Research Article Open Access
All Hazards Receipt Facility (AHRF) Testing Algorithm Modifications for Enhanced Screening and Safety
Michael J Perry1*, Alan J Antenucci1, Cassandra D Kelly-Cirino2, Monica Heyl3, Kenneth M Aldous1, and Christina T Egan1
1New York State Department of Health-Wadsworth Centre, 120 New Scotland Avenue, Albany, NY 12208, USA
2DNA Genotek INC, 2 Beaverbrook Road, Kanata, Ontario, Canada
3Monica Heyl & Associates, LLC, P.O. Box 152, Joppa, MD 21085, USA
- *Corresponding Author:
- Perry M
New York State Department of Health-Wadsworth Centre
120 New Scotland Avenue, Albany
NY 12208, USA
Tel: 518-402-4455
E-mail: michael.perry@health.ny.gov
Received Date: November 03, 2015; Accepted Date: February 01, 2016; Published Date: February 08, 2016
Citation: Perry MJ, Antenucci AJ, Kelly-Cirino CD, Heyl M, Aldous KM, et al. (2016) All Hazards Receipt Facility (AHRF) Testing Algorithm Modifications for Enhanced Screening and Safety. J Bioterror Biodef 7:139 doi: 10.4172/2157-2526.1000139
Copyright: © 2016 Perry MJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Bioterrorism & Biodefense
Abstract
After the terrorist events of 2001, it became rapidly apparent that a comprehensive laboratory detection and response capacity was urgently needed. In the past decade significant work has been done at the local, state and federal level to increase our nation’s emergency response framework. An integral part of this system was the development and construction of the All Hazards Receipt Facility by the Department of Homeland Security. The AHRF is a mobile, selfcontained laboratory designed to screen uncharacterized suspicious substances for chemical, radiological, nuclear, and explosive threats prior to acceptance at a Laboratory Response Network facility, or other testing laboratory. The Wadsworth Centre, New York State Department of Health was one of two sites selected to evaluate the prototype AHRF unit and exercise the testing algorithms for rule out of acute hazards. The original testing algorithm, developed by the Department of Homeland Security and Environmental Protection Agency, has been revised and updated by the Wadsworth Centre’s Biodefense Laboratory. The improved screening protocol enhances the safety of laboratory staff, mitigates hazards that could cause serious damage to the facility and increases public safety. In addition, the modified testing algorithm was developed to streamline sample testing. The changes have significantly decreased result turnaround- times, allow for increased sample throughput thus enhancing workflow. The All Hazards approach to sample analysis requires comprehensive testing of multiple threats with minimal sample destruction to ensure sufficient sample remains for both evidentiary and confirmatory testing. Our screening protocol combines non-destructive handheld detectors and limited sample volume colorimetric test kits to achieve this goal.
Keywords
All Hazards; Receipt facility; Terrorism; Screening; Training
Abbreviations
AHRF: All Hazards Receipt Facility; APHL: Association of Public Health Laboratories; ASZM-TEDA: Activated Carbon, Impregnated with Copper, Silver, Zinc, Molybdenum, and Triethylenediamine; BSC: Biosafety Cabinet; BSL: Biosafety Level; CBRE: Chemical, Biological, Radiological, and Explosive; CoC: Chain of Custody; CWA: Chemical Warfare Agent; DHS: Department of Homeland Security; EPA: Environmental Protection Agency; FSP: Flame Spectrophotometer; HEPA: High-efficiency Particulate Arrestance; HVAC: Heating, Ventilation and Air Conditioning; IMS: Ion Mobility Spectrometer; LRN: Laboratory Response Network; PHL: Public Health Laboratories
Significance of Research
After the terrorist events of 2001, it became rapidly apparent that a comprehensive laboratory detection and response capacity was urgently needed. In the past decade significant work has been done at the local, state and federal level to increase our nation’s emergency response framework. An integral part of this system was the development and construction of the All Hazards Receipt Facility (AHRF) by the Department of Homeland Security (DHS). The AHRF is a mobile, self-contained laboratory designed to screen uncharacterized suspicious substances for chemical, radiological, nuclear, and explosive threats prior to acceptance at a Laboratory Response Network (LRN) facility, or other testing laboratory. The Wadsworth Centre, New York State Department of Health, was one of two sites selected to evaluate the prototype AHRF unit and exercise the testing algorithms for rule out of acute hazards. The original testing algorithm, developed by the DHS and Environmental Protective Agency (EPA), has been revised and updated by the Wadsworth Centre’s Biodefense Laboratory. The improved screening protocol enhances the safety of laboratory staff, mitigates hazards that could cause serious damage to the facility and increases public safety. In addition, the modified testing algorithm was developed to streamline sample testing. The changes have significantly decreased results turn-around-times, allow for increased sample throughput while enhancing workflow. The All Hazards approach to sample analysis requires comprehensive testing of multiple threats with minimal sample destruction to ensure sufficient sample remains for both evidentiary and confirmatory testing. Our screening protocol combines the use of non-destructive handheld detectors and limited sample volume colorimetric test kits to achieve this goal.
Introduction
In 2002, the Association of Public Health Laboratories (APHL) completed the first ever assessment of Public Health Laboratories (PHL) readiness for chemical terrorism as part of the Chemical Terrorism Project. Upon completion of their assessment, APHL published its findings and recommendations in a document entitled ready or not: Findings and Recommendations of the APHL Chemical Terrorism Project [1]. First among the numerous key findings was the determination of inadequate worker safety and facility security measures at the majority of PHLs tasked with testing chemical threats. APHL formally recommended that federal support be provided to state governments to enhance the PHLs ability to address chemical terrorism response both safely and securely. In addition, APHL also recommended the development of high throughput field screening, sample triage and sample testing algorithms for use during an emergency response situation. Beginning in 2004, the Department of Homeland Security (DHS) took the lead to develop a mobile facility capable of screening uncharacterized chemical, biological, radiological, and explosive (CBRE) agents, while maintaining the highest protection for laboratory personnel [2]. DHS was tasked as the lead agency for the structural development of the All Hazards Receipt Facility (AHRF), while the Environmental Protection Agency (EPA) would lead the development of a rapid screening method for CBRE agents. The AHRF facility was meant to provide a technologically advanced, isolated laboratory that would protect PHLs from the potentially serious consequences of accepting and testing CBRE containing samples. By segregating CBRE testing to the AHRF, the main PHL infrastructure would not be jeopardized from an accidental, and devastating, release of a CBRE agent. The associated testing 2006 protocol was intended to not only provide laboratory staff preliminary results on the nature of the sample, but also encompass safe high throughput, non-destructive testing for multiple agents while simultaneously protecting laboratory staff and facilities from potential unknown hazards [2]. Two prototype AHRF’s were constructed and deployed in 2007, the first to the EPA Region 1 laboratory in Massachusetts and the second to the New York State Department of Health, Wadsworth Centre in Albany, New York. After deployment of the alpha units to each site, the AHRF protocol underwent a rigorous 2-day evaluation and a secondary week-long follow-up assessment a few months later [3]. Information gathered during the prototype testing phase led to modifications of the 2006 protocol and the eventual release of EPA 2008 protocol [4]. It is important to note that this protocol does not include methods for the determination or characterization of biological threat agents. Testing laboratories must continue to rely on approved Laboratory Response Network methodologies for biological threat testing.
Although the original intent of this endeavour was to develop a protocol specifically for use in the AHRF, it became apparent that an adaptable version was required so that PHLs without access to the financial resources to purchase an AHRF could still implement the screening methods in their fixed-site laboratories in order to provide enhanced protection to their facility and staff [5]. We have enhanced the EPA 2008 protocol so that it can be easily adopted by laboratories with varying capacity and equipment resources. In addition, we have developed and delivered an extensive training program aimed to provide PHLs with the hands-on experience needed to safely implement these screening techniques within their laboratories.
All hazards receipt facility overview
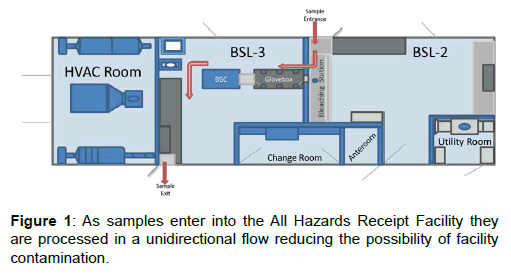
The AHRF is a single-wide trailer that is 48-ft long, 14-ft wide, and 13-ft high. The AHRF is divided into six separate sections consisting of Biosafety Level (BSL) -2 and BSL-3 laboratories, a changing room, an anteroom, and two utility areas. Each area is separated using tight sealing doors and space pressurization is actively monitored with pressure transducers, magnehelic and digihelic gauges. Air flow in the AHRF is filtered by both high-efficiency particulate arrestance (HEPA) and carbon filters to provide the maximum amount of infrastructure, community and personnel protection. Two air handling units supply HEPA filtered air into the AHRF, while the exhaust air from the Class III glove box in the BSL-3 laboratory is filtered with inline HEPA and activated carbon, impregnated with copper, silver, zinc, molybdenum, and triethylenediamine (ASZM-TEDA) filters. Filters are arranged in a Bag In/Bag Out housing allowing for minimal worker exposure when replacing filters.
Figure 1 depicts the floor plan of the AHRF and the red arrows show the path a potential CBRE containing sample will take during screening. Samples are brought into the AHRF through an airlock sample pass-through opening directly into a six foot chemical fume hood, operating with an average face velocity of 100 fpm, within the BSL-2 laboratory. The sequential outer packaging layers of the samples undergo preliminary screening within the chemical fume hood. Once the sample has been screened to its secondary layer of containment, the samples are passed into a second airlock pass-through directly into a Class III double-sided glove box specifically designed for use with chemical warfare agents (CWA). The glove box operates at a minimum pressure of -0.5” H2O and is housed inside the BSL-3 laboratory. Manipulation of the sample is restricted to the BSL-3 laboratory and must occur within the Class III glove box to ensure complete protection of the facility and the workers. Once the sample has been screened for CBRE, the remaining sample can be repacked for transport to the appropriate confirmatory laboratory and removed from the Class III cabinet via a third airlock pass-through directly into a Class II Type A2 Biosafety Cabinet (BSC). A minute amount of sample can be removed in a sealed container for flame susceptibility testing within the BSC. When the repackaged sample is ready to be transported from the AHRF to the confirmatory laboratory, a fourth airlock sample pass-through is used to pass the sample from the BSL-3 laboratory to the outside of the AHRF. This complex series of pass-through and hard linked enclosures ensure maximal safety of the community, the infrastructure and the workers.
Enhanced screening protocol for chemical, radiological and explosive threats handheld detection equipment
There are a number of different handheld detection platforms available on the market, each with specific capabilities, limitations and advantages. The EPA completed an assessment of the technologies available and chose to incorporate several platforms into the screening protocol. Recommendations from the assessment included an alpha/beta/gamma scintillator with attached data logger for radiological surveys, an ion mobility spectrometer (IMS), and a flame spectrophotometer (FSP) for chemical threats be used during screening [4]. Original screening equipment included a SAM935TM (Berkeley Nucleonic Corporation, San Rafael, CA) for the detection of gamma radiation, Ludlum 2929 and 2360 (Ludlum Measurements Inc, Sweetwater, TX) for the detection of alpha and beta radiation, MultiRae Plus (Rae Systems, San Jose, CA) for detection of oxygen, lower explosive limits, hydrogen cyanide, and volatile organic compounds, an AP4Ce FSP (Proengin Inc, Plantation, FL) for the detection of nerve, blister, and blood agents, and a LCD3.2E IMS (Smiths Detection, Edgewood, MD) for the detection of never and blister agents. In the years since the first EPA protocol was written, technologies have improved providing laboratories with additional detection equipment. Facilities utilizing the all hazards protocol should assess its requirements and capabilities when selecting new instruments. An ideal source of possible handheld detection equipment is EPA’s Supplemental Screening Protocol which contains an extensive list of available handheld detectors with their respective cost and capabilities [5].
The EPA 2006 protocol only incorporated the use of one set of handheld detectors during sample screening in an effort to reduce the equipment cost associated with implementing this method. However, the use of only one set of equipment significantly increased both the amount of time necessary to process a sample and the risk of crosscontamination of samples due to the fact that the handheld detectors needed to be thoroughly decontaminated (surface and internal air chambers) before being passed from the transport container screening area in the BSL-2 laboratory into the direct sample screening area in the BSL-3 laboratory. The extensive decontamination required when transferring the detectors between laboratories caused significant delays in testing turn-around-times as well as reducing surge capacity.
In order to increase sample throughput, decrease results turnaround- time, and limit potential cross-contamination of samples, we recommend purchasing two sets of handheld detectors. Each set should be dedicated to the BSL-2 or BSL-3 laboratory eliminating serious bottlenecks during a large scale event response and decreasing the amount of decontamination required between samples. We recognize that the high cost associated with the handheld detection units may prohibit laboratories from purchasing two full sets of equipment. We highly recommend laboratories evaluate their physical laboratory space to determine if two sets are required, and if so which technologies are best suited to their specific testing needs. At a minimum we recommend using an AP4Ce FSP and a Ludlum 2360 due to the versatility and sensitivity of the units.
Sample receipt form
When samples arrive at the testing facility under escort from a law enforcement agent or first responder, a background gathering interview is completed to determine the nature of the event and any pertinent sample information. In 2006, the interview process was approximately two hours in length period. Ultimately, a long interview process can leave first responder delivery personnel standing outside in undesirable conditions. To alleviate the interview process, the sample receipt form was significantly reduced to essential questions necessary for assessing the sample condition and the nature of the threat. The interview process is now more representative of an enhanced chain of custody (CoC).
All information acquired during the interview process is captured into an electronic document on a ruggedized, fully deconnable, computer tablet. Moving away from paper record keeping to a shorter electronic form allows for quicker data input and the ability to store information on a secure network. All information is quickly and easily accessible from any networked computer with approved access credentials. Additionally, the sample receipt form can easily be modified to incorporate new information as times and situations change.
Sample processing flow
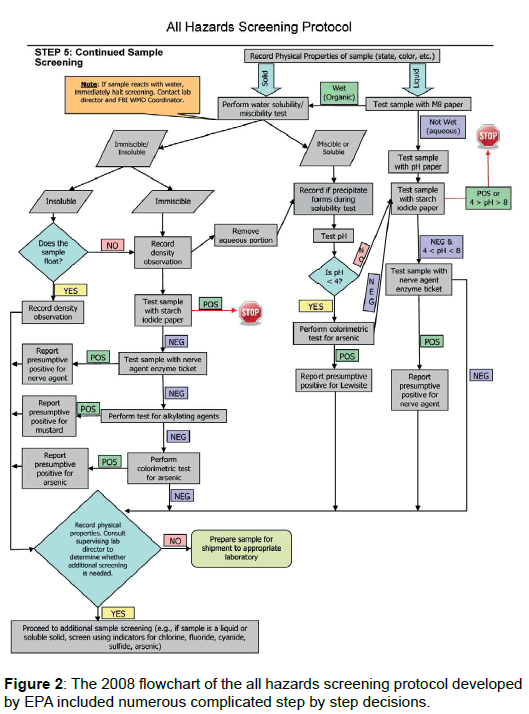
The EPA 2008 protocol consists of a complex matrix of tests originally designed to generate a class of hazards. This method was ambiguous and time intensive as various assays were only run in complicated progressions Figure 2. This procedure resulted in the original sample being handled numerous times increasing the chance of laboratory contamination and sample cross-contamination as chemical agents tend to be extremely volatile and persistent. We have streamlined the approach by having all colorimetric tests prepared before the sample container is opened. A single aliquot is taken for requesting laboratories and colorimetric tests are sequentially evaluated. Removing only one aliquot prevents re-entry into the original sample, reduces the possibility of sample contamination, and decreases the time required to process samples.
Thermal susceptibility
An early test in the original protocol, thermal susceptibility, required a small amount of sample to be transferred using a sample pass-through of the Class III glove box to a Class II biological safety cabinet on a metal spatula or wire loop. The sample was subjected to a flame to determine if an explosive hazard was present. The AHRF Screening Protocol advices that the thermal susceptibility should not be performed if a chemical weapon is suspected; however this is one of the initial tests performed. As no other testing had been completed at this point, this method introduced serious safety concerns should the sample contain a nerve, blood, or blistering agent.
To increase the safety of the protocol, the thermal susceptibility test was moved to the end of the protocol and is only performed once nerve, blister, and blood agents have been ruled out. In addition, peroxide, E.L.I.T.ETM (Field Forensics, St. Petersburg, FL) and/or Drop Ex PlusTM (Plexus Scientific, Columbia, MD) tests are implemented earlier on, inside the glove box, to establish the explosive hazard of the sample.
Protocol Optimization
Colorimetric kits
A majority of the samples that are sent to PHL for suspected bio threat agent screening tend to be powders. Typically, the powders that are received by laboratories are not bio threat agents. A major concern is that these powder samples have the potential to be highly explosive compounds. The all hazards protocol takes laboratorians apprehension in testing these samples into consideration. The EPA 2008 protocol suggested, when sufficient sample was present (>2 g or 2 mL), samples should be screened with the E.L.I.T.E.TM colorimetric explosive detection tickets. These tickets screen for nitro aromatics, aliphatic, and nitrate based explosives. However, the hazard associated with this kit is increased because a heat source is required to observe chemical changes on the detection ticket. Placing a heat source in a glove box or biosafety cabinet can add a new level of hazard and requires great care and constant awareness of surroundings. There is always a potential that samples, supplies, and gloves in the enclosure can be compromised if they come into contact with the heat source, or that non-intrinsically safe equipment could explode.
Other explosive kits are available that are equally specific and sensitive and do not require a heat source. An alternative explosive detection kit that was implemented in 2009 by the Wadsworth Centre was the Drop Ex PlusTM. The Drop Ex PlusTM tests for poly-nitroaromatics, nitrate-esters, nitramines, inorganic nitrate compounds, peroxides, chlorates, bromates, and urea based explosives. Besides for the ability to test for additional explosive compounds, the added benefit is that no heat source is required to observe chemical changes.
While the battery operated E.L.I.T.E.TM ticket heater is a safety hazard, utilizing a hot plate increase the hazards exponentially. The original protocol included the DB-3 dye test for identification of alkylating agents, such as mustard agents. The DB-3 dye test consists of two chemical solutions utilized in succession. The chemical solutions can be made in house, making it a quick and low cost screening tool. Unfortunately, the DB-3 dye test requires the use of a hot plate to bring the chemical reaction to completion. If it is decided that the DB-3 dye test is to be used during sample screening, it should be noted that the battery operated heater that accompanies the E.L.I.T.ETM test kit produces enough heat to be used in place of a heat block. The advantage of using the battery operated heater is that it is safer than a hot plate, does not require an electrical outlet quickly heats up and cools down and it is less likely to compromise screening materials and PPE. However, eliminating all sources form the glove box is highly recommended.
Peroxide tests
In New York State, samples that require all hazards screening, arrive triple contained and collected according to the CODE RED protocol [6]. Upon arrival each layer of containment is screened to ensure that samples are not leaking or off gassing. Screening of the outer packaging layers were not originally conducted for explosives, instead M8 paper which screens for CWAs was the only tested utilized. A peroxide based explosives test was not originally included until later in the protocol, once the primary sample was opened and screened using a peroxide test strip. As a safety precaution, a peroxide test was introduced during the BSL-2 screening portion of the outer sample containers. Peroxide based explosives are detectable by peroxide test strips and the Drop Ex PlusTM test kit. Introduction of sample container screening allows for an earlier identification of explosives resulting in safer sample handling.
Limitations/Interpretations
Reference cards
When the protocol was first implemented, it was immediately evident that serious difficulty arose in the subjective interpretations of the test results. While the protocol lists expected tests results, such as colour changes, recognizing the degree of colour changes can be difficult. The Wadsworth Centre Biodefense Laboratory developed reference cards to facilitate test interpretation Figure 3. The reference card is broken down into three easy to use sections.
The first section is a detailed description of colorimetric test results Figure 3. Colorimetric tests are highly subjective and can lead an inexperienced analyst to misinterpret results. The purpose of each test is listed along with its expected outcomes (e.g. if the peroxide strip turns blue, this indicates the presence of an oxidizer).
The second section of the reference card is a list of handheld detection equipment used during the screening process. A brief synopsis of the detection technology is discussed along with abridged operational instructions and response times.
Finally, the third section lists possible chemical, radioactive, and explosive agents that a technician might encounter while screening unknown samples. Critical agents are described by common sample abbreviations, key screening detection results and general agent characteristics. Ready knowledge and familiarity with key agent characteristics is extremely important during the initial first responder interview as important sample information might be inadvertently offered. For example, the delivery personnel might indicate that the sample appears to have a garlic odour and has caused several exposed victims to blister after coming in contact with the sample. A well trained technician with easy access to the reference cards would be quickly alerted to the high likely-hood of a mustard agent being present in the sample.
Biological testing
The screening protocol intentionally excludes specific biological sample screening tests due to the lack of rapid, low sample consumption tests available. Although not thought of as a biological screening method, the original screening protocol includes the use of a density test in determining potential weaponized biological agents. Since a weaponized biological agent will be extremely light making it easy to aerosolize and disseminate, if it were to be placed in water, the sample should float.
The Wadsworth Centre Biodefense Laboratory has removed the float test from its revised screening protocol as this test uses valuable sample which is better used for definitive biological testing. Once samples have been ruled-out for a potential explosive, radiological, and/or chemical agent, the sample is forwarded on to the appropriate Laboratory Response Network (LRN) lab for confirmatory bio threat agent testing.
Training Program
In order to ensure that laboratory staffs performing All Hazards screening procedures are adequately trained and knowledgeable, the Department of Homeland Security awarded the Wadsworth Centre Biodefense Laboratory with funding in 2009 to provide a national All Hazards Training Program. Four courses were developed and offered onsite at the Wadsworth Centre that are specifically focused on highly complex topics including 1) design and engineering considerations, 2) technician training in laboratory All Hazard laboratory procedures, 3) critical decisions making training for laboratory directors, and 4) fundamentals of high-containment laboratory work.
The All Hazards Receipt Facility training for engineering and support personnel focused on teaching facilities staff and engineers on AHRF heating, ventilation and air conditioning (HVAC), environmental enclosures, IT/communications, design considerations, maintenance, and security. Attendees were given the opportunity to discuss critical considerations needed for the integration of a standalone AHRF, infrastructure and minimal requirements for a retro-fit to an existing lab space, routine maintenance and upkeep of highly specialized engineering controls including the Class III glove box, and understanding of the requirements for annual certification.
The all hazards response training for laboratory personnel module focused on the all hazards approach to identifying unknown threat substances based on the modified EPA 2008 testing algorithms. Along with hands-on training, participants were introduced to containment requirements, utilization of handheld detectors, hazard mitigation, testing and workflow considerations, and key characteristics of hazardous agents.
The third course focused on consequence management of the all hazard screening testing algorithms. Critical guidance is given to public health officials, lab directors and other authorities who will be making significant public health and safety decisions based on the results of the all hazards screening method, such as the predictive value of the different colorimetric tests, the potential impact of any hazards identified, and the potential response actions.
The fourth module provided broad training for technicians working in a high containment laboratory. Laboratorians were given training in BSL-2 and -3 lab procedures, with emphasis being placed on safe working techniques for testing uncharacterized samples within both traditional and enhanced BSL-3 labs. Participants were given the opportunity to work both in a traditional Biosafety cabinet as well as a Class III glove box.
Each course provided participants with extensive hands-on time, and was tailored to specifically address the participants’ particular concerns, such as the implementation of the all hazards screening protocol within an existing lab versus a newly designed and built facility [7].
With funding from DHS, and generous travel support from APHL, the Wadsworth Centre has offered, free of charge, 13 training sessions to more than 50 participants from 18 states.
We expect that as the national emergency response initiative continues to move towards a comprehensive response plan, the need for highly trained laboratory staff will continue to grow. The Wadsworth Centre Biodefense Laboratory plans to continue supporting the all hazards training program to ensure that the public health lab sector is ready to rapidly respond to all types of CBRE threat events.
Conclusions
The all hazards protocol is used as a guide for screening unknown and uncharacterized samples for chemical, radiological, and explosive hazards. The original protocol has had vast improvements since its initial introduction. The New York State Department of Health Wadsworth Centre evaluated the protocol and tailored it to address the deficiencies and short comings of the already introduced protocol [1]. With the original protocol, samples can be screened in a minimum of 6 hours however, protocol modification allows for streamlining sample testing and as a result, screening can be resolved within 3 hours. In addition, modifications improved the overall safety for laboratory personnel and the testing facility.
Acknowledgments and Disclaimer
The All Hazards training program was supported in part by the Department of Homeland Security All Hazards Training Program award HSHQC09C00013. References to any specific commercial product do not necessarily constitute an endorsement from the New York State Department of Health.
References
- Association of Public health Laboratories (2003) Ready or not: findings and recommendations of the APHL chemical terrorism project.
- United States Environmental Protection Agency and Department of Homeland Security (2006) Draft interim All hazards receipt facility protocol standard operating procedures (guidance).
- United States Environmental Protection Agency and Department of Homeland Security (2010) Final report – Assessment of all hazards receipt facility (AHRF) screening protocol.
- United States Environmental Protection Agency and Department of Homeland Security (2008) all hazards receipt facility protocol.
- United States Environmental Protection Agency (2010) Supplement to all hazards receipt facility (AHRF) protocol.
- Kelly C, Egan C, Cirino N (2006) The CODE RED solution: biothreat response training for first responders. Biosecurity and Bioterrorism4: 391-396.
- United States Army Edgewood Chemical Biological Center and Department of Homeland Security (2011) All hazards receipt facility best practices guidelines: A tiered approach.
Relevant Topics
- Anthrax Bioterrorism
- Bio surveilliance
- Biodefense
- Biohazards
- Biological Preparedness
- Biological Warfare
- Biological weapons
- Biorisk
- Bioterrorism
- Bioterrorism Agents
- Biothreat Agents
- Disease surveillance
- Emerging infectious disease
- Epidemiology of Breast Cancer
- Information Security
- Mass Prophylaxis
- Nuclear Terrorism
- Probabilistic risk assessment
- United States biological defense program
- Vaccines
Recommended Journals
Article Tools
Article Usage
- Total views: 11424
- [From(publication date):
March-2016 - Apr 17, 2025] - Breakdown by view type
- HTML page views : 10607
- PDF downloads : 817