteria (n=19) versus a best response at any point during the trial of stable disease or progressive disease by Choi criteria (n=10). Logrank test for difference between curves p-value of 0.03.
Discussion
This is the first trial to evaluate the utility of Choi Criteria in the response of anti-angiogenic TKI’s in R/M HNSCC. This retrospective exploratory analysis suggests that the Choi Criteria are able to identify response rates to targeted therapy with axitinib more accurately than RECIST v1.0. Although stable disease and disease control rates (CR, PR, or SD as defined by RECIST v1.0) may serve to demonstrate a better response to axitinib, radiographic measures are needed to distinguish whether the SD is due to indolent disease or treatment effect. In this analysis, the use of Choi Criteria was able to delineate treatment responses in a subset of patients labeled with SD by RECIST v1.0.
The observations made in our study are important since traditional cytotoxic chemotherapeutic agents lead to cell death via direct intracellular damage and apoptosis, while tyrosine kinase inhibitors target particular upstream molecular pathways. As a result of this inhibition, cells may go into arrest and slow necrosis rather than immediate apoptosis. Accordingly, TKIs are sometimes referred to as cytostatic rather than cytotoxic agents. This alternate mechanism of action has been noted to result in differential radiographic responses in other tumor types. In these tumors, treatment response has been observed radiographically as cystic attenuation and occasionally increase in tumor size [16,17]. Although initially thought to represent progression, biopsies of these lesions demonstrated intratumoral hemorrhage and myxoid degeneration consistent with treatment effect [16].
Traditional RECIST criteria may not adequately characterize responses amongst all chemotherapeutic agents [18,19]. Response to immunotherapy, for example, has sometimes demonstrated disease “flare” including enlargement of tumor mass and new lesions interpreted as progression by traditional RECIST criteria. Based on this observation a modified criteria has been proposed: immune-related RECIST (irRECIST) [19,20].
The application of Choi Criteria has been explored in malignancies treated with anti-angiogenic TKIs. Initial analysis of responses GIST patients treated with the imatinib, a multi-receptor TKI, was performed by comparing CT response to matched PET/CT responses, whereby, the majority of patients (73%) had a decreased tumor size (mean decrease of 13%), i.e., SD by RECIST. When the scans were compared to PET/CT, 70% labeled with SD by RECIST had metabolic evidence of response, thus the advent of Choi Criteria [15]. Additionally, RECIST underestimated clinical benefit and tumor progression compared to Choi Criteria [13]. These unique radiologic findings are thought to be due to the anti-PDGFR mechanism of imatinib which, downstream, leads to anti-angiogenesis [15]. In renal cell carcinoma, the use of Choi Criteria correlated better with survival than RECIST [14].
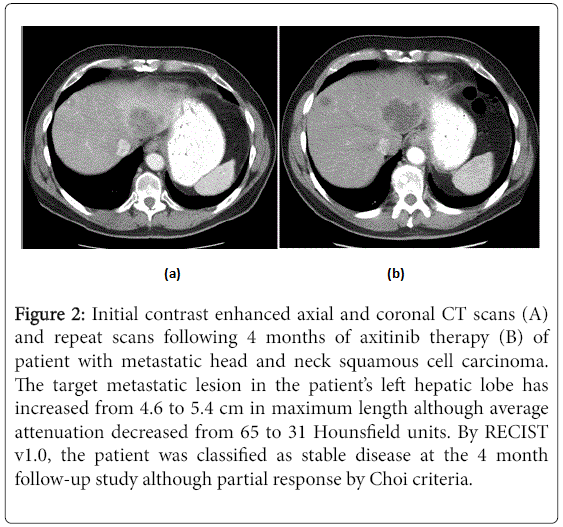
Axitinib is a multi-receptor TKI and is a particularly potent inhibitor of angiogenesis. In our study, patients treated with axitinib had radiographic evidence of response suggested by tumor swelling and internal cystic degeneration similar to that seen with in GISTs, leading to our hypothesis of the Choi Criteria as a rational alternative measure of disease response. Re-analysis of the imaging of our patients demonstrated a substantial cohort who attained partial responses by Choi Criteria due do a change in the contrast enhanced CT attenuation of their metastatic lesions that were classified as SD by the RECIST criteria; an example of a patient from our study is provided in Figure 2. This decrease in attenuation values could reflect tissue necrosis or another effective post-chemotherapy change from the axitinib drug investigated by this trial. The significant difference in overall survival between patients that demonstrated partial response by the Choi Criteria versus patients without disease response suggests that these changes in radiologic findings reflect important underlying changes in the biology of the patient’s neoplasm better captured by the Choi Criteria than the more frequently applied RECIST criteria.
Figure 2: Initial contrast enhanced axial and coronal CT scans (A) and repeat scans following 4 months of axitinib therapy (B) of patient with metastatic head and neck squamous cell carcinoma. The target metastatic lesion in the patient’s left hepatic lobe has increased from 4.6 to 5.4 cm in maximum length although average attenuation decreased from 65 to 31 Hounsfield units. By RECIST v1.0, the patient was classified as stable disease at the 4 month follow-up study although partial response by Choi criteria.
Our study is limited based on its exploratory, retrospective nature. Imaging studies were obtained and initially interpreted utilizing RECIST v1.0; the use of a second imaging criteria increases the risk of a type-I error. Although the Choi criteria can utilize many of the same imaging studies as the RECIST criteria, the Choi criteria may be subject to greater, inter-rater variability in attenuation measurement based on the regions of interest boundaries for metastatic lesions. Also, the reviewer judging response by Choi Criteria was not blinded to response by RECIST, which theoretically could influence judgement. Therefore, treatment effect on survival may be underestimated.
Conclusion
The Choi Criteria warrants further evaluation for assessing treatment responses, especially for anti-angiogenic TKI therapy in R/M HNSCC. Future studies utilizing axitinib in this population should consider utilizing Choi Criteria to guide treatment decisions based suggestion that these response criteria may more accurately identify patients garnering benefit compared to RECIST v1.0.
Acknowledgement
The authors thank the many investigators in the University of Michigan Head and Neck Specialized Program of Research Excellence for their contributions to patient recruitment, assistance in data collection and encouragement including Mary Beth DeRubeis, NP-C, M.S.N., Heidi Mason, NP-C, M.S.N., Leah Shults, RN and Terri Jobkar, RN.
Contract Grant Sponsor
This work was supported by the University of Michigan Head and Neck Specialized Program of Research Excellence NIH/NCI P50CA097248 and NIH/NIDCD T32 DC005356, as well as University of Michigan Cancer Center Core Grant NIH/NCI P3O CA046592
References
- Clavel M, Vermorken JB, Cognetti F, Cappelaere P, de Mulder PH, et al. (1994) Randomized comparison of cisplatin, methotrexate, bleomycin and vincristine (CABO) versus cisplatin and 5-fluorouracil (CF) versus cisplatin (C) in recurrent or metastatic squamous cell carcinoma of the head and neck. A phase III study of the EORTC head and neck cancer cooperative group. Ann Oncol 5: 521-526.
- Forastiere AA (1992) Chemotherapy of head and neck cancer. Ann Oncol 3: 11-14.
- Gibson MK, Li Y, Murphy B, Hussain MH, DeConti RC, et al. (2005) Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): An intergroup trial of the Eastern cooperative oncology group. J Clin Oncol 20: 3562-3567.
- Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, et al. (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359: 1116-1127.
- Stewart JS, Cohen EE, Licitra L, Van Herpen CM, Khorprasert C, et al. (2009) Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [corrected]. J Clin Oncol 27: 1864-1871.
- Wheeler DL, Dunn EF, Harari PM (2010) Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat Rev Clin Oncol 7: 493-507.
- Grandis JR, Zeng Q, Drenning SD, Tweardy DJ (1998) Normalization of EGFR mRNA levels following restoration of wild-type p53 in a head and neck squamous cell carcinoma cell line. Int J Oncol 13: 375-378.
- Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, et al (2002) Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res 62: 7350-7356.
- Chung CH, Ely K, McGavran L, Varella-Garcia M, Parker J, et al. (2006) Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J Clin Oncol 24: 4170-4176.
- Hu-Lowe DD, Zou HY, Grazzini ML, Hallin ME, Wickman GR, et al (2008) Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res 14: 7272-7283.
- Swiecicki PL, Zhao L, Belile E, Sacco AG, Chepeha DB, et al. (2015) A phase II study evaluating axitinib in patients with unresectable, recurrent or metastatic head and neck cancer. Invest New Drugs 33: 1248-1256.
- Benjamin RS, Choi H, Macapinlac HA, Burgess MA, Patel SR, et al. (2007) We should desist using RECIST, at least in GIST. J Clin Oncol 25: 1760-1764.
- Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, et al. (2007) Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: Proposal of new computed tomography response criteria. J Clin Oncol 25: 1753-1759.
- Goh V, Ganeshan B, Nathan P, Juttla JK, Vinayan A, et al. (2011) Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: CT texture as a predictive biomarker. Radiology 261: 165-171.
- Choi H, Charnsangavej C, de Castro Faria S, Tamm EP, Benjamin RS, et al. (2004) CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: A quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol 183: 1619-1628.
- Bechtold RE, Chen MY, Stanton CA, Savage PD, Levine EA (2003) Cystic changes in hepatic and peritoneal metastases from gastrointestinal stromal tumors treated with Gleevec. Abdom Imaging 28: 808-814.
- Linton KM, Taylor MB, Radford JA (2006) Response evaluation in gastrointestinal stromal tumours treated with imatinib: Misdiagnosis of disease progression on CT due to cystic change in liver metastases. Br J Radiol 79: e40- e44.
- George S, Motzer RJ, Hammers HJ, Redman BG, Kuzel TM, et al. (2016) Safety and efficacy of nivolumab in patients with metastatic renal cell carcinoma treated beyond progression: A subgroup analysis of a randomized clinical trial. JAMA Oncol 2: 1179-1186.
- Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, et al. (2016) Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol 34: 1510-1517.
- Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, et al. (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res 15: 7412-7420.
Citation: Swiecicki PL, Dickerson E, Bellile E, Srinivasan A, Worden FP (2017) Alternative Imaging Response Criteria with the Use of Axitinib in Head and Neck Cancer: An Exploratory Analysis Utilizing the Choi Criteria. Otolaryngol (Sunnyvale) 7:326. DOI: 10.4172/2161-119X.1000326
Copyright: © 2017 Swiecicki PL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4905
- [From(publication date): 0-2017 - Apr 06, 2025]
- Breakdown by view type
- HTML page views: 4021
- PDF downloads: 884