Research Article Open Access
Altered Structural-functional Maturation of the Right Amygdala in Healthy Adolescents Exposed to Traumatic Events
Bernal-Casas David1*, Pincham H2, Harding E2, Prabhu G1, Fearon P2,3 and Dolan R11Department of Neurology, Wellcome Trust Centre for Neuroimaging, Institute of Neurology, University College London, London, UK
2Department of Neurology, Developmental Neuroscience Unit, The Anna Freud Centre, UK
3Department of Clinical Research, Educational and Health Psychology, University College London, UK
- *Corresponding Author:
- Bernal-Casas David
Department of Neurology, Wellcome Trust Centre for Neuroimaging
Institute of Neurology, University College London
12 Queen Square - London - WC1N 3BG, UK
Tel: ++44 (0) 20 3448 4362
Fax: ++44 (0) 20 7813 1420
E-mail: bernalgps@gmail.com
Received Date: August 11, 2015 Accepted Date: September 15, 2015 Published Date: September 22, 2015
Citation: David BC, Pincham H, Harding E, Prabhu G, Fearon P, et al. (2015) Altered Structural-functional Maturation of the Right Amygdala in Healthy Adolescents Exposed to Traumatic Events. J Child Adolesc Behav 3:248. doi:10.4172/2375-4494.1000248
Copyright: © 2015 David BC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Child and Adolescent Behavior
Abstract
Trauma is defined as a physical or psychological threat or assault to an individual’s physical integrity, sense of self, safety or survival or to the physical safety of a significant other. The most common long-term mental health conditions resulting from trauma are post-traumatic stress disorder (PTSD) and depression. A number of studies using neuroimaging methodologies have implicated the amygdala and hippocampus in emotion processing in mood disorders, and adult depression studies suggest amygdala-hippocampal functional connectivity deficits. However, while trauma contributes to depression in a variety of ways, the neurobiological mechanisms underlying exposure to traumatic experiences have been poorly studied. In order to address this question, we used voxel based morphometry (VBM) and spectral dynamic causal modelling (spectral DCM; spDCM) approaches to analyse structural and restingstate functional magnetic resonance imaging and examine grey matter volume, white matter volume, and effective connectivity within the amygdala-hippocampal network in adolescents without psychiatric diagnoses, who had or had not been previously exposed to traumatic life events. Our results indicated greater intrinsic connectivity within the right amygdala in individuals with traumatic experiences compared to controls. Likewise we observed reduced white matter volume within the same region in those individuals, compared with controls. Together, these findings are suggestive of altered maturation of the right amygdala in healthy adolescents following trauma exposure. We interpret such brain changes as a plausible mechanism that may make individuals more vulnerable to developing psychopathology later in life.
Keywords
MRI; Structural MRI; Resting-state fMRI (rs-fMRI); VBM; Spectral DCM; Amygdala; Hippocampus; Trauma
Introduction
Exposure to traumatic life events and experiences during childhood is associated with an increased risk of later psychopathology, as well as impaired neurobiological and neurocognitive functioning [1,2]. Even though the neurobiological mechanisms remain poorly understood, there is likely a complex interplay between environmental experiences and individual differences in risk versus protective genes, which influence the brain circuitry underpinning psychological and emotional development [3]. Stressful events in childhood and adolescence are proposed to alter outputs of the hypothalamic-pituitary-adrenal axis, resulting in sustained high levels of glucocorticoid steroids. The structure and function of the limbic system, specifically the hippocampus and amygdala, appear particularly vulnerable to these changes [4]. For instance, reduced hippocampal volumes in adults with PTSD and a history of childhood physical and sexual abuse has been reported5. Further, reduced resting-state functional connectivity between the right amygdala and a number of limbic structures, including the hippocampus and putamen, in individuals drawn from the Netherlands Study of Depression and anxiety was found. Notably, much existing research has examined alterations in the neural architecture of patient cohorts, such as those with post-traumatic stress disorder [5-13] or trauma-induced depression [14]. Notwithstanding the usefulness of these studies, it is important to consider whether the same findings hold in young people who have been exposed to stressful life events but are currently free of comorbid psychopathology. This is important to enable the comparison between trauma-exposed and non-exposed participants to be matched for current psychopathology. To that end, the current study was interested in whether young people who have previously experienced a stressful life event (but are currently psychologically well) demonstrate abnormalities in either brain structure or functional connectivity compared to an age-matched control group.
A number of studies have examined structural brain changes following trauma exposure in individuals without psychiatric diagnoses. While many have reported volumetric reductions in the hippocampus [15-17] or amygdala [18], others have found no change in either hippocampal or amygdala volume [19,20]. Resting-state functional imaging studies have also produced varied results, with some research reporting increased amygdala-hippocampal connectivity associated with greater levels of self-reported trauma symptoms [21] or following stress induction [22], and other work reporting no trauma-related connectivity changes [18]. Variability in type of trauma exposure, and imaging analysis may play a role in explaining these discrepancies. Through the use of combined structural and functional measures, we aimed to contribute understanding on the relevance of amygdalahippocampal interactions in trauma exposure.
Our focus on young people was driven by the notion that trauma exposure is likely to be most influential in shaping neural structures during developmentally sensitive periods [23,24]. Adolescence is a sensitive period for both cognitive and neural development [25], and is associated with an increased incidence of psychiatric disorder onset. In keeping with the view of adolescence as a time of particular vulnerability to developmental perturbations, cross-sectional studies reveal that regionally specific effects of early stressors in the amygdala are most prominent during adolescence [18]. We therefore examined currently healthy young people who self-reported early exposure to a range of extreme stressors. Self-report measures of early trauma (natural disasters, family illness/death, experiences of bullying etc.) have been previously linked to reduced amygdala volumes in young people [18,26,27], however the impact of such stressors on functional connectivity in youth have not been widely explored [28]. Concurrent examinations of trauma-related changes in both brain structure and functional connectivity have been limited to adult populations or to task-specific activation paradigms [16,29].
The current study employed structural and functional magnetic resonance imaging to examine grey and white matter volume estimates by means of voxel based morphometry (VBM), as well as effective functional connectivity by means of spectral dynamic causal modelling (spectral DCM; spDCM) within the amygdala-hippocampal network in currently-healthy adolescents. Importantly, any neural differences between stress exposed individuals versus unexposed individuals should not be driven by current psychopathology, as all participants were free from current psychopathology.
Materials and Methods
Participants and centers
A large cohort of 298 healthy adolescents [152 males, range: 14-24 years, mean = 19.1 ± 2.9 (SD); 146 females, range: 14-24 years, mean = 19.1 ± 2.9] were scanned over 1½ years at 3 sites: (1) Wellcome Trust Centre for Neuroimaging (WTCN), London, (2) Medical Research Council Cognition and Brain Sciences Unit (MRC CBSU), Cambridge, and (3) Wolfson Brain Imaging Centre (WBIC), Cambridge. The study received ethical approval from the NRES Committee East of England - Cambridge Central (12/EE/0250) and all participants gave written informed consent. This study was conducted by the NeuroScience in Psychiatry Network (NSPN), which addresses how psychiatric disorders are related to abnormal maturation of brain systems.
Selection of participants with histories of abuse and neglect
The Structured Clinical Interview for DSM-IV (SCID) was administered by a trained research assistant and audio recordings were made with the informed consent of participants. During the course of these interviews 29 participants were identified (10 female, mean age 20.45 years) who self-reported histories of trauma (a solitary trauma), including experiences of physical or sexual abuse, having been in a lifethreatening situation (e.g. natural disaster, car accident, drowning), physical/sexual assault, death of a parent or sibling or witnessing/ hearing about actual or threatened death to others over the course of childhood. The control group (mean age: 20.53 years) was created by matching each trauma-exposed participant to a non-exposed participant in terms of gender, age and parental education levels (as a proxy for socio-economic status). There was no significant difference in handedness between the two groups. Table 1 shows demographic data of participants.
| Trauma-exposed participants (N = 29) |
Non-exposed participants (N = 29) |
p-values | |
| Age [years] | 20.53 ± 2.77 range 16-25 |
20.45 ± 2.61 range 16-25 |
0.9087 |
| Sex [Male/Female] | 19/10 | 19/10 | 1.0000 |
| Parental education [years] | 18.05 ± 8.03 | 16.15 ± 6.18 | 0.3153 |
Table 1: Demographic data of participants.
Data acquisition and preprocessing
Structural MRI: All multi-parameter maps (MPM) were acquired on 3T whole body MRI systems (Magnetom TIM Trio, Siemens Healthcare, Erlangen, Germany; VB17 software version) operated with the standard 32-channel radio-frequency (RF) receive head coil and RF body coil for transmission. The MPM comprised three multi-echo 3D fast low angle shot (FLASH) scans with PD (TR/α = 23.7 ms/60), T1 (TR/α = 18.7 ms/200), and MT (TR/α = 23.7 ms/60) - weighted contrast, one RF transmit (B1) field map and one static magnetic (B0) field map scan [30].
The MPM acquisition and pre-processing were developed and optimized in previous studies and are widely described elsewhere [30-36]. The post-processed MT maps resulting from this step were used in our VBM analyses.
Functional MRI: At all three sites, fMRI data were acquired on 3T whole body MRI systems (Magnetom TIM Trio, Siemens Healthcare, Erlangen, Germany; VB17 software version) operated with the standard 32-channel radio-frequency (RF) receive head coil and RF body coil for transmission. 269 contiguous multi-slice images were obtained with a multi-echo-planar sequence (orientation = AC-PC line, number of slices = 34; slice thickness = 3.8 mm; FOV = 240 mm; TE1 = 13 ms; TE2 = 31 ms; TE3 = 48 ms; TR = 2.420 s; flip angle = 90°; matrix size = 64×64×34; voxel size = 3.8×3.8×3.8 mm3).
The fMRI data were analysed using procedures implemented in Statistical Parametric Mapping (SPM8, Welcome Trust Centre for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm). First, the fMRI data were summed up over the three echoes. Data were then realigned, co-registered, anatomical images were normalized to MNI space, and the resultant normalization matrix was then used to normalize the fMRI data. Finally, the data were visually inspected and spatially smoothed using a 6 mm Gaussian kernel. Ultra-low frequency fluctuations were removed using a high-pass filter (1/128 s, 0.0078 Hz). Confound time-series were extracted from predefined coordinates of extra-cerebral compartments (the pons: x, y, z = 0, -24, -33; and lateral ventricle: x, y, z = 1, -43, 6).
We extracted data exhibiting physiologically-relevant resting-state (i.e. low frequency) dynamics from our region(s) of interest (ROIs): left and right amygdala, and left and right hippocampus, which were anatomically defined using the PickAtlas software (WFU PickAtlas, ANSIR Laboratory, Winston-Salem, NC, USA; http://fmri.wfubmc. edu/software/PickAtlas). The resting-state was thus modelled using a General Linear Model (GLM) with a discrete cosine basis set (GLMDCT) consisting of 130 functions with frequencies characteristic of resting-state dynamics (0.0078 – 0.1 Hz [37-40]), six nuisance regressors capturing head motion, and the confound time-series from the extra-cerebral compartments. The regional BOLD signal was summarized with the principal eigenvariate (adjusted for confounds: head movements and extra-cerebral compartments) of voxels within 6 mm of the subject’s peak coordinate, as identified using statistical parametric mapping. For those familiar with the process of extracting ROIs, this was achieved by using an F-contrast including the discrete cosine set modelling the resting-state. This procedure allowed us to extract physiologically relevant resting-state data from the anatomically defined regions for each hemisphere.
Spectral dynamic causal modelling (spDCM): Effective connectivity estimates
Spectral dynamic causal modelling (spectral DCM; spDCM) is based on deterministic models that generate predicted crossed spectra from a biophysically plausible model of coupled neuronal fluctuations in a distributed neuronal network [41]. In this setting, the nature of the endogenous fluctuations (and observation noise) has to be parameterized. The most parsimonious and general form, is a power law or scale free form that can be motivated from a large body of work on noise in fMRI [42] and underlying neuronal activity [43,44].
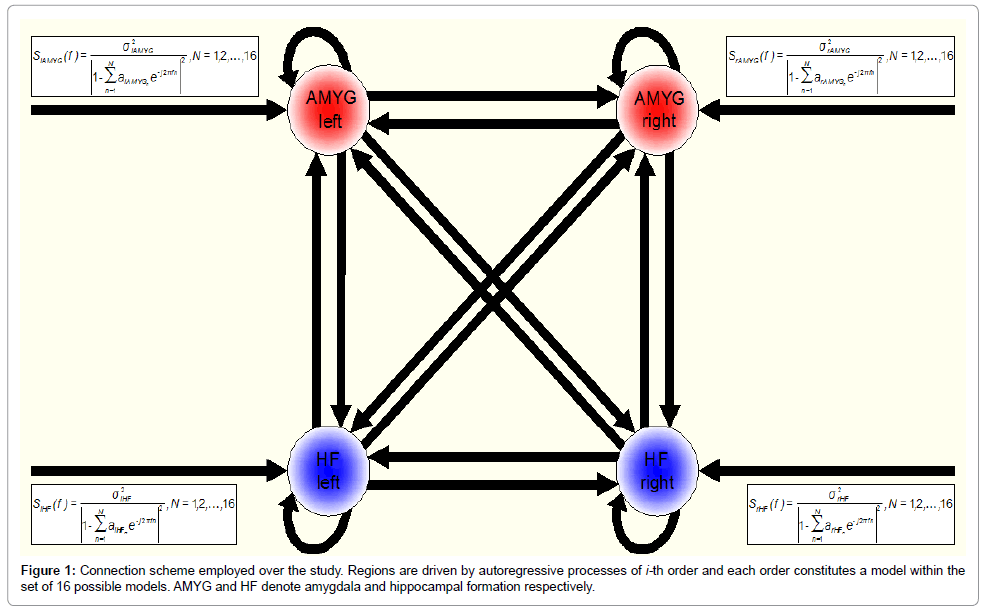
In this study, we intended, first, to improve the description of the endogenous fluctuations (and observation noise) by using auto-regressive (AR) models of different order, and second, to run formal model comparisons in order to select the best AR model, or the best combination of AR models that provide the best balance between accuracy and complexity for explaining the measured data. Accordingly, we modelled endogenous fluctuations (and observation noise) within the amygdala-hippocampal network with AR models from order-1 up to order-16 (N = 1, 2, …, 16). This was done by modifying the deterministic functions that model the neuronal fluctuations (and observation noise) in the source code (they are specified in spm_csd_fmri_mtf.m). Thus, we fitted a total of 16 models to each subject’s data. Figure 1 shows the connection scheme employed over this study (Figure 1).
For the analyses presented in this paper, we used spectral DCM for fMRI as implemented in the software package SPM12 (beta version), code release 5831.
Bayesian model selection (BMS)
To select the optimal fluctuation model (given a set of 16 alternatives), we used Bayesian model selection (BMS) for fMRI responses as implemented elsewhere [45,46].
Voxel-based morphometry (VBM): Grey and white matter volume estimates: For voxel-based morphometry (VBM) analyses, the magnetization transfer (MT) saturation maps were processed in VBM8 (http://dbm.neuro.uni-jena.de/vbm/) with the default settings and classified into different tissue classes: grey matter (GM), white matter (WM), and cerebral-spinal fluid (CSF). The reason for using MT maps is that even though T1w images are commonly used for brain segmentation, the MT maps have been shown to improve the segmentation of subcortical areas [33,47]. Moreover, aiming at optimal anatomical precision we applied the diffeomorphic registration algorithm DARTEL [48]. The warped GM and WM probability maps were scaled by the Jacobian determinants of the deformation fields to account for local compression and expansion [49], resulting in GM and WM volume (GVM/WMV) maps. The GMV/WMV maps were then smoothed by convolution with an isotropic Gaussian kernel of 6 mm full-width-at-half-maximum (FWHM).
We then extracted data from our ROIs which were previously defined using the PickAtlas software (see above). More specifically, we computed the averaged GMV/WMV across voxels within our a priori defined ROIs for each individual. This was followed by a multiple regression analysis.
Multiple linear regression models: To test the hypotheses that spDCM parameter estimates were different between groups, we carried out linear regression analyses.
We first calculated the averaged spDCM parameters from restingstate fMRI data. This was followed by a multiple regression analysis explaining spDCM estimates by a constant, age, differentiating between males and females (in order to identify gender-specific effects), poverty scores, group, and scanner site. The correlation was modelled as:
spDCMi = β1× [constantmale]+β2× [agemale]+β3× [constantfemale]+β4× [agefemale]+β5× [parental education]+β6× [group]+β7i× [site] (1)
where i runs from 1 to Np, being Np = 84 the number of spDCM parameter estimates (16 connectivity estimates plus 68 fluctuation estimates).
All independent variables in Eq. (1) were mean corrected. This multiple linear regression was performed on a parameter-by-parameter basis beyond the statistical threshold p-valueFDR < 0.05 in the paired t-test of group.
We then considered the same correlation model for grey matter volume (GMV) and white matter volume (WMV) estimates within each ROI separately; we also included the total intracranial volume (TICV) as independent variable.
GMVi = β1×[constantmale]+β2× [agemale]+β3× [constantfemale]+β4× [agefemale]+β5× [parental educaiton]+β6× [group]+β7× [site]+β8× [TICV] (2)
and
WMVi = β1× [constantmale]+β2× [agemale]+β3× [constantfemale]+β4× [agefemale]+β5× [parental education]+β6× [group]+β7× [site]+β8× [TICV] (3)
where i runs from 1 to Nr, being Nr = 4 the number of ROIs.
Results
BMS analyses
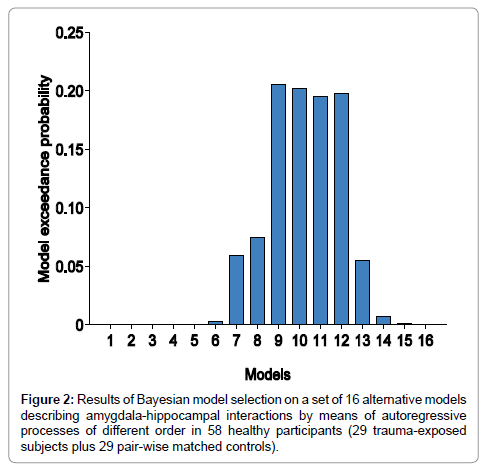
We used random effects Bayesian model selection (BMS) to determine, from our model space of 16 alternative spectral DCMs (Figure 1), the model that provided the best balance between accuracy and complexity for explaining the measured data. The analyses did not reveal any winning model in this group of 58 healthy adolescents (29 trauma-exposed participants plus 29 controls), but a subgroup of models having comparable exceedance probabilities.
Figure 2 shows the results of BMS Random Effects (RFX). The exceedance probability of models 9, 10, 11, and 12 (i.e., the probability that this model is a more likely model than any other model considered) was around 0.2. These models describe the neuronal and observation fluctuations by using autoregressive models of order 9, 10, 11, and 12, respectively.
Given the fact that we did not obtain a winning model, we used Bayesian model averaging (BMA) to compute reliable parameter estimates that account for model uncertainty. Recall BMA averages parameter estimates over models by weighting estimates by their corresponding posterior model probabilities [50] (Figure 2).
Statistical analyses
Following BMA, we used the resulting posterior means from the averaged spectral DCMs for examining correlations (or statistical differences) between spDCM parameter estimates and healthy adolescents with or without a trauma experience. Recall we defined group as a regressor or independent variable in the correlation model accounting for the effects of having or not a trauma experience during childhood (Yes = 1; Not = 0). Table 1 shows the significance levels (p-values) for negative associations between group and the averaged spectral DCM estimates (Table 2).
| spDCMi= f(group) Negative associations p-values (unc.) |
spDCMi = f(group) Negative associations p-values (unc.) |
||
| (a) Connectivity estimates 16 parameters rAMYG →rAMYG rAMYG → rHF rAMYG → lAMYG rAMYG → lHF |
0.9997* 0.5671 0.4811 0.1197 |
(b) Fluctuation estimates 68 parameters σ2 (rAMYG) AR1 (rAMYG) AR2 (rAMYG) AR3 (rAMYG) AR4 (rAMYG) AR5 (rAMYG) AR6 (rAMYG) AR7 (rAMYG) AR8 (rAMYG) AR9 (rAMYG) AR10 (rAMYG) AR11 (rAMYG) AR12 (rAMYG) AR13 (rAMYG) AR14 (rAMYG) AR15 (rAMYG) AR16 (rAMYG) |
0.6541 0.4411 0.8249 0.7715 0.4907 0.1627 0.1883 0.1832 0.1188 0.1081 0.1922 0.5164 0.6989 0.7003 0.4763 0.3937 0.4984 |
| rHF→ rAMYG rHF → rHF rHF→ lAMYG rHF → lHF |
0.6314 0.8852 0.7642 0.5934 |
σ2 (rHF) AR1 (rHF) AR2 (rHF) AR3 (rHF) AR4 (rHF) AR5 (rHF) AR6 (rHF) AR7 (rHF) AR8 (rHF) AR9 (rHF) AR10 (rHF) AR11 (rHF) AR12 (rHF) AR13 (rHF) AR14 (rHF) AR15 (rHF) AR16 (rHF) |
0.5531 0.8224 0.8759 0.1048 0.7591 0.9252 0.8422 0.8449 0.8485 0.8353 0.7492 0.5981 0.5924 0.6913 0.5425 0.3870 0.3835 |
| lAMYG →rAMYG lAMYG → rHF lAMYG →lAMYG lAMYG → lHF |
0.0610 0.3836 0.7804 0.6479 |
σ2 (lAMYG) AR1 (lAMYG) AR2 (lAMYG) AR3 (lAMYG) AR4 (lAMYG) AR5 (lAMYG) AR6 (lAMYG) AR7 (lAMYG) AR8 (lAMYG) AR9 (lAMYG) AR10 (lAMYG) AR11 (lAMYG) AR12 (lAMYG) AR13 (lAMYG) AR14 (lAMYG) AR15 (lAMYG) AR16 (lAMYG) |
0.1225 0.3732 0.9816 0.5900 0.0047 0.1568 0.1833 0.5858 0.9635 0.9410 0.7895 0.9080 0.9579 0.8968 0.7080 0.6656 0.5913 |
| lHF → rAMYG lHF →rHF lHF → lAMYG lHF → lHF |
0.6637 0.1806 0.3339 0.8291 |
σ2 (lHF) AR1 (lHF) AR2 (lHF) AR3 (lHF) AR4 (lHF) AR5 (lHF) AR6 (lHF) AR7 (lHF) AR8 (lHF) AR9 (lHF) AR10 (lHF) AR11 (lHF) AR12 (lHF) AR13 (lHF) AR14 (lHF) AR15 (lHF) AR16 (lHF) |
0.7177 0.6833 0.3848 0.3832 0.3945 0.3741 0.3818 0.5733 0.6086 0.4697 0.5589 0.6696 0.7899 0.7499 0.5744 0.4438 0.4810 |
We observed a statistically significant difference in the selfconnection within the right amygdala in individuals with a trauma experience compared to controls (p-value = 0.0003). Individuals with a trauma experience showed a greater intrinsic connectivity within the right amygdala compared to controls. This connectivity estimate survived multiple comparison problem by means of FDR (p-valueFDR = 0.0225). Recall we correct for 84 parameters or tests.
Following statistical analyses on the spectral DCM parameter estimates, we performed statistical analyses on grey and white matter volume estimates within each ROI separately. Tables 2 and 3 show the significance levels (p-values) for negative associations between group and GMV, and group and WMV respectively (Tables 3 and 4).
| GMVi = f(group) Negative associations p-values(unc.) |
|
|---|---|
| GMV (rAMYG) GMV (rHF) GMV (lAMYG) GMV (lHF) |
0.3627 0.2938 0.4837 0.1808 |
Table 3: Significance levels (p-values) for negative associations between group and grey matter volume (GMV) rAMYG, rHF, lAMYG, lHF denote right amygdala, right hippocampal formation, left amygdala, and left hippocampal formation respectively.
| WMVi = f(group) Negative associations p-values (unc.) |
|
|---|---|
| WMV (rAMYG) WMV (rHF) WMV (lAMYG) WMV (lHF) |
0.0208 0.0711 0.1790 0.2451 |
Table 4: Significance levels (p-values) for negative associations between group and white matter volume (WMV) rAMYG, rHF, lAMYG, lHF denote right amygdala, right hippocampal formation, left amygdala, and left hippocampal formation respectively. Significant p-values (unc.) are highlighted in bold: negative correlations are shown in red, positive correlations in blue.
We observed a statistically significant difference in white matter volume (WMV) within the right amygdala in individuals with a trauma experience compared to controls (p-value = 0.0208). Individuals with a trauma experience showed a lesser WMV within the right amygdala compared to controls. Even though this difference did not survive multiple comparison problems by means of FDR (p-valueFDR = 0.0832), it was very close to significance level (here we corrected for 4 parameters or tests) and more interestingly, it was highlighted the same brain aspect as in previous connectivity analyses: the right amygdala.
At this stage, we may conclude that changes in the self-connection within the right amygdala may be driven by changes in white matter volume within the same aspect. We interpret these findings as suggestive of abnormal brain maturation after exposure to traumatic experiences.
Discussion
While traumatic early life events might lead to the onset of PTSD or depression, the underlying neurobiological mechanisms have not been elucidated. We used voxel based morphometry (VBM) to examine differences in grey and white matter volume, and spectral dynamic causal modelling (spectral DCM; spDCM) to investigate effective connectivity within the amygdala-hippocampal network in healthy adolescents. The primary objective was to identity abnormal patterns of structural-functional brain maturation in young people exposed to traumatic early life experiences in comparison to controls.
Using estimates of grey and white matter volume and effective connectivity, the results indicate greater intrinsic connectivity (i.e. greater excitation) within the right amygdala in individuals with early traumatic experiences compared to controls. Reduced white matter volume (e.g. lesser anatomical wiring) was also observed in the right amygdala for the trauma-exposed group. These findings suggest altered maturation of the right amygdala in young people following trauma exposure.
Based on previous research, our study investigated the impact of trauma on the amygdala-hippocampal network. We did not observe any compelling statistical evidence concerning involvement of the hippocampus, suggesting that amygdala (more specifically the right hemisphere) might be preferentially implicated in traumatic events. It is important to reiterate that the differences we observed should not be attributed to differences in current levels of psychopathology. Instead, the observed maturational brain changes induced by traumatic events might make individuals more susceptible to develop a psychopathology such as PTSD or depression later in life.
A major strength of the present study includes the combined use of various MRI analyses (VBM of grey and white matter, and effective connectivity by means of spectral DCM). Further, inclusion of participants without current psychopathology removes a confound that is present in many previous examinations of this topic. Despite these strengths, some limitations of the study require acknowledgment. First, the spectral DCM algorithm together with the Bayesian comparison procedure implemented here may be further improved by searching for optimal region-specific fluctuation models (and observation noise) rather than assuming the same generative model across regions (from AR1 up to AR16 processes). Although that approach may have advantages and potentially reveal some other statistical effects, it is computationally very expensive due to estimation of a very large number of models. Second, analyses suggest the use of other statistical correction strategies rather than FDR (e.g. a random field theory approach). The reason for this is that FDR does not take into account autoregressive models (i.e. the temporal structure), nor brain regions (i.e. the spatial structure) and deals with all parameter estimates as independent of each other. Third, VBM is commonly directed at examining gray matter but it can also be used to examine white matter. In the latter case, however, the sensitivity is limited because white matter areas are characterized by large homogeneous regions with only subtle changes in intensity [51]. Finally, the use of the SCID interview to select participants with traumatic life events may be criticized as being too structured (and diagnostically focused), potentially not enabling all former traumatic experiences to be revealed.
In summary, our findings demonstrated abnormal structuralfunctional maturation of the right amygdala in currently-healthy young people exposed to traumatic events. Together, these findings are suggestive of potential biological markers over the course of adolescence that may have prognostic utility for PTSD or depression. Indeed, our observations, both in white-matter and intrinsic connectivity within right amygdala are very interesting and intriguing, however the reason, i.e. the underlying biological mechanisms that lead to these observations, remain an open question since only a small body of research has been conducted on this topic so far.
Acknowledgements
This work was funded by grants from the Wellcome Trust. Authors acknowledge support by NSPN (NeuroScience in Psychiatry Network) Principals, NSPN Research Assistant team, NSPN Data Management team, and U-CHANGE (Understanding and Characterizing Adolescent-to-Adult Neurodevelopmental Growth Effects) team. D. Bernal-Casas wishes to thanks relatives and friends for their continued moral and financial support.
References
- Beitchman JH, Zucker KJ, Hood JE, daCosta GA, Akman D, et al. (1992) A review of the long-term effects of child sexual abuse. Child Abuse Negl 16: 101-118.
- Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009) Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10: 434-445.
- McCrory E, De Brito SA, Viding E (2012) The link between child abuse and psychopathology: a review of neurobiological and genetic research. J RSoc Med 105: 151-156.
- McEwan K, Waddell C, Barker J (2007) Bringing children's mental health "out of the shadows". CMAJ 176: 471-472.
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, et al. (1997) Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse--a preliminary report. Biol Psychiatry 41: 23-32.
- van der Werff SJ, Pannekoek JN, Veer IM, van Tol MJ, Aleman A, et al. (2013) Resting-state functional connectivity in adults with childhood emotional maltreatment. Psycho med 43: 1825-1836.
- Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, et al. (2001) Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry 50: 943-951.
- Carrion VG, Weems CF, Reiss AL (2007) Stress predicts brain changes in children: a pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics119: 509-516.
- De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, et al. (1999) A.E. Bennett Research Award. Developmental traumatology. Part II: Brain development. Biological psychiatry 45: 1271-1284.
- De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G (2001) A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol psychiatry 50: 305-309.
- De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, et al. (2002) Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biolpsychiatry 52: 1066-1078.
- Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, et al. (2012) Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci 37: 241-249.
- Bluhm RL, Williamson PC, Osuch EA, Frewen PA, Stevens TK, et al. (2009) Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J Psychiatry Neurosci 34: 187-194.
- Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, et al. (2002) Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry 159: 2072-2080.
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, et al. (2008) Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry ClinNeurosci 20: 292-301.
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, et al. (2012) Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry 71: 286-293.
- Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, et al. (2007) Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. Neuroimage 35: 795-803.
- Korgaonkar MS, Antees C, Williams LM, Gatt JM, Bryant RA, et al. (2013) Early exposure to traumatic stressors impairs emotional brain circuitry. PLoS One 8: e75524.
- Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, et al. (2006) Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry 59: 975-982.
- Baker LM, Williams LM, Korgaonkar MS, Cohen RA, Heaps JM, et al. (2013) Impact of early vs. late childhood early life stress on brain morphometrics. Brain Imaging Behav 7: 196-203.
- Nooner KB, Mennes M, Brown S, Castellanos FX, Leventhal B, et al. (2013) Relationship of trauma symptoms to amygdala-based functional brain changes in adolescents. J Trauma Stress 26: 784-787.
- Vaisvaser S, Lin T, Admon R, Podlipsky I, Greenman Y, et al. (2013) Neural traces of stress: cortisol related sustained enhancement of amygdala-hippocampal functional connectivity. Front Hum Neurosci7: 313.
- Casey BJ, Giedd JN, Thomas KM (2000) Structural and functional brain development and its relation to cognitive development. BiolPsychol 54: 241-257.
- Knudsen EI (2004) Sensitive periods in the development of the brain and behavior. J CognNeurosci 16: 1412-1425.
- Blakemore SJ (2012) Imaging brain development: the adolescent brain. Neuroimage 61: 397-406.
- Aas M, Navari S, Gibbs A, Mondelli V, Fisher HL, et al. (2012) Is there a link between childhood trauma, cognition, and amygdala and hippocampus volume in first-episode psychosis? Schizophr Res 137: 73-79.
- De Brito SA, Viding E, Sebastian CL, Kelly PA, Mechelli A, et al. (2013) Reduced orbitofrontal and temporal grey matter in a community sample of maltreated children. J Child Psychol Psychiatry 54: 105-112.
- Dean AC, Kohno M, Hellemann G, London ED (2014) Childhood maltreatment and amygdala connectivity in methamphetamine dependence: a pilot study. Brain Behav 4: 867-876.
- Ganzel BL, Kim P, Glover GH, Temple E (2008) Resilience after 9/11: multimodal neuroimaging evidence for stress-related change in the healthy adult brain. Neuroimage 40: 788-795.
- Weiskopf N, Lutti A, Helms G, Novak M, Ashburner J, et al. (2011) Unified segmentation based correction of R1 brain maps for RF transmit field inhomogeneities (UNICORT). Neuroimage 54: 2116-2124.
- Helms G, Dathe H, Dechent P (2008) Quantitative FLASH MRI at 3T using a rational approximation of the Ernst equation. MagnReson Med 59: 667-672.
- Helms G, Dathe H, Kallenberg K, Dechent P (2008) High-resolution maps of magnetization transfer with inherent correction for RF inhomogeneity and T1 relaxation obtained from 3D FLASH MRI. MagnReson Med 60: 1396-1407.
- Helms G, Draganski B, Frackowiak R, Ashburner J, Weiskopf N (2009) Improved segmentation of deep brain grey matter structures using magnetization transfer (MT) parameter maps. Neuroimage 47: 194-198.
- Helms G, Dathe H, Weiskopf N, Dechent P (2011) Identification of signal bias in the variable flip angle method by linear display of the algebraic Ernst equation. MagnReson Med 66: 669-677.
- Lutti A, Hutton C, Finsterbusch J, Helms G, Weiskopf N (2010) Optimization and validation of methods for mapping of the radiofrequency transmit field at 3T. MagnReson Med 64: 229-238.
- Lutti A, Stadler J, Josephs O, Windischberger C, Speck O, et al. (2012) Robust and fast whole brain mapping of the RF transmit field B at 7T. PLoS One 7: e32379.
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. MagnReson Med 34: 537-541.
- Fransson P (2005) Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum brain mapp 26: 15-29.
- Fox MD, Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8: 700-711.
- Deco G, Jirsa VK, McIntosh AR (2011) Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat Rev Neurosci 12: 43-56.
- Friston KJ, Kahan J, Biswal B, Razi A (2014) A DCM for resting state fMRI. Neuroimage 94: 396-407.
- Bullmore E, Long C, Suckling J, Fadili J, Calvert G, et al. (2001) Colored noise and computational inference in neurophysiological (fMRI) time series analysis: resampling methods in time and wavelet domains. Hum brain mapp 12: 61-78.
- Shin CW, Kim S (2006) Self-organized criticality and scale-free properties in emergent functional neural networks. Physical review E, Statistical, nonlinear, and soft matter physics 74: 045101.
- Stam CJ, de Bruin EA (2004) Scale-free dynamics of global functional connectivity in the human brain. Hum Brain Mapp 22: 97-109.
- Penny WD, Stephan KE, Mechelli A, Friston KJ (2004) Comparing dynamic causal models. Neuroimage 22: 1157-1172.
- Stephan KE, Penny WD, Daunizeau J, Moran RJ, Friston KJ (2009) Bayesian model selection for group studies. Neuroimage 46: 1004-1017.
- Tardif CL, Collins DL, Pike GB (2009) Sensitivity of voxel-based morphometry analysis to choice of imaging protocol at 3 T. Neuroimage 44: 827-838.
- Ashburner J (2007) A fast diffeomorphic image registration algorithm. Neuroimage 38: 95-113.
- Ashburner J, Friston KJ (2000) Voxel-based morphometry--the methods. Neuroimage 11: 805-821.
- Penny WD, Stephan KE, Daunizeau J, Rosa MJ, Friston KJ, et al. (2010) Comparing families of dynamic causal models. PLoSComputBiol 6: e1000709.
- Kurth F, Luders E, Gaser C (2015) Voxel-Based Morphometry. In: Toga AW (Edn). Brain Mapping. Academic Press: Waltham pp: 345-349.
Relevant Topics
- Adolescent Anxiety
- Adult Psychology
- Adult Sexual Behavior
- Anger Management
- Autism
- Behaviour
- Child Anxiety
- Child Health
- Child Mental Health
- Child Psychology
- Children Behavior
- Children Development
- Counselling
- Depression Disorders
- Digital Media Impact
- Eating disorder
- Mental Health Interventions
- Neuroscience
- Obeys Children
- Parental Care
- Risky Behavior
- Social-Emotional Learning (SEL)
- Societal Influence
- Trauma-Informed Care
Recommended Journals
Article Tools
Article Usage
- Total views: 15424
- [From(publication date):
October-2015 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 10758
- PDF downloads : 4666