Research Article Open Access
Alterations of ASL Blood Perfusion in the Thalamus and Ventral Intermediate Thalamic Nucleus in Parkinson's Disease Patients
Hong-Ying Zhang1, Yao Xu2, Jia-Xing Cheng2, Wen-Xin Chen1, Jing Ye1, Jing-Tao Wu1, Lan-Lan Chen2 and Jun Xu2,3*1Department of Radiology, Subei People’s Hospital of Jiangsu Province, Yangzhou University, Yangzhou, 225001, China
2Department of Neurology, Subei People’s Hospital of Jiangsu Province, Yangzhou University, Yangzhou, 225001, China
3Jiangsu Key Laboratory of Integrated Traditional Chinese and Western Medicine for Prevention and Treatment for Senile Diseases, School of Medicine, Yangzhou University, Yangzhou, 225001, China
- *Corresponding Author:
- Jun Xu
Department of Neurology
Subei People’s Hospital of Jiangsu Province,
Yangzhou University, Yangzhou, 225001, China
Tel: +86 136 1157 2068
E-mail: 13611572068@126.com
Received date: September 19, 2016; Accepted date: November 01, 2016; Published date: November 08, 2016
Citation: Zhang HY, Xu Y, Cheng JX, Chen WX, Ye J, et al. (2016) Alterations of ASL Blood Perfusion in the Thalamus and Ventral Intermediate Thalamic Nucleus in Parkinson’s Disease Patients. J Alzheimers Dis Parkinsonism 6: 281. doi: 10.4172/2161-0460.1000281
Copyright: ©2016 Zhang HY, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Parkinson’s disease (PD) is characterized by brain metabolic abnormalities, particularly in subcortical regions. However, previously reported observations of subcortical blood perfusion are inconsistent, especially in the thalamus. This study aimed to investigate the alterations of thalamic regional cerebral blood flow (CBF) in PD by using a recently developed advanced 3D pseudo-continuous pulse arterial spin labeling (ASL) perfusion magnetic resonance imaging approach. Twenty-three PD patients with mild-to-moderate stage disease and 29 normal control subjects underwent ASL MRI. Regional mean CBF values in the thalamus and ventral intermediate thalamic nucleus (Vim) were measured and compared between the groups by one-way analysis of variance. The PD patients showed significantly lower CBF than controls in the bilateral thalamus and Vim. Our findings indicate that hypoperfusion in the thalamus and Vim could be an imaging marker of PD.
Keywords
Arterial spin labeling; Parkinson’s disease; Thalamus; MRI
Introduction
The thalamus is a critical structure for sensorimotor information integration and is an intermediate node within the well-documented complicated extrapyramidal circuitry that has been seriously implicated in the course of Parkinson’ disease (PD) [1-3]. Treatments such as deep brain stimulation (DBS) targeted to the substructure of the thalamus have been shown to be effective for PD symptoms. Functional imaging of the subcortical nucleus has attracted increasing attention in investigations of the intrinsic alterations in PD patients and magnetic resonance imaging (MRI) and positron emission tomography (PET) are the main approaches.
PET is a traditional method for measurement of cerebral blood flow (CBF) and 18F-fluorodeoxyglucose (FDG) metabolism. CBF and glucose metabolism are tightly coupled in normal and diseased brains [4-6]. Multiple PET studies have reported significant abnormal changes in PD patients in terms of CBF, metabolism and selective neurotransmitters receptor activities. Arterial spin labeling (ASL) MRI is increasingly applied in clinical research. ASL perfusion MRI is able to offer absolute quantification of CBF in units of milliliters of blood per 100 g of tissue per minute, which is comparable with measurements acquired by 15O-PET at both resting and task-activation states [6-9] and a few studies have applied ASL MRI in studies of PD. However, previous studies have reported inconsistent observations regarding alterations of subcortical CBF and metabolism in PD brains; for example, a PD-related covariance pattern (PDRP) has been proposed, which indicates that the PD brain is characterized by increased CBF and FDG hypermetabolism in a set of subcortical regions such as pallidumthalamus and pons, along with decreased CBF and hypometabolism in some frontoparietal regions [5,10-13]. In contrast, some PET studies indicated that subcortical blood flow and glucose metabolism were decreased in the PD brain and assumed that the often-reported PDRP could be caused by an artifact of global mean normalization during data preprocessing [14,15]. Motor improvement in PD patients treated with apomorphine or DBS was suggested to be associated with increases in CBF in a series of subcortical as well as cortical regions [16,17]. Another three investigations using ASL MRI did not reveal any differences in subcortical CBF between PD patients and controls, except in some cortex areas [18-20]. The studies described above demonstrate that the underlying metabolic alterations in subcortical regions are elusive in the PD brain. In the present study, we aimed to establish the regional thalamic CBF using an alternative recently developed 3D pseudocontinuous pulse ASL (pCASL) MRI approach.
Most previous ASL-based research employed either conventional two-dimensional multi-slice or three-dimensional gradient echobased techniques and suffered from motion and susceptibility artifacts. Recently, a new pCASL method with three-dimensional fast spin echo (3D-FSE) with spiral readout was introduced and has drawn increasing attention, because it offers a greatly increased signal-to-noise ratio and reductions in motion artifacts and distortion in regions of high magnetic susceptibility. Due to the advances in ASL techniques, 3D pCASL is increasingly applied in clinical neuroimaging protocols and research [21].
Materials and Methods
Subjects
We recruited PD patients for this prospective study between October 2014 and May 2016 from the Department of Neurology of our hospital. Participants included PD patients with mild-to-moderate stage disease without dementia and healthy age-matched normal controls. Participants were examined by structural MRI and 3D-FSE pCASL MRI. All PD patients were diagnosed by neurologists and fulfilled the United Kingdom Parkinson’s Disease Society Brain Bank criteria. Hoehn and Yahr (HY) scores for the patients ranged from 1 to 3. Motor symptoms were evaluated with the Unified Parkinson Disease Rating Scale part III (UPDRS-III). Six PD patients had never received any anti-PD medication therapy and the others had been off medication for at least 24 h before MRI. The demographic and clinical details for the study groups are listed in Table 1. This study was approved by the Ethics Review Board of our hospital and informed consent was obtained from all study participants.
Imaging protocol
All MRI examinations were performed at 3T MR imaging system (Discovery 750, GE Healthcare, Milwaukee, WI) with an 8-channel phased array head coil. Foam pads were put into the coil to prevent head motion and reduce noise. The MRI protocols included axial diffusion weighted imaging (WI), T1WI, T2WI and sagittal T2WI. The 3D ASL parameters were as follows: 36 axial slices parallel to the AC-PC line, covering the whole brain; TR 4844 ms; TE 10.5 ms; slice thickness 4.0 mm; slice gap 0 mm; FOV 24 × 24 cm; bandwidth 62.5 kHz; NEX 3; post labeling delay time 2025 ms. Multiarm spiral imaging was used with 8 arms and 512 points acquired on each arm. The scanning time was 4 min 41 s. Finally, a BRAVO T1 weighted three-dimensional (3D) high-resolution sequence that covered the whole brain was acquired with the following parameters: TR 12 ms; TE 5.1 ms; TI 450 ms; flip angle 20; slice thickness 1 mm; slice gap 0 mm; FOV 256 mm; and matrix 256 × 256.
| Number | F/M | Age (years) | MMSE | UPDRS-III | |
|---|---|---|---|---|---|
| PD | 23 | 6/17 | 64.3 ± 8.6 | 25.5 ± 3.5 | 46.0 ± 19.2 |
| Controls | 29 | 12/17 | 64.1 ± 9.5 | 28.2 ± 1.1 | NA |
PD: Parkinson��?s Disease; F: Female; M: Male; UPDRS: Unified Parkinson Disease Rating Scale; NA: Not Applicable.
Table 1: Demographic and clinical data for PD patients and healthy control participants.
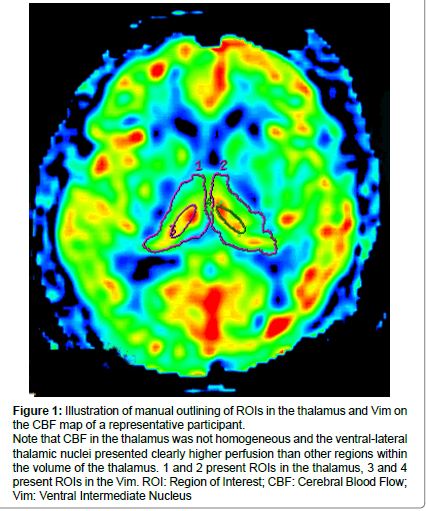
Figure 1: Illustration of manual outlining of ROIs in the thalamus and Vim on the CBF map of a representative participant.
Note that CBF in the thalamus was not homogeneous and the ventral-lateral thalamic nuclei presented clearly higher perfusion than other regions within the volume of the thalamus. 1 and 2 present ROIs in the thalamus, 3 and 4 present ROIs in the Vim. ROI: Region of Interest; CBF: Cerebral Blood Flow; Vim: Ventral Intermediate Nucleus.
Data processing and analysis
ASL data post-processing was performed on the GE AW4.6 workstation. The 3D ASL software toolkit was used to generate raw CBF maps. Absolute thalamic regional CBF (rCBF) was measured by drawing a region of interest (ROI) on the slice with the largest area of the thalamus for each participant by referring to the high-resolution 3D T1 MRI. The borders of the left and right thalamus were defined through manual drawing. All ROIs were drawn blinded to the patient or control status.
During our pre-experiment, we observed that the CBF maps of the thalamus were not homogeneous and the ventral intermediate thalamic nucleus (Vim) presented the highest perfusion among the whole thalamus area, The Vim could be easily positioned and thus, we also drew ROIs on the bilateral Vim to detect CBF in these regions. Figure 1 illustrates the positions of ROIs in a representative case.
Statistical analyses of differences in demographic data and rCBF between the groups were conducted by one-way analysis of variance using SPSS software, version 15.0.
Results
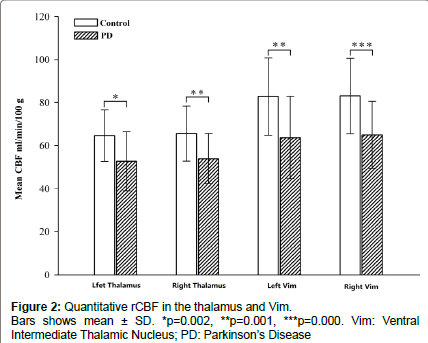
The demographic characteristics and neuropsychological scores of the study participants are shown in Table 1. No significant differences in age or sex were noted between groups. The mean rCBF values obtained in the two groups are shown in Figure 2. The absolute mean rCBF values (± SD) in the left and right thalamus and Vim in PD patients were 52.6 ± 13.7, 53.9 ± 11.6, 63.7 ± 19.1 and 64.9 ± 15.4 ml/min/100 g, respectively. The mean rCBF values (± SD) in the left and right thalamus and Vim in the control group were 64.6 ± 12.0, 65.5 ± 12.7, 82.8 ± 18.0 and 83.0 ± 17.6 ml/min/100 g, respectively. The PD group showed significantly lower rCBF in the left and right thalamus and Vim than the control group with F=11.1, 11.3, 13.5, 15.0 and p=0.002, 0.001, 0.001 and 0.000, respectively. No significant differences in the rCBF between the left and right thalamus and Vim were observed in the within group analysis. No significant correlations were found between the rCBF values and UPDRS scores for the patients.
Discussion
In this prospective study, we aimed to illuminate the changes in regional blood flow in the thalamus of PD patients using a new 3D-FSE pCASL perfusion MRI method. Our findings showed significantly lower regional CBF in the bilateral thalamus in PD patients when compared with the control group, in particular, in the thalamic substructure of the Vim.
The thalamus is the core brain region that integrates sensorimotor information and plays a relaying role within the cortical-basal gangliathalamo- cortical extrapyramidal circuitry. Decreased thalamic neuronal electrophysiology activity in both Parkinsonian animal models and human patients has been demonstrated in previous studies and is considered as an important factor underlying the movement deficits of PD [3,22-24]. Through noninvasive blood flow measurement, our findings confirmed a decreased level of blood perfusion in the thalamus as well as in a substructure, the Vim, in PD patients and these findings are consistent with those of previous studies. We speculate that the possible of mechanism for the subcortical hypoperfusion is related to the defects in neurotransmitters and consequent impairment of neurogenic vasoregulation. Loss of dopaminergic neurons is the most prominent pathological feature in PD and stimulation of the dopaminergic system was shown to induce widespread increases in CBF in both animal models and humans [14,25].
Our findings indicate that the pattern of CBF in the thalamus is heterogeneous and the Vim presents the highest activity within the thalamus. This imaging feature may be attributed to the intensive modulation function of sensorimotor activity in the Vim. PET and SPECT studies indicated that Levodopa and DBS in PD patients could induce improvement of the CBF deficit in the Vim [17,26]. However, some previous studies using ASL did not reveal reduced rCBF in the thalamus [10,11,18-20], which is inconsistent with our findings. This discrepancy could be ascribed to differences in the methodology employed. A statistical parametric map with whole brain CBF normalization was applied in previous studies. However, different brain areas, such as the posterior and anterior circulating, cortical and subcortical regions, actually possess very different rCBF values and whole brain CBF normalization liked introduced bias. In our method, careful ROIs were carefully drawn for acquisition of absolute rCBF data. Our results suggest that thalamic hypoperfusion could be a characteristic of the PD brain.
There are some methodological limitations in our study. First, we focused on the thalamus as the ROI, rather than whole brain regions, based on the consideration that principal pathological features of PD can be attributed to disorders of subcortical neurotransmitters. Second, we only acquired CBF data to quantify blood perfusion due to the restrictions of the imaging technique. Other ASL parameters such as the arterial transmission time and blood flow volume could provide further insight into the mechanisms underlying the pathology of PD [18]. Additional perfusion parameters should be taken into account in future investigations.
In summary, compared with healthy controls, PD patients showed significantly lower rCBF in the thalamus as well as in its substructure, the Vim. Based on the excellent ability to measure CBF deep in the brain, 3D ASL pseudo-continuous pulse perfusion MRI is a suitable approach for detecting biomarkers of PD and could be used for treatment and disease monitoring in the future.
Acknowledgement
This work was supported by Grants 81471642, 81571652, 81271211 and 81471215 from the National Natural Science Foundation of China, Grants BE2015665, BK20151592 from the Science and Technology Department of Jiangsu Province and Grant 2012-ws-002, 2015-WSN-112 from the Six Talent Peaks Project of Jiangsu Province.
References
- Henderson JM, Carpenter K, Cartwright H, Halliday GM (2000) Loss of thalamic intralaminar nuclei in progressive supranuclear palsy and Parkinson's disease: Clinical and therapeutic implications. Brain 123: 1410-1421.
- Brodkey JA, Tasker RR, Hamani C, McAndrews MP, Dostrovsky JO, et al. (2004) Tremor cells in the human thalamus: Differences among neurological disorders. J Neurosurg 101: 43-47.
- Bosch-Bouju C, Smither RA, Hyland BI, Parr-Brownlie LC (2014) Reduced reach-related modulation of motor thalamus neural activity in a rat model of Parkinson's disease. J Neurosci 34: 15836-15850.
- Zou Q, Wu CW, Stein EA, Zang Y, Yang Y (2009) Static and dynamic characteristics of cerebral blood flow during the resting state. Neuroimage 48: 515-524.
- Ma Y, Tang C, Spetsieris PG, Dhawan V, Eidelberg D (2007) Abnormal metabolic network activity in Parkinson's disease: Test-retest reproducibility. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism 27: 597-605.
- Cha YH, Jog MA, Kim YC, Chakrapani S, Kraman SM, et al. (2013) Regional correlation between resting state FDG PET and pCASL perfusion MRI. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism 33: 1909-1914.
- Feng CM, Narayana S, Lancaster JL, Jerabek PA, Arnow TL, et al. (2004) CBF changes during brain activation: fMRI vs. PET. Neuroimage 22: 443-446.
- Ye FQ, Berman KF, Ellmore T, Esposito G, van Horn JD, et al. (2000) H(2)(15)O PET validation of steady-state arterial spin tagging cerebral blood flow measurements in humans. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine 44: 450-456.
- Zhang K, Herzog H, Mauler J, Filss C, Okell TW, et al. (2014) Comparison of cerebral blood flow acquired by simultaneous [15O]water positron emission tomography and arterial spin labeling magnetic resonance imaging. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism 34: 1373-1380.
- Eckert T, Barnes A, Dhawan V, Frucht S, Gordon MF, et al. (2005) FDG PET in the differential diagnosis of parkinsonian disorders. Neuroimage 26: 912-921.
- Huang C, Tang C, Feigin A, Lesser M, Ma Y, et al. (2007) Changes in network activity with the progression of Parkinson's disease. Brain 130: 1834-1846.
- Borghammer P, Hansen SB, Eggers C, Chakravarty M, Vang K, et al. (2012) Glucose metabolism in small subcortical structures in Parkinson's disease. Acta Neurol Scand 125: 303-310.
- Ma Y, Huang C, Dyke JP, Pan H, Alsop D, et al. (2010) Parkinson's disease spatial covariance pattern: Non-invasive quantification with perfusion MRI. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism 30: 505-509.
- Borghammer P, Chakravarty M, Jonsdottir KY, Sato N, Matsuda H, et al. (2010) Cortical hypometabolism and hypoperfusion in Parkinson's disease is extensive: Probably even at early disease stages. Brain Struct Funct 214: 303-317.
- Borghammer P, Cumming P, Aanerud J, Gjedde A (2009) Artefactual subcortical hyperperfusion in PET studies normalized to global mean: Lessons from Parkinson's disease. Neuroimage 45: 249-257.
- Hosey LA, Thompson JL, Metman LV, van den Munckhof P, Braun AR (2005) Temporal dynamics of cortical and subcortical responses to apomorphine in Parkinson disease: An H2(15)O PET study. Clinical neuropharmacology 28: 18-27.
- Fukuda M, Barnes A, Simon ES, Holmes A, Dhawan V, et al. (2004) Thalamic stimulation for parkinsonian tremor: Correlation between regional cerebral blood flow and physiological tremor characteristics. Neuroimage 21: 608-615.
- Al-Bachari S, Parkes LM, Vidyasagar R, Hanby MF, Tharaken V, et al. (2014) Arterial spin labelling reveals prolonged arterial arrival time in idiopathic Parkinson's disease. Neuroimage Clin 6: 1-8.
- Kamagata K, Motoi Y, Hori M, Suzuki M, Nakanishi A, et al. (2011) Posterior hypoperfusion in Parkinson's disease with and without dementia measured with arterial spin labeling MRI. Journal of magnetic resonance imaging: JMRI 33: 803-807.
- Le Heron CJ, Wright SL, Melzer TR, Myall DJ, MacAskill MR, et al. (2014) Comparing cerebral perfusion in Alzheimer's disease and Parkinson's disease dementia: An ASL-MRI study. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism 34: 964-970.
- Amukotuwa SA, Yu C, Zaharchuk G (2016) 3D Pseudocontinuous arterial spin labeling in routine clinical practice: A review of clinically significant artifacts. J Magn Reson Imaging 43: 11-27.
- Basha D, Dostrovsky JO, Lopez Rios AL, Hodaie M, Lozano AM, et al. (2014) Beta oscillatory neurons in the motor thalamus of movement disorder and pain patients. Experimental neurology 261: 782-790.
- Chen H, Zhuang P, Miao SH, Yuan G, Zhang YQ, et al. (2010) Neuronal firing in the ventrolateral thalamus of patients with Parkinson's disease differs from that with essential tremor. Chinese Medical Journal 123: 695-701.
- Galvan A, Devergnas A, Wichmann T (2015) Alterations in neuronal activity in basal ganglia-thalamocortical circuits in the parkinsonian state. Frontiers in neuroanatomy 9: 5.
- Leenders KL, Wolfson L, Gibbs JM, Wise RJ, Causon R, et al. (1985) The effects of L-DOPA on regional cerebral blood flow and oxygen metabolism in patients with Parkinson's disease. Brain 108: 171-191.
- Sestini S, Ramat S, Formiconi AR, Ammannati F, Sorbi S, et al. (2005) Brain networks underlying the clinical effects of long-term subthalamic stimulation for Parkinson's disease: A 4 year follow-up study with rCBF SPECT. J Nucl Med 46: 1444-1454.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 3725
- [From(publication date):
November-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 2846
- PDF downloads : 879