Research Article Open Access
Alterations in the Secretory Behavior of Salivary Glands of Rhipicephalus Sanguineus Females Latreille Acari Ixodidae Feeding in Resistant Rabbit
Karim Christina Scopinho Furquim, Maria Izabel Camargo Mathias*, Letícia Maria Gráballos Ferraz Hebling, Gislaine Cristina Roma and Gervásio Henrique BecharaUNESP, Via de Acesso Prof. Paulo Castellane, s/n, CEP: 14884-900, Jaboticabal, S.P., Brazil
- *Corresponding Author:
- Maria Izabel Camargo Mathias
UNESP, Via de Acesso Prof. Paulo Castellane
s/n, CEP: 14884-900, Jaboticabal, S.P. Brazil
Fax: +55 19 35264100
E-mail: micm@rc.unesp.br
Received Date: July 22, 2013; Accepted Date: October 15, 2013; Published Date: October 21, 2013
Citation: SFurquim KCS, Camargo-Mathias MI, Ferraz Hebling LMG, Roma GC, Bechara GH (2013) Alterations in the Secretory Behavior of Salivary Glands of Rhipicephalus sanguineus Females (Latreille, 1806) (Acari: Ixodidae) Feeding in Resistant Rabbit. Air Water Borne Diseases 2:114. doi:10.4172/2167-7719.1000114
Copyright: © 2013 Scopinho Furquim KC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Air & Water Borne Diseases
Keywords
Rhipicephalus sanguineus; Immunization; Glandular extract; Secretory cycle; Glycoprotein; Lipoprotein; Calcium and acid phosphatase
Introduction
The species Rhipicephalus sanguineus, known as dog tick, is a vector of several pathogens that affect animals and also the human being [1]. This species is currently considered an “urban plague”, since it infests dogs in urban areas [2-4].
As ticks are obligatory hematophage organisms, the secretion of their salivary glands is considered fundamental for their survival, once it is a complex and efficient mixture, constituted by several bioactive elements which modulate the immune-inflammatory and hemostatic responses of the host [5-8], allowing the ectoparasites to remain fixed on the host consuming blood for several days or even weeks.
The saliva of the ticks is a complex and sophisticated pharmacological arsenal, constituted by protein, glycoprotein, lipoprotein and lipid [9-12], such as acid phosphatase, esterases, aminopeptidases, metalloproteases, calreticulin, prostaglandins, prostacyclins, lipocalins [8,9,12-19], among many others. Such molecules interact with the elements of the host’s immune system blocking their defenses on the first days of feeding [16,20]. Moreover, according to Wikel [21], the same salivary molecule can present different biological activities in the modulation of the local homeostatic responses of the host, which also contributes to the high functional complexity of the saliva in modulating the host’s defense system.
Among the elements with pharmacological and immunological properties synthesized by the salivary glands of the ticks are: a) enzymes b) enzyme inhibitors c) host-homologous protein d) immunoglobulin binding protein e) amine-binding lipocalin f) receptor agonists/ antagonists g) elements that carry calcium (calreticulin) h) cytokine expression modulators, as well as other bioactive lipid components, such as prostaglandin (PGE2) [8,12,16].
The diversified biochemistry of the ticks’ saliva reflects the morphofunctional complexity of their salivary glands. In the females, these glands are constituted by acini types I, II and III [9,13,22-24] and the morphohistochemical alterations, which occur exclusively in acini II and III result in differences in the production of secretion over the glandular cycle [9]. Acini II contain different secretory cells (a, b, c1, c2, c3 e c4) [9]. In R. sanguineus females, in addition to these, c5 and c6 were recently described [24]. Types d, e and f are observed in acini III [9,13,22,24].
The pharmacological and immunological characteristics of the bioactive elements present in the ticks’ saliva demonstrate that this mixture is an important source of antigens, able to modify the immunobiology of the tick-host interface and also to impair the physiology of the ectoparasite [8,16,25], also demonstrating that it can be used to control these arthropods.
Thus, this study aimed to histochemically and cytochemically investigate the secretory cycle of salivary glands of R. sanguineus females fed for 2, 4 and 6 days on rabbits previously immunized with salivary glands extracts from females of this same species fed for 2 days.
Materials and Methods
Material
Salivary glands of adult R. sanguineus females fed for 2, 4 and 6 days subjected to infestation on New Zealand White rabbits immunized with glandular extract of 2-day fed females were used in this study. Unfed individuals (males and females) from a colony kept in BOD incubator, in controlled conditions (29°C, 80% of humidity and photoperiod of 12 hours), in the Biotery of the Biology Department of UNESP campus Rio Claro (SP) were used in the infestations A, B and C made in rabbits, according to the procedure described by Bechara et al. [26].
-Infestation A: Performed with naive rabbits using 25 couples of R. sanguineus couples/host, for the acquisition of 2-day fed females (55 individuals), to obtain the glandular extract SGE2=glandular extract of 2-day fed females.
The extract was processed and inoculated in the hosts subjected to infestation B.
- Infestation B (test group=TG): Performed with 4 rabbits inoculated with SGE2 extract, subjected to challenge infestation with 15 couples of adult R. sanguineus ticks/host.
- Infestation C (control group=PCG): Performed with 4 naive rabbits, subjected to challenge infestation with 15 couples of adult R. sanguineus ticks/host.
This experiment was approved by the Ethics Committee in Research and Scientific Merit–P-UNIARARAS, Protocol n°021/2009.
Methods
In the Laboratory of the Department of Molecular Biology of UNESP Rio Claro (SP), Brazil, the salivary glands were put in eppendorf tube containing 200 μL of phosphate buffer pH 7.4. Then the glands were macerated, centrifuged for 30 minutes at 10.000×g, the supernatant was collected and put for proteins dosage, according to the methodology described by Sedmark and Grossberg [27] (Bradford method), which should be of at least 0.2 μg/μL.
After the determination of the protein content, the extract was filtered with the help of sterile filtering units (JBR610303, disposable filtering unit Millex GV, durapore membrane PVDF, Millipore, MilliUni), of 0.22 μm and 13 mm of diameter, attached to hypodermic syringes in the interior of a pre-sterile vertical laminar flow. The extract was then divided in volumes of 50 μL and kept in freezer at -20°C. Only at the moment of inoculation, the extract was mixed (50 μL of extract/ host) with 50 μL of complete Freund adjuvant (reference #F 5881, Sigma-Aldrich), procedures that are also made in pre-sterile vertical laminar flow.
After, rabbits from TG had the right dorsal side sheared and were subcutaneously inoculated with SGE2 extract, via hypodermic syringe, for three times in intervals of 21 days. Only after 15 days from the last inoculation, all the hosts from TG and CG were subjected to infestation challenge with 15 couples of R.sanguineus/host.
The R. sanguineus females fed for 2, 4 and 6 days were removed from the rabbits inoculated with SGE2 (TG) extract, as well as from those not inoculated (CG) and subjected to histochemical analysis.
In the Histology Laboratory of the Biology Department of UNESP campus Rio Claro (SP), Brazil, salivary glands of R. sanguineus females of each group (TG and CG) fed for 2, 4 and 6 days were removed in buffered saline solution (7.5 g NaCl+2.38 g Na2HPO4+2.72 g KH2PO4 in 1000 mL distilled water) and fixed.
Part of the material was fixed in 4% paraformaldehyde at 4°C, after fixation, the material was dehydrated in a series of increasing concentrations of ethanol (70%, 80%, 90% and 95%), embedded in resin (Leica), and sectioned at 3 μm thickness. Sections were mounted on glass slides and histochemical tests were applied to detect the presence of the following compounds: proteins (Bromophenol blue), as proposed by Pearse [28]; polysaccharides (PAS-Periodic Acid Schiff), as proposed by McManus [29], and counterstained with methyl green; calcium (von Kossa) as proposed by Junqueira and Junqueira [30] and lipids [31].
The other part of the material was fixed in 10% buffered neutral formalin and acetone (9:1) for one hour and thirty minutes at 4°C, then processed according to the methods described by Hussein et al. [32], for detection of acid phosphatase activity. The material was then dehydrated in increasing concentrations of ethanol (70%, 80%, 90% and 95%), embedded in resin (Leica), and sectioned at a thickness of 7 μm. The sections were placed on glass slides and counterstained with hematoxylin for 2 minutes. The control samples were incubated without substrate.
The slides of the all histochemical tests were mounted with Canada balsam and examined and photographed under Motic BA 300 light microscope.
Results
Acini I were not considered in the analyses of the salivary glands once they are agranular, and do not present secretory function, acting only in the hydric balance of the ectoparasite [33,34].
Control Group (CG)
Females fed for 2 days
Acinus II
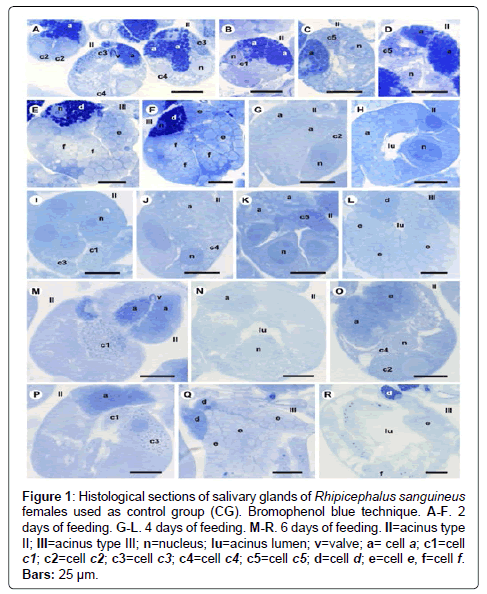
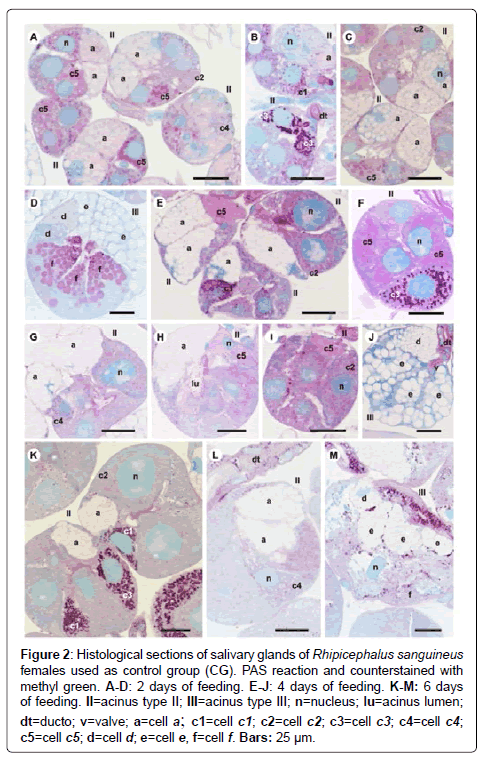
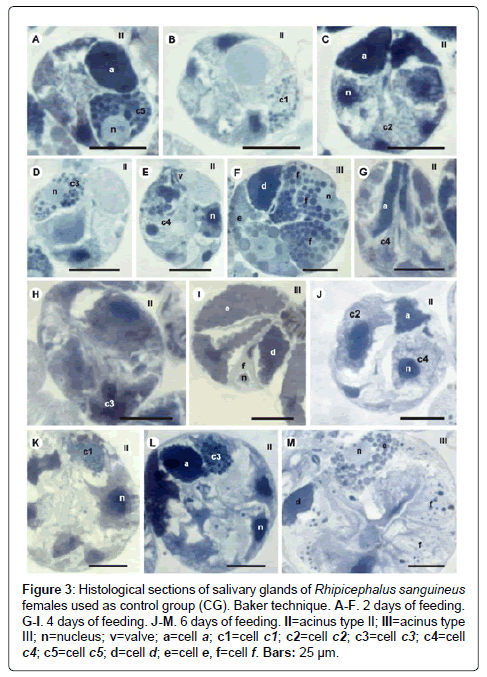
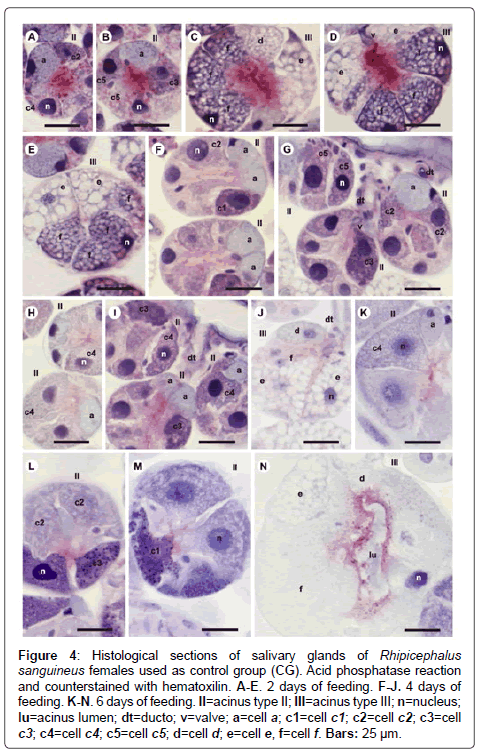
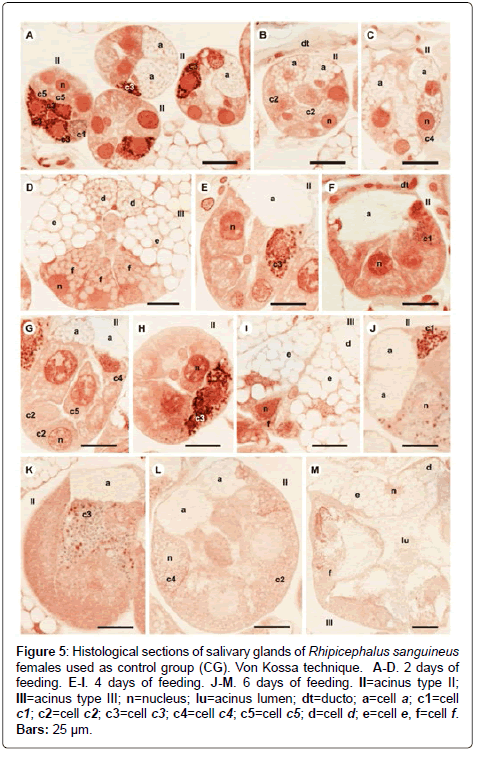
-a cells: The secretion granules are moderately (Figure 1A and 1C) or strongly (Figure 1B and 1D) stained for bromophenol blue, weakly stained for PAS (Figure 2A and 2C), strongly stained for Baker (Figure 3A and 3C) and negative for acid phosphatase (Figure 4A and 4B) and von Kossa (Figure 5A and 5C).
Figure 1: Histological sections of salivary glands of Rhipicephalus sanguineus females used as control group (CG). Bromophenol blue technique. A-F. 2 days of feeding. G-L. 4 days of feeding. M-R. 6 days of feeding. II=acinus type II; III=acinus type III; n=nucleus; lu=acinus lumen; v=valve; a= cell a; c1=cell c1; c2=cell c2; c3=cell c3; c4=cell c4; c5=cell c5; d=cell d; e=cell e, f=cell f. Bars: 25 ├?┬Ám.
Figure 2: Histological sections of salivary glands of Rhipicephalus sanguineus females used as control group (CG). PAS reaction and counterstained with methyl green. A-D: 2 days of feeding. E-J: 4 days of feeding. K-M: 6 days of feeding. II=acinus type II; III=acinus type III; n=nucleus; lu=acinus lumen; dt=ducto; v=valve; a=cell a; c1=cell c1; c2=cell c2; c3=cell c3; c4=cell c4; c5=cell c5; d=cell d; e=cell e, f=cell f. Bars: 25 ├?┬Ám.
Figure 3: Histological sections of salivary glands of Rhipicephalus sanguineus females used as control group (CG). Baker technique. A-F. 2 days of feeding. G-I. 4 days of feeding. J-M. 6 days of feeding. II=acinus type II; III=acinus type III; n=nucleus; v=valve; a=cell a; c1=cell c1; c2=cell c2; c3=cell c3; c4=cell c4; c5=cell c5; d=cell d; e=cell e, f=cell f. Bars: 25 ├?┬Ám.
Figure 4: Histological sections of salivary glands of Rhipicephalus sanguineus females used as control group (CG). Acid phosphatase reaction and counterstained with hematoxilin. A-E. 2 days of feeding. F-J. 4 days of feeding. K-N. 6 days of feeding. II=acinus type II; III=acinus type III; n=nucleus; lu=acinus lumen; dt=ducto; v=valve; a=cell a; c1=cell c1; c2=cell c2; c3=cell c3; c4=cell c4; c5=cell c5; d=cell d; e=cell e, f=cell f. Bars: 25 ├?┬Ám.
Figure 5: Histological sections of salivary glands of Rhipicephalus sanguineus females used as control group (CG). Von Kossa technique. A-D. 2 days of feeding. E-I. 4 days of feeding. J-M. 6 days of feeding. II=acinus type II; III=acinus type III; n=nucleus; lu=acinus lumen; dt=ducto; a=cell a; c1=cell c1; c2=cell c2; c3=cell c3; c4=cell c4; c5=cell c5; d=cell d; e=cell e, f=cell f. Bars: 25 ├?┬Ám.
-c1 cells: The granules are strongly stained for bromophenol blue (Figure 1B) and strongly stained for PAS (Figure 2B), Baker (Figure 3B) and von Kossa (Figure 5A). Such cells were not observed in the technique for the detection of acid phosphatase.
-c2 cells: The granules are weakly stained for bromophenol blue (Figure 1A), weakly (Figure 2A) or moderately (Figure 2C) stained for PAS, weakly stained for Baker (Figure 3C), moderately stained for acid phosphatase (Figure 4A) and negative for von Kossa (Figure 5B).
-c3 cells: The granules are moderately stained for bromophenol blue (Figure 1A) and acid phosphatase (Figure 4B), and strongly stained for PAS (Figure 2B) and Baker (Figure 3D). For von Kossa technique, the same granule presented variations, being strongly stained in some regions and negative in others (Figure 5A).
-c4 cells: The granules do not react to the specific markers (Figures 1A, 2A, 3E and 5C), except for acid phosphatase, being moderately stained for this marker (Figure 4A).
-c5 cells: Granules are weakly stained for bromophenol blue (Figure 1C) or present variations (weakly stained in some regions and moderately stained in others) (Figure 1D) and weakly stained for PAS (Figure 2A and 2C). For Baker, staining is also varied, from moderately stained to strongly stained (Figure 3A). The granules do not react for von Kossa (Figure 5A), but are moderately stained for acid phosphatase (Figure 4B).
Acinus III
-d cells: The granules are strongly stained for bromophenol blue (Figure 1E and 1F) and Baker (Figure 3F); negative for PAS (Figure 2D), acid phosphatase (Figure 4C) and von Kossa (Figure 5D).
-e cells: Granules are weakly (Figure 1E) or moderately stained for bromophenol blue (Figure 1F); negative for PAS (Figure 2D), acid phosphatase (Figures 4C-4E) and von Kossa (Figure 5D) and moderately stained for Baker (Figure 3F).
-f cells: The granules are negative (Figure 1E) or weakly (Figure 1F) stained by bromophenol blue and weakly (Figure 4E) or moderately (Figures 4C-4D) stained by acid phosphatase. Concerning phosphatase, this result is mainly observed in the granules found in the basal region of the cells (Figures 4C-4E). The granules are moderately stained for PAS stained (Figure 2D), or the same granule can present heterogeneous staining, being moderately stained in one region stained and strongly stained in others (Figure 2D). Staining is also varied for Baker, being moderately stained in a region and strongly stained in others (Figure 3F). Granules do not react to von Kossa (Figure 5D).
Females fed for 4 days
Acinus II
-a cells: The granules are weakly stained (Figure 1G and J) or presented varied staining (weakly stained in a region and moderately stained in others) (Figure 1H and 1K) for bromophenol blue and negative for PAS (Figures 2E and 2G-2H), acid phosphatase (Figures 4F and 4G, 4I) and von Kossa (Figures 5E-5G). Granules are strongly stained for Baker technique (Figure 3G).
-c1 cells: The granules are moderately stained for bromophenol blue (Figure 1I) and strongly stained for PAS (Figure 2E) and von Kossa (Figure 5F). However, they are negative to acid phosphatase (Figure 4F). Such cells are not observed through the application of Baker technique.
-c2 cells: Granules are weakly stained for bromophenol blue (Figure 1G), moderately for PAS (Figures 2E and 2) and acid phosphatase (Figures 4F-4G), and negative for von Kossa (Figure 5G). Such cells are not observed through Baker technique.
-c3 cells: Granules are moderately stained for bromophenol blue (Figure 1I) and strongly stained for PAS (Figure 2F) and Baker (Figure 3H). They present varied staining for von Kossa, showing negative (Figure 5E) and strongly stained (Figure 5H) regions, phosphatase staining is not observed (Figures 4G and 4I).
-c4 cells: Granules do not react to bromophenol blue (Figure 1J), PAS (Figure 2G), Baker (Figure 3G) and von Kossa (Figure 5G), and are weakly stained (Figure 4I) or negative (Figure 4H) for acid phosphatase.
-c5 cells: The granules are moderately stained for bromophenol blue (Figure 11K) and acid phosphatase (Figure 4G); weakly (Figure 2H) or moderately (Figures 2E and 2I) stained for PAS. Varied staining is also observed, where the same granule was weakly stained in one region and moderately stained in others (Figure 2F). No reaction for von Kossa (Figure 5G). These cells are not observed through Baker technique.
Acinus III
-d cells: The granules are moderately stained for bromophenol blue (Figure 1L); negative for PAS (Figure 2J), acid phosphatase (Figure 4J) and von Kossa (Figure 5I) and strongly stained for Baker technique (Figure 3I).
-e cells: The granules are weakly stained for bromophenol blue (Figure 1L); negative for PAS (Figure 2E), acid phosphatase (Figure 4J) and von Kossa (Figure 5I), and moderately stained for Baker (Figure 3I).
-f cells: These cells do not contain secretion granules in this phase of the glandular cycle (Figures 3I, 4J and 5I).
Females fed for 6 days
Acinus II
-a cells: The secretion granules are weakly (Figure 1N) or moderately stained (Figures 1M and 1O-1P) for bromophenol blue; negative for PAS (Figure 2K and 2L), acid phosphatase (Figure 4K) and von Kossa (Figures 5J-5L) and strongly stained for Backer (Figure 3J and 3L).
-c1 cells: The granules are moderately stained for bromophenol blue (Figure 1M and 1P) and acid phosphatase (Figure 4M); strongly stained for PAS (Figure 2K) and Baker (Figure 3K) and negative for von Kossa (Figure 5J).
-c2 cells: The granules are weakly stained for bromophenol blue (Figure 1O) and acid phosphatase (Figure 4L); moderately stained for PAS (Figure 2K) and Baker (Figure 3J) and negative for von Kossa (Figure 5L).
-c3 cells: The granules are moderately stained for bromophenol blue (Figure 1P) and von Kossa (Figure 5K); strongly stained for PAS (Figure 2K) and Baker (Figure 3L), and negative for acid phosphatase (Figure 4L).
-c4 cells: The granules of these cells are negative to all applied techniques (Figures 1O, 2L, 3J, 4K and 5L).
-c5 cells: These cells are not observed as they are inactive in this phase.
Acinus III
-d cells: The secretion granules are moderately (Figure 1Q) or strongly (Figure 1R) stained for bromophenol blue; strongly stained for Baker (Figure 3M) and negative for PAS (Figure 2M), acid phosphatase (Figure 4N) and von Kossa (Figure 5M).
-e cells: The granules are weakly stained for bromophenol blue (Figure 1Q and 1R); negative to PAS (Figure 2M), acid phosphatase (Figure 4N) and von Kossa (Figure 5M), and moderately stained for Baker (Figure 3M).
-f cells: These cells do not contain secretion granules in this phase of the glandular cycle (Figures 1R, 2M, 3M, 4F and 5M).
Test group
Females fed for 2 days
Acinus II
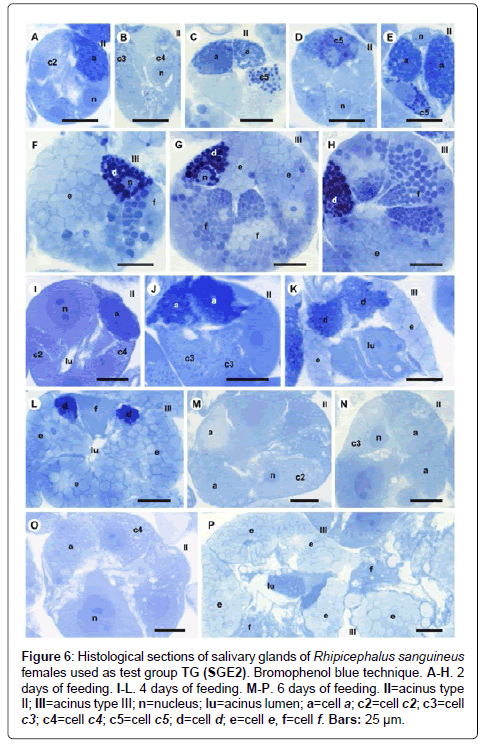
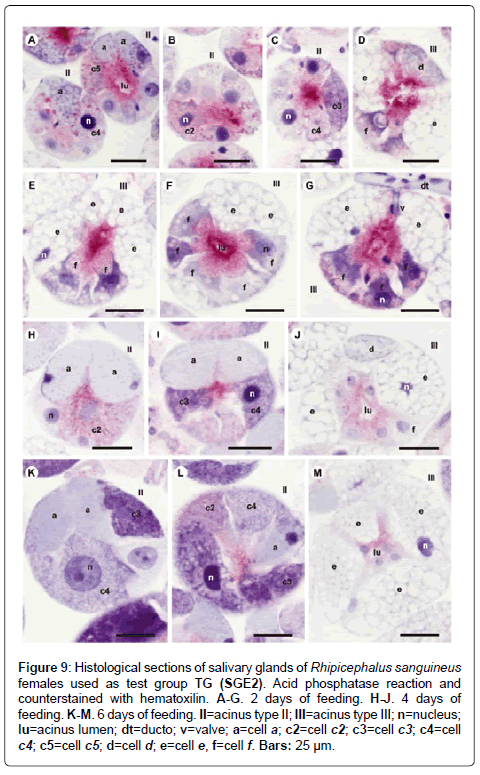
-a cells: Results are the same as the ones obtained in CG (Figures 7A and 7B, 8A-8C, 9A, 10A-10C), except that they are moderately (Figure 6A and 6C) or strongly stained for bromophenol blue (Figure 6E).
Figure 6: Histological sections of salivary glands of Rhipicephalus sanguineus females used as test group TG (SGE2). Bromophenol blue technique. A-H. 2 days of feeding. I-L. 4 days of feeding. M-P. 6 days of feeding. II=acinus type II; III=acinus type III; n=nucleus; lu=acinus lumen; a=cell a; c2=cell c2; c3=cell c3; c4=cell c4; c5=cell c5; d=cell d; e=cell e, f=cell f. Bars: 25 ├?┬Ám.
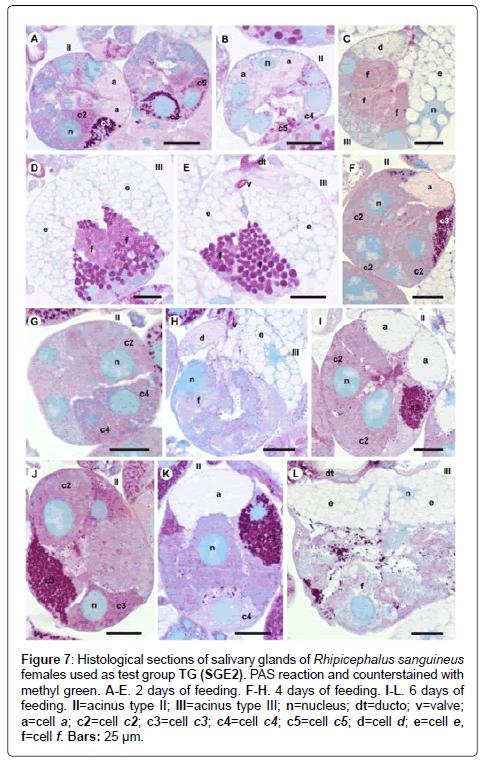
Figure 7: Histological sections of salivary glands of Rhipicephalus sanguineus females used as test group TG (SGE2). PAS reaction and counterstained with methyl green. A-E. 2 days of feeding. F-H. 4 days of feeding. I-L. 6 days of feeding. II=acinus type II; III=acinus type III; n=nucleus; dt=ducto; v=valve; a=cell a; c2=cell c2; c3=cell c3; c4=cell c4; c5=cell c5; d=cell d; e=cell e, f=cell f. Bars: 25 ├?┬Ám.
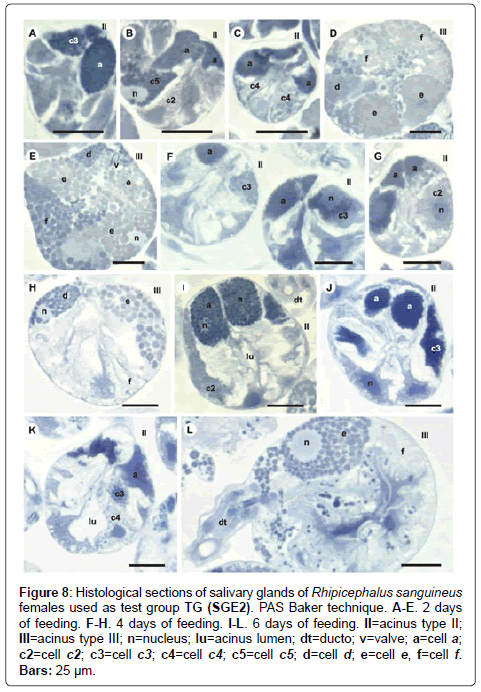
Figure 8: Histological sections of salivary glands of Rhipicephalus sanguineus females used as test group TG (SGE2). PAS Baker technique. A-E. 2 days of feeding. F-H. 4 days of feeding. I-L. 6 days of feeding. II=acinus type II; III=acinus type III; n=nucleus; lu=acinus lumen; dt=ducto; v=valve; a=cell a; c2=cell c2; c3=cell c3; c4=cell c4; c5=cell c5; d=cell d; e=cell e, f=cell f. Bars: 25 ├?┬Ám.
Figure 9: Histological sections of salivary glands of Rhipicephalus sanguineus females used as test group TG (SGE2). Acid phosphatase reaction and counterstained with hematoxilin. A-G. 2 days of feeding. H-J. 4 days of feeding. K-M. 6 days of feeding. II=acinus type II; III=acinus type III; n=nucleus; lu=acinus lumen; dt=ducto; v=valve; a=cell a; c2=cell c2; c3=cell c3; c4=cell c4; c5=cell c5; d=cell d; e=cell e, f=cell f. Bars: 25 ├?┬Ám.
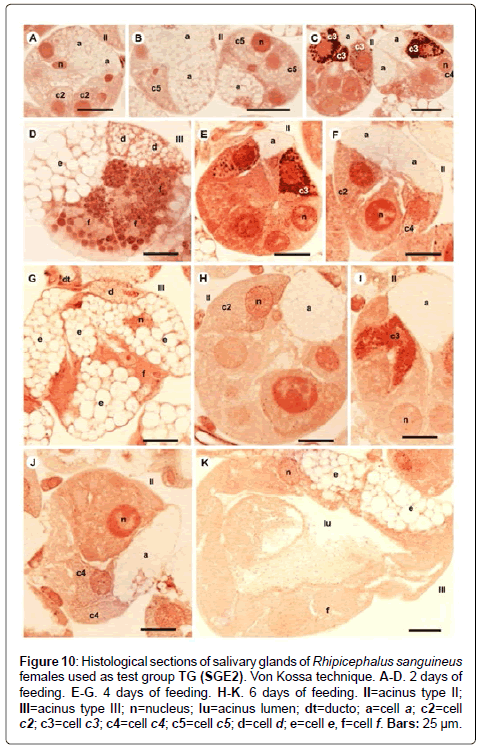
Figure 10: Histological sections of salivary glands of Rhipicephalus sanguineus females used as test group TG (SGE2). Von Kossa technique. A-D. 2 days of feeding. E-G. 4 days of feeding. H-K. 6 days of feeding. II=acinus type II; III=acinus type III; n=nucleus; lu=acinus lumen; dt=ducto; a=cell a; c2=cell c2; c3=cell c3; c4=cell c4; c5=cell c5; d=cell d; e=cell e, f=cell f. Bars: 25 ├?┬Ám.
-c1 cells: These cells are not observed as they are inactive.
-c2 cells: The results are the same as the ones obtained for CG (Figures 6A, 8B, 9B and 10A), except that these cells are moderately stained for PAS (Figure 7A).
-c3 cells: The results are the same as the ones obtained in CG (Figures 6B, 7A, 8A and 10C), except that these cells are moderately stained for phosphatase (Figure 9C).
-c4 cells: The results are the same as the ones obtained for CG (Figures 6B, 7B, 8C and 10C), except that these cells are weakly stained for phosphatase (Figure 9A and 9C).
-c5 cells: The granules are moderately (Figure 6C) or strongly stained (Figure 6E) for bromophenol blue. Granules with varied staining are also observed–some regions are strongly stained and others moderately stained by bromophenol blue (Figure 6D). These granules are moderately stained for PAS (Figure 7A and 7B) and phosphatase (Figure 9A); strongly stained for Baker (Figure 8B) and negative for von Kossa (Figure 10B).
Acinus III
-d cells: The granules are strongly (Figure 6F) or intensively (Figure 6G and 6H) stained for bromophenol blue; moderately stained for Baker (Figure 8D and 8E); weakly stained for acid phosphatase (Figure 9D) and negative to PAS (Figure 7C) and von Kossa (Figure 10D).
-e cells: The granules are weakly (Figure 6F) or moderately (Figure 6G and 6H) stained for bromophenol blue; weakly stained for Baker (Figure 8D and 8E) and negative for PAS (Figures 7C-7E), acid phosphatase (Figures 9D-9G) and von Kossa (Figure 10D).
-f cells: The granules are weakly (Figure 6G and 9E), moderately (Figure 6G and 9F) or strongly (Figure 6H and 9G) stained for bromophenol blue and acid phosphatase. For acid phosphatase, the weakly stained granules occur more frequently. The granules are moderately (Figure 7C) or strongly stained (Figure 7E) for PAS, and some present varied staining (moderately stained in a region and strongly stained in others) (Figure 7D). The granules are moderately stained (Figure 8E) or present varied staining (weakly stained in a region and moderately stained in others) (Figure 8D) for Baker; varied staining was also observed for von Kossa (negative in some regions and strongly stained in others) (Figure 10D).
Females fed for 4 days
Acinus II
-a cells: The granules are moderately (Figure 6I) or strongly (Figure 6J) stained for bromophenol blue; weakly stained for PAS (Figure 7F); strongly stained for Baker (Figure 8F and 8G) and negative for acid phosphatase (Figure 9H and 9I) and von Kossa (Figure 10E and 10F).
-c1 cells: These cells are not observed as they are inactive.
-c2 cells: The granules are weakly stained for bromophenol blue (Figure 6I); moderately stained for PAS (Figures 7F and 7G) and Baker (Figure 8G); strongly stained for acid phosphatase (Figure 9H) and negative for von Kossa (Figure 10F).
-c3 cells: The granules are moderately stained for bromophenol blue (Figure 6J); strongly stained for PAS (Figure 7F) and Baker (Figure 8F) and negative for acid phosphatase (Figure 9I). For von Kossa technique, varied staining is observed in the same granule (negative and strongly stained regions) (Figure 10E).
-c4 cells: The granules are negative for bromophenol blue (Figure 6I), acid phosphatase (Figure 9I) and von Kossa (Figure 10F) and weakly stained for PAS (Figure 7G). Such cells are not observed through Baker technique.
-c5 cells: These cells are not observed as they are inactive.
Acinus III
-d cells: The granules are moderately (Figure 6K) or strongly (Figure 6L) stained for bromophenol blue; weakly stained for PAS (Figure 7H); strongly stained for Baker (Figure 8H) and negative for acid phosphatase (Figure 9J) and von Kossa (Figure 10G).
-e cells: The granules are weakly stained for bromophenol blue (Figure 6K) or presented varied staining (weakly stained in a region and moderately in others) (Figure 6L). Granules are moderately stained for Baker (Figure 8H) and negative for PAS (Figure 7H), acid phosphatase (Figure 9J) and von Kossa (Figure 10G).
-f cells: These cells do not contain secretion granules in this phase of the secretory cycle (Figures 6K, 7H, 8H, 9J and 10G).
Females fed for 6 days
Acinus II
-a cells: The granules are weakly stained for bromophenol blue (Figures 6M and 6O); negative for PAS (Figures 7I-7K), acid phosphatase (Figures 9K and 9L) and von Kossa (Figures 10H-10J) and strongly stained for Baker (Figures 8I- 8K).
-c1 cells: These cells are not observed as they are inactive.
-c2 cells: Granules are weakly stained for bromophenol blue (Figure 6M); negative for von Kossa (Figure 10H) and moderately stained for PAS (Figures 7I and 7J), Baker (Figure 8I) and acid phosphatase (Figure 9L).
-c3 cells: Granules are weakly stained for bromophenol blue (Figure 6N); moderately or strongly stained for PAS (Figures 7I-7K); strongly stained for Baker (Figure 8J and 8K) and negative for acid phosphatase (Figure 9K and 9L) and von Kossa (Figure 10I).
-c4 cells: The granules are negative for all the tests applied (Figures 6O, 7K, 8K. 9K and 9L and 10J).
-c5 cells: These cells are not observed as they are inactive.
Acinus III
-d cells: These cells are not observed as they are inactive.
-e cells: The granules are weakly stained for bromophenol blue (Figure 6P); negative to PAS (Figure 7L), acid phosphatase (Figure 9M) and von Kossa (Figure 10K) and moderately stained for Baker (Figure 8L).
-f cells: These cells do not contain secretion granules in this phase of the secretory cycle (Figures 6P, 7L, 8L and 10K).
The results for salivary glands of the females from groups CG and TG fed for 2, 4 and 6 days are summarized in Tables 1-8.
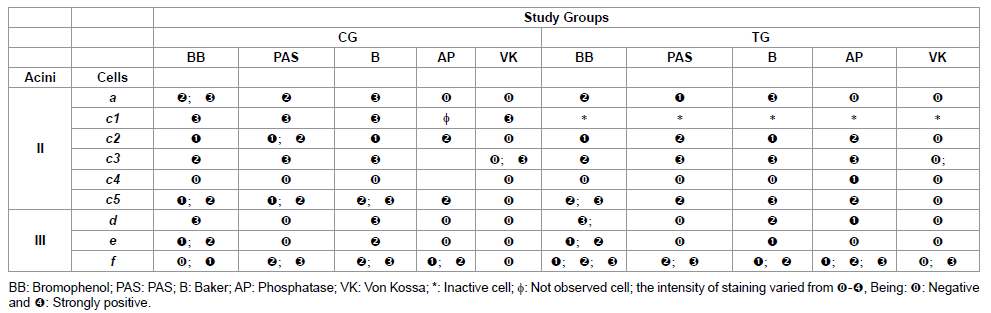
Table 1: Histochemistry of the glandular acini secretion of R. sanguineus females from groups CG and TG fed for 2 days.

Table 2: Histochemistry of the glandular acini secretion of R. sanguineus females from groups CG and TG fed for 4 days.
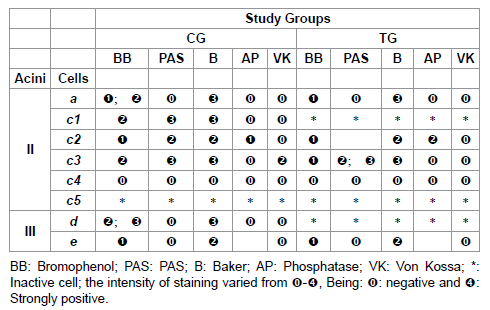
Table 3: Histochemistry of the glandular acini secretion of R. sanguineus females from groups CG and TG fed for 6 days.
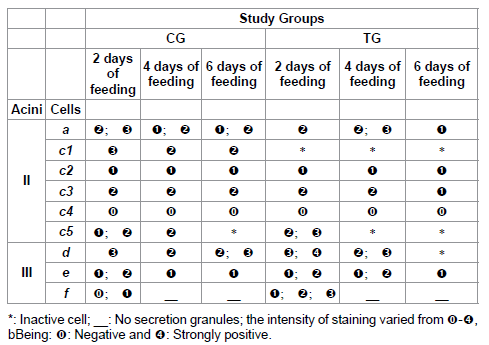
Table 4: Protein staining for acini II and III of salivary glands of R. sanguineus females from groups CG and TG fed for 2, 4 and 6 days.
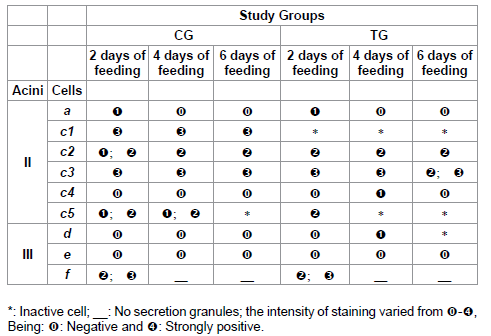
Table 5: Polysaccharides staining for acini II and III of salivary glands of R. sanguineus females from groups CG and TG fed for 2, 4 and 6 days.
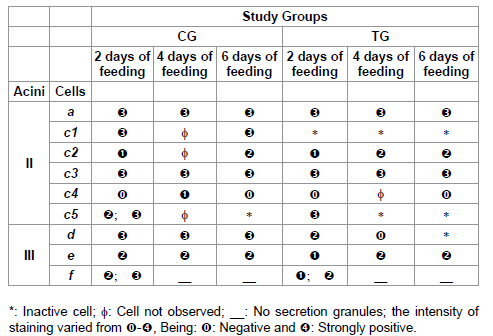
Table 6: Lipids staining for acini II and III of salivary glands of R. sanguineus females from groups CG and TG fed for 2, 4 and 6 days.
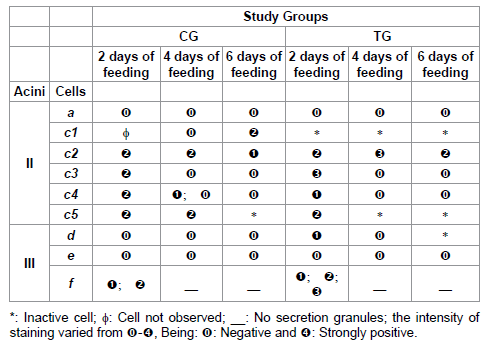
Table 7: Acid phosphatase staining for acini II and III of salivary glands of R. sanguineus females from groups CG and TG fed for 2, 4 and 6 days.
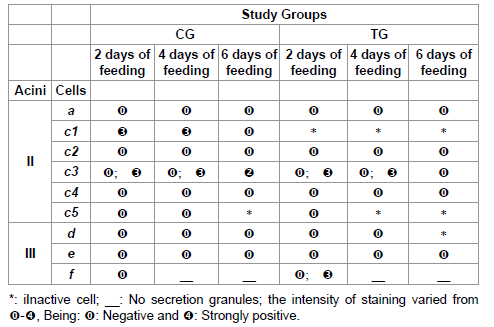
Table 8: Calcium staining for acini II and III of salivary glands of R. sanguineus females from groups CG and TG fed for 2, 4 and 6 days.
Discussion
This study showed the alterations detected over the secretory cycle of salivary glands of R. sanguineus females, in response to the host’s immunization, which occurred, a) in the protein, polysaccharide, lipid, mineral (calcium) and enzyme (acid phosphatase) contents of the glandular secretion and b) in the secretory activity of c1 cells (glands of 2-day fed females), c1 and c5 (4-day fed females) and c1 and d (6-day fed females), corroborating Furquim et al. [35] confirms that the immunization of]. The observed alterations clearly show that the pharmacology of ticks’ saliva can be molded according to the specific homeostatic defenses of the host, as suggested by Ribeiro [36].
There are few studies in literature addressing the morphohistochemistry of the alterations undergone by glandular cells of ticks fed on resistant hosts, either by inoculation of antigens or reinfestations. Moreover, these studies describe superficially the cellular alterations. These studies only focus the occurrence of precocious glandular degeneration [25,37,38]. On the other hand, it is important to obtain these data, once they reveal information about the immunogenic capacity of each component secreted by the salivary glands, indicating their implication in the modulation of the host.
The alterations in the glandular secretory cycle demonstrate that the hosts (rabbits immunized with glandular extract SGE2) developed some type of response (resistance) to the inoculated antigens (obtained from glandular tissues of 2-day fed females - SGE2), corroborating other authors who reported that the contact of the host with the ticks’ saliva would stimulate. For example, laboratory rabbits, to produce immunological response to several saliva antigens, due to their antigenic properties [39]. However, the authors of this study discuss if the resistance acquired by the hosts causes alterations in the glandular secretory cycle, or if the salivary glands only modified their secretory cycle to compensate the acquisition of resistance by the hosts. This study showed that histochemical alterations in the salivary glands of the individuals from TG varied in the different periods of the glandular cycle (2, 4 or 6 days), as well as in the different types of secretory cells present in acini II and III.
This study confirms that the immunization of the hosts with SGE2 extract affects exclusively acini II and III, corroborating Jittapalapong et al. [25], Furquim et al. [35] and Nunes et al. [38]. This probably occurs because the secretory cells of acini II and III are responsible for the secretion of immunogenic molecules, which will modulate the immune-inflammatory and homeostatic systems of the host, corroborating Ferreira et al. [40].
Data here obtained clearly show that among the salivary glands of individuals from TG (2, 4 or 6 days), the 2-day fed females presented the most severe alterations. Moreover, most of these alterations are related to an increase in the intensity of staining of the components; i.e. a more intense staining of protein, polysaccharides, calcium and acid phosphatase was verified while comparing the results to CG. In addition to this, in TG group, it was in the glands obtained from 2-day fed females that the most intense staining were found for all the elements here analyzed, and it was also in this situation (feeding period) that the greatest number of alteration in the greatest number of cells was observed. These alterations could be explained, once the inoculation of glandular extract SGE2 made the hosts specifically resistant (immune) to the antigens of the 2-day fed females’ salivary glands, which affected the blood consumption of the ticks mainly in this feeding period. As a response, the females of TG (mainly 2-day fed females) modified the composition of their saliva through histochemical alterations in their salivary glands, which allow them to counter-attack the resistance acquired by the host.
The present study demonstrated that it was in the 6-day glands that the greatest number of cellular alterations occurred (increase of phosphatase and decrease of proteins, polysaccharides and calcium), and that the alterations in the salivary glands of 4-day fed females would be more related to the increase in the intensity of staining of the components, and not to their reduction of synthesis. In addition, between both glandular tissues (4 and 6 days) from TG, it was in the 4-day fed females’ glandular tissue that greater intensity of staining for protein, polysaccharides, lipids, acid phosphatase and calcium was detected, and also alterations in most types of glandular cells.
The salivary glands obtained from individuals from TG which underwent most alterations (following 2-day fed females) were those from the 4-day fed females. It was observed that the hosts were more resistant to the antigens present in the saliva of 4-day fed females than those from 6-day fed females, and the 4-day glands presented more modifications than 6-day glands.
Considering that the composition of the ticks’ saliva varies over the glandular cycle [9,11,17,41-43], according to the ectoparasite’s needs, while modulating the host’s immune-inflammatory and hemostatic system, and that the components by them secreted have different biological activities [44], it was verified that the glands of 2 and 4-day fed females from GT presented an increase in the staining of their components, once they were in periods of the glandular cycle that would antecede the phase of blood consumption and, therefore, the salivary secretion introduced into the host would be able to modulate/ overcome the host’s defense system, neutralizing/inhibiting the resistance acquired allowing the ectoparasite to complete the feeding process.
As for the alterations in the different types of cells, the affected ones were: a, c1, c2, c3, c4, c5, d, e, f (in the females fed for 2 days); a, c1, c2, c4, c5, d, e (in 4-day fed females); and c1, c2, c3, d (in 6-day fed females). Nunes et al. [38] observed that the most affected cells by the re-infestation in Amblyomma cajennense were b, c2, c3 and d in the semi-engorged females and c1, c3, c4 and f in the completely engorged.
Thus, this study demonstrated that over the glandular cycle (2-6 days) of R. sanguineus females from TG some cells were more affected than others, probably due to the hosts’ immunization. The most affected were the f cells, followed by c5; d; c3; a, c2, c4, and finally the less affected were type e. According to literature, the secretion synthesized by f and c cells would be involved in the modulation of the local homeostatic reactions of the host [9,22,23].
For the 2-day fed females from TG group, the most pronounced histochemical alterations occurred in c5 cells (increase in the intensity of protein, polysaccharides and lipid staining) and in f (increase in the staining of protein, polysaccharides, calcium and acid phosphatase and decrease in lipid staining). In the 4-day fed females, most histochemical alterations were observed in a cells (increase in protein and polysaccharides), c4 (increase in polysaccharides and decrease in acid phosphatase) and d (increase in proteins and polysaccharides), and in the 6-day fed females, most alterations were found in c3 cells (reduction of polysaccharides and calcium). Thus, it can be inferred that the most affected cells were the f cells, due to the presence of their secretion in SGE2, which presents high immunogenic capacity and great actuation in the beginning of the modulation of the immuneinflammatory and hemostatic systems of the host, which occurs in R. sanguineus exclusively between 2-3 days.
As for c4 and c5 cells, these are important in the modulation of the host and also in the immunogenic capacity. The c5 cells underwent alterations only in the 2-day fed individuals, and they might be involved in the beginning of the host’s modulation. The c4 cells underwent alterations mainly in the 4-day fed individuals. Thus, these might participate in the modulation of the hemostatic system of the host, as the fourth day of feeding is marked by the beginning of a period of great blood consumption by R. sanguineus. These data corroborate Walker et al. [22] and Sonenshine [23], who stated that the secretion produced by group c cells would be associated to the manipulation of the host’s immune response. Walker et al. [22], in addition, the glycoproteins of this secretion would act as anticoagulants [23]. As for a and d cells, it can be inferred that the histochemical alterations would be related to the immunogenic capability and the modulation action of their secretions. Such statement corroborates data from literature, showing that these cells are responsible for the synthesis of the proteins, which act both on the modulation of the host and on the formation and maintenance of the feeding lesion [17]; in addition, some of these proteins would be antigenic [45]. Concerning a cells, alterations were exclusively detected in the 4-day glands; therefore, it can be proposed that they would be involved in the initial modulation of the immune-inflammatory system of the host. As for d cells, alterations were observed in the 2-day and 4-day glands; therefore, it can be inferred that they would be involved both in the initial modulation of the immune-inflammatory system and hemostatic system, once 4 days of feeding would be the moment when the phase of high consumption of blood begins. The modifications observed in c3 cells of the 6-day glands could be a consequence of the finalization of the secretory activity, when the females would be in the final feeding phase, not needing the salivary glands’ activity anymore.
Data obtained in this study lead to the conclusion that the variation in the intensity of the histochemical alterations on the salivary glands of 2 and 6-day females from TG would be a consequence of the different immunogenic capacities of the glandular molecules present in SGE2 extract (used to immunize the hosts), considering that the greater the immune response to a certain glandular antigen, the greater the alterations in this same component present in the glands of the ticks fed on immunized host, Wheeler et al. [10], who reported that not all the components present in the salivary secretion of the host would have antigenic activity.
Considering that each cellular type present in the salivary glands synthesizes secretions with specific constitution concerning the nature and proportion of compounds [9,24], this study demonstrated that the variations in the individuals from TG in relation to CG over the glandular cycle were more severe in the proteins, followed by polysaccharides/acid phosphatase, lipids and calcium, showing the importance of the relation quality/quantity of each of these bioactive elements in the modulation of the immune-inflammatory response of the host. Moreover, the great immunogenic capacity of the elements was demonstrated, corroborating Gill et al. [44], who reported intense immunological response of the host to glycoprotein, acid phosphatase, esterase and aminopeptidase secreted by salivary glands of Hyalomma anatolicum anatolicum.
The results here obtained suggest that the salivary glands would have the capacity to adequate to the synthesis and secretion of bioactive components, according to the requirements to manipulate the host, so that the ectoparasites could perform their feeding process efficiently, fighting the host’s defenses. These data corroborate Ribeiro [5], who stated that the pharmacology of the ticks’ saliva can be molded according to the specific hemostatic defenses of the host.
According to literature, the protein and glycoprotein elements are the main substances secreted by the ticks’ salivary glands and have important functions in the modulation of the host [8,10,13,16], this explains why such elements underwent intense modifications over the glandular cycle of females from TG.
As for the lipid components present in the ticks’ saliva [9,11], it is known that these molecules also participate in the constitution of the cement, with the function of maintaining the ectoparasite fixed to the host. However, it is also known that this mixture contains proteins (lipoproteins), which act both in the modulation of the host’s defense system and in the formation and maintenance of the feeding lesion [17]. Thus, the cement would be constituted by a mixture of immunogenic and non-immunogenic proteins, containing lipid and carbohydrates [45].
As for the lipid elements, prostaglandins would be more specifically found in the ticks’ saliva [8,12,46], purine nucleoside adenosine [12] and prostacyclins [47], molecules which are part of the sophisticated pharmacological arsenal for the modulation of the hemostatic reactions in the host, which actuation affects the capacity of permanence and blood consumption of the ectoparasite [20].
According to literature, the saliva of R. sanguineus contains prostaglandin and purine nucleoside adenosine, which act in the host’s immune-inflammatory system, allowing the ectoparasite to feed completely [12]. According to Bishop et al. [48], a lipoprotein of the cement would have been recognized in the secretion granules of e cells in R. appendiculatus, which would have high immunogenic capacity, data that explain the fact that the lipid staining in this study presented modifications in the glands of individuals from TG, in comparison with those from CG to compensate the acquisition of resistance by the hosts, affecting the fixation, permanence and blood consumption of R. sanguineus females.
As for the calcium, it deserves special attention due to the alterations in staining face to the immunization of the hosts. The literature describes the presence and the function of this element in the salivary glands of several species of ticks [8,15,16,25,49-51]. According to Jaworski et al. [15], binding proteins called calreticulins are among the components secreted by the ticks’ saliva, acting as facilitators of the feeding through their immunosupressing and antihemostatic action [8,16,25]. In addition, the calreticulins would modulate the immunity of the host, mimetizing or inducing the host’s self-immunity [8].
The calreticulins could also inhibit the hemostatic response of the host binding calcium ions, cofactors of the enzymes involved in the coagulation process; however, in this case, the calreticulins would bind calcium only in the host’s organism, not corroborating the results here obtained, which demonstrate the presence of calcium in the secretion of some salivary glands cells [8].
The calcium staining for the individuals here studied could be related to the presence of calreticulin, which presence in salivary glands of R. sanguineus has already been demonstrated [52]. Considering that the alterations in calcium staining in the individuals from the TG were pronounced, with an increase of this element in 2-day f cells, it could be inferred that there is a close relation between this molecule and the modulation of the immune-inflammatory and hemostatic systems of the host. In addition to this, the calcium/calreticulin complex would present high immunogenic capacity. Considering this information, as well as all the alterations here detected in f cells from TG, the bioactive molecules present in the secretion would very possibly be excellent candidates for the production of a vaccine.
Thus, considering all the data obtained here, it can be concluded that the histochemical alterations detected in the salivary glands of individuals from the TG occurred due to the immunization of the hosts and resulted in modifications with consequent efficacy of the salivary secretion in their modulation, process that occurred to compensate or even overcome the resistance developed by the hosts, allowing the ectoparasites’ fixation, permanence and feeding.
Acknowledgements
This research has been supported by FAPESP (Sao Paulo State Research Foundation) (Grants no. 08/58443-7 and 07/59020-0) and CNPq (National Council for Scientific and Technological Development) (Grant no. 308733/2006-1 and M.I. Camargo-Mathias and G.H. Bechara academic carrier research fellowships). Part of this work has been facilitated through the Integrated Consortium on Ticks and Tick-borne Diseases (ICTTD-3) supported by the European Union under contract number 510561-INCO.
References
- Dantas-Torres F (2010) Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit Vectors 3: 26.
- Labruna MB, Homem VS, Heinemann MB, Ferreira Neto JS (2000) Ticks (Acari: Ixodidae) associated with rural dogs in Uruar, Eastern Amazon, Brazil. J Med Entomol 37: 774-776.
- Labruna MB (2004) Biologica-ecologia de Rhipicephalus sanguineus (Acari: Ixodidae). Revista Brasileira de Parasitologia Veterinria 1: 123-124.
- Labruna MB, Camargo LMA, Terrassini F, Ferreira F, Schumaker TTS, et al. (2005) Ticks (Acari: Ixodidae) from the state of Rondnia, Western Amazon, Brazil. Syst Appl Acarol 10: 17-32.
- Ribeiro JM, Makoul GT, Levine J, Robinson DR, Spielman A (1985) Antihemostatic, antiinflammatory, and immunosuppressive properties of the saliva of a tick, Ixodes dammini. J Exp Med 161: 332-344.
- Wikel SK (1999) Tick modulation of host immunity: An important factor in pathogen transmission. Int J Parasitol 29: 851-859.
- Sauer JR, Essenberg RC, Bowman AS (2000) Salivary glands in ixodid ticks: Control and mechanism of secretion. J Insect Physiol 46: 1069-1078.
- Steen NA, Barker SC, Alewood PF (2006) Proteins in the saliva of the Ixodida (ticks): Pharmacological features and biological significance. Toxicon 47: 1-20.
- Binnington KC (1978) Sequential changes in salivary gland structure during attachment and feeding of the cattle tick, Boophilus microplus. Int J Parasitol 8: 97-115.
- Wheeler CM, Coleman JL, Benach JL (1991) Salivary gland antigens of Ixodes dammini are glycoproteins that have interspecies cross-reactivity. J Parasitol 77: 965-973.
- Shipley MM, Dillwith JW, Bowman AS, Essenberg RC, Sauer JR (1993) Changes in lipids of the salivary glands of the lone star tick, Amblyomma americanum, during feeding. J Parasitol 79: 834-842.
- Oliveira CJ, S-Nunes A, Francischetti IM, Carregaro V, Anatriello E, et al. (2011) Deconstructing tick saliva: non-protein molecules with potent immunomodulatory properties. J Biol Chem 286: 10960-10969.
- Gill HS, Walker AR (1988) The salivary glands of Hyalomma anatolicum anatolicum: Nature of salivary gland components and their role in tick attachment and feeding. Int J Parasitol 18: 83-93.
- Harnnoi T, Sakaguchi T, Nishikawa Y, Xuan X, Fujisaki K (2007) Molecular characterization and comparative study of 6 salivary gland metalloproteases from the hard tick, Haemaphysalis longicornis. Comp Biochem Physiol B Biochem Mol Biol 147: 93-101.
- Jaworski DC, Simmen FA, Lamoreaux W, Coons LB, Muller MT, et al (1995) A secreted calreticulin protein in ixodid tick (Amblyomma americanum) saliva. J Insect Physiol 41: 369-375.
- Brossar M, Wikel SK (2004) Tick immunobiology. Parasitology 129: S161-S176.
- Mulenga A, Blandon M, Khumthong R (2007) The molecular basis of the Amblyomma americanum tick attachment phase. Exp Appl Acarol 41: 267-287.
- Kaewhom P, Stich RW, Needham GR, Jittapalapong S (2008) Molecular analysis of calreticulin expressed in salivary glands of Rhipicephalus (Boophilus) microplus indigenous to Thailand. Animal Biodiversity and Emerging Diseases 1149: 53-57.
- Konnai S, Nishikado H, Yamada S, Imamura S, Ito T, et al (2011) Molecular identification and expression. Molecular identification and expression analysis of lipocalins from blood feeding taiga tick, Ixodes persulcatus schulze. Experiment Parasitol 127 : 467-474.
- Francischetti IM, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JM (2009) The role of saliva in tick feeding. Front Biosci (Landmark Ed) 14: 2051-2088.
- Wikel SK (1996) Host immunity to ticks. Annu Rev Entomol 41: 1-22.
- Walker AR, Fletcher JD, Gill HS (1985) Structural and histochemical changes in the salivary glands of Rhipicephalus appendiculatus during feeding. Int J Parasitol 15: 81-100.
- Sonenshine DE (1991) Biology of ticks. Oxford University Press, New York, USA.
- Camargo-Mathias MI, Furquim KCS (2013) The histology as a tool for the understand of the morphophysiology of the brown dog tick (Rhipicephalus sanguineus). In: Advances in Zoology Research, Jenkins OP (Ed.), 1st Ed., Nova Science Publishers, New York, USA 5: 167-192.
- Jittapalapong S, Stich RW, Gordon JC, Wittum TE, Barriga OO (2000) Performance of female Rhipicephalus sanguineus (Acari: Ixodidae) fed dogs exposed to multiple infestations or immunization with tick salivary gland or midgut tissues. J Med Entomol 37: 601-611.
- Bechara GH, Szab MPJ, Ferreira BR, Garcia MV (1995) Rhipicephalus sanguineus tick in Brazil: Feeding and reproductive aspects under laboratorial conditions. Braz J Vet Parasitol 4: 61-66.
- Sedmak JJ, Grossberg SE (1977) A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem 79: 544-552.
- Pearse AGE (1985) Histochemistry. Theoretical and Applied. Analytic Technology, vol. 2, Churchill Livingston, New York, USA.
- McManus JF (1946) Histological demonstration of mucin after periodic acid. Nature 158: 202.
- Junqueira LCU, Junqueira LMMS (1983) Tcnicas bsicas de citologia e histologia. Editora Santo, So Paulo, Brazil 48-81.
- Baker JR (1946) The histochemical recognition of lipine. Q J Microsc Sci 87: 441-470.
- Hussein MA, Bowen ID, Lewis GH (1990) The histochemical localization of ATPase, cholinesterase and acid phosphatase activity in Culex pipiens (Diptera, Culicidae) larvae using a methacrylate embedding technique. Cell Biol Int Rep 14: 775-781.
- McMullen HL, Sauer JR, Burton RL (1976) Possible role in uptake of water vapour by ixodid tick salivary glands. J Insect Physiol 22: 1281-1285.
- Rudolph D, Knlle W (1978) Uptake of water vapour from air: process, site, and mechanism in ticks. In: Comparative physiology: Water, ions and fluid mechanics. Cambridge University Press, UK 84-85.
- Furquim KC, Mathias MI, Hebling LM, Roma GC, Bechara GH (2011) Ticks' response to feeding on host immunized with glandular extracts of Rhipicephalus sanguineus females fed for 2, 4, and 6 days. I. Inactivity or early degeneration of salivary glands? Parasitol Res 109: 147-162.
- Ribeiro JM (1987) Role of saliva in blood-feeding by arthropods. Annu Rev Entomol 32: 463-478.
- Almeida AP, Bechara GH, Varma RM (1994) Cross-reactivity between hard tick antigens. Braz J Med Biol Res 27: 697-707.
- Nunes PH, Bechara GH, Camargo Mathias MI (2011) Secretory process of salivary glands of female Amblyomma cajennense (Acari: Ixodidae) ticks fed on resistant rabbits. Exp Appl Acarol 53: 179-187.
- Wheeler CM, Coleman JL, Benach JL (1991) Salivary gland antigens of Ixodes dammini are glycoproteins that have interspecies cross-reactivity. J Parasitol 77: 965-973.
- Ferreira BR, Machado RZ, Bechara GH (1996) Western blot analysis of tick antigens from a Rhipicephalus sanguineus unfed larval extract and identification of antigenic sites in tick sections using immunohistochemistry. A comparative study between resistant and susceptible host species. Vet Parasitol 62: 161-174
- McSwain JL, Essenberg RC, Sauer JR (1982) Protein changes in the salivary glands of the female lone star tick, Amblyomma americanum, during feeding. J Parasitol 68: 100-106.
- Sanders ML, Scott AL, Glass GE, Schwartz BS (1996) Salivary gland changes and host antibody responses associated with feeding of male lone star ticks (Acari:Ixodidae). J Med Entomol 33: 628-634.
- Xiang F, Zhang J, Zhou Y, Li Z, Gong H, Zhou J (2009) Proteomic analysis of proteins in the salivary glands of the fed and unfed female tick Rhipicephalus haemaphysaloides. Agric Sci China 8: 121-127.
- Gill HS, Boid R, Ross CA (1986) Isolation and characterization of salivary antigens from Hyalomma anatolicum anatolicum. Parasite Immunol 8: 11-25.
- Sonenshine DE (1993) Biology of Ticks. Oxford University Press, New York, USA.
- Harris SG, Padilla J, Koumas L, Ray D, Phipps RP (2002) Prostaglandins as modulators of immunity. Trends Immunol 23: 144-150.
- Ribeiro JM, Makoul GT, Robinson DR (1988) Ixodes dammini: Evidence for salivary prostacyclin secretion. J Parasitol 74: 1068-1069.
- Bishop R, Lambson B, Wells C, Pandit P, Osaso J, et al. (2002) A cement protein of the tick Rhipicephalus sanguineus, located in the secretory e cell granules of the type III salivary gland acini, induces strong antibody response in cattle. Int J Parasitol 32: 833-842.
- Needham GR, Sauer JR (1979) Involvement of calcium and cyclic AMP in controlling ixodid tick salivary fluid secretion. J Parasitol 65: 531-542.
- Qian Y, Yuan J, Essenberg RC, Bowman AS, Shook AL, et al. (1998) Prostaglandin E2 in the salivary glands of the female tick, Amblyomma americanum (L.): calcium mobilization and exocytosis. Insect Biochem Mol Biol 28: 221-228.
- Maritz-Olivier C, Louw AI, Neitz AW (2005) Similar mechanisms regulate protein exocytosis from the salivary glands of ixodid and argasid ticks. J Insect Physiol 51: 1390-1396.
- Xu G, Fang QQ, Keirans JE, Durden LA (2004) Cloning and sequencing of putative calreticulin complementary DNAs from four hard tick species. J Parasitol 90: 73-78.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14083
- [From(publication date):
October-2013 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 9560
- PDF downloads : 4523