Research Article Open Access
Aerobical Physical Activities Improve Quality of Cardiac Health in an Animal Model: Parameters of Calorimetry, Profile Lipids and Oxidative Stress
Pedro OB*, Klinsmann CS, Camila B and Ana Angélica HFSão Paulo State University, São Paulo, Brazil
- Corresponding Author:
- Pedro OB
São Paulo State University
Department of Chemistry and Biochemistry
São Paulo, São Paulo, Brazil
Tel: 1130917568
E-mail: pedro.barbanera@gmail.com
Received date: May 10, 2017; Accepted date: June 14, 2017; Published date: June 21, 2017
Citation: Pedro OB, Klinsmann CS, Camila B, Angélica HFA (2017) Aerobical Physical Activities Improve Quality of Cardiac Health in an Animal Model: Parameters of Calorimetry, Profile Lipids and Oxidative Stress. Biochem Physiol 6:219. doi:10.4172/2168-9652.1000219
Copyright: © 2017 Pedro OB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Occidental life styles, including physical inactivity, are related to risk of developing heart diseases. To prevent or even mitigate these effects, the frequent recommendation has been the practice of physical exercise as a measure of therapeutic interaction in preventive medicine and rehabilitation. Thus, our study was carried out to evidence the effects of aerobic physical exercise regularly practiced, through swimming, calorimetry, lipid profile, and oxidative stress in myocardium. Thirty-two Winstar rats, males, 75 days old were used. Animals were divided into two groups: C (n=8) as the control, which received food and water ad libitum and TR (n=8) as the trained group received the same treatment as C and animals in this group were submitted to swim. Swimming was used as a model of exercise with moderate intensity training for eight weeks. The experimental design was completely randomized with 16 treatments and eight replications, with a significance level of 5% probability. Calorimetry shows lower carbohydrate oxidation and increased lipid oxidation in C when compared with TR. The trained group had higher glucose, higher HDL-cholesterol and lower accumulation of triglycerides, and verified the protective effect in cardiac tissue. Thus, we can conclude that the swimming protocol was effective in controlling deleterious effects caused by poor habits on the myocardium.
Keywords
Myocardium; Oxidative stress; Physical exercise
Introduction
Worldwide, cardiovascular diseases are the main cause of death in the occidental lifestyle [1]. In the countries of America, 75% of deaths are associated with heart diseases [2].
The risk of developing heart diseases can be explained by sidereactions of the metabolism, which has been shown to promote oxidative stress through the elevated reactive oxygen species (ROS) production and/or reduced antioxidant defense that provokes the decrease of health and well-being of biological systems and their functions [3-6].
According to biochemical theories, lipotoxicity may be an important target for the deleterious action of free radicals and trigger oxidative stress in the cardiac tissue [7,8], due to the imbalance between lipid entry in cardiac tissue and its ability to oxidize them [9].
In addition, it was possible to evidence that, in young people, risk factors for the development of diseases were the high serum concentration of total cholesterol and low density lipoprotein C (LDL-C) and low high-density lipoprotein C (HDL-C) [10]. Salvaro and Ávila Jr [11] analyzed profile lipids of women and verified an association between sedentariness and high level of LDL-C, triglycerides (TG) and total cholesterol on the blood, beside the low level of HDL-C.
A non-pharmacological treatment is physical exercise. The positive effects obtained by practicing physical activity upon health, in general, are unquestionable. Among implications for the cardiovascular system, there may be the reduction of total cholesterol, LDL-C, TG and HDL-C, besides the contribution to the glycemic control, for the physiological responses related to the increase of the metabolic demand and to the oxygen supply associated with muscular expenses [12].
The beneficial effects of exercise practiced regularly on metabolic disorders and directly on the heart tissue [13,14] decreases the chances of dying from cardiovascular disease by the reduction of risk factors, such as hypertension and hyperlipidemia, and by practicing physical activities; it also enhances longevity by mechanisms independent of these risk factors [15,16]. In addition, the protocol of the moderate intensity of exercise can promote improvement in diastolic function in rats [17] and generates a physiological molecular pattern, different from that in pathological cardiac hypertrophy [18].
The prescription of the exercise involves bouts between 60 and 100% VO2 max heart rate [19], training volumes and rest periods to lead to benefits of physical activity [19,20].
However, inspite of the consensus regarding direct and indirect beneficial effects provided by the practice of physical activity, concerning the cardiovascular parameters, our study was carried out to evidence the effects of aerobic physical exercise regularly practiced, through swimming, calorimetry, lipid profile and the oxidative stress in myocardium.
Material and Methods
Experimental animals
Male Winstar rats [200-250 g, bw] aging 75 days were maintained in polypropylene cages under constant temperature [24 ± 1°C], 12 h light/ dark cycle and relative humidity of 55 ± 5%. They, received standard chow [Purina Labina, Campinas/SP, Brazil, containing by weight: 22.0% protein, 3.8% fat, 44.5% carbohydrate and 3.81 kcal/g of metabolizable energy] and water ad libitum. Experimental procedures were approved [protocol no. 421] by the Ethical Committee for Conduction of Animal Studies at the Institute of Biosciences of Botucatu, Universidade Estadual Paulista [UNESP], in accordance with guidelines of the Canadian Council on Animal Care as outlined in “Guide to the Care and Use of Experimental Animals”. The animals were randomly divided into two groups [n=8]: sedentary control [C] and group submitted to swimming sessions, considered trained [TR]. Food and water intake was daily measured [9:00-10:00 a.m.] and body weight was weekly measured.
Training protocol
The rats were submitted to swim in a rectangular polyethylene tank with dimensions 80 cm deep, 80 cm wide and 100 cm length, containing water at 31 ± 1°C. The adaptation period was initiated at a depth of 10 cm of water for 10 minutes, which gradually increased 10 cm/day and 10 min/day, for six consecutive days. After this period, the animals initiated swimming training sessions at 60 cm deep for 60 min daily [always between 18:00 and 19:00 p.m.], five times per week during eight weeks [experimental period]. The exercise intensity was obtained by placing small weights in the thoracic region of the animal, corresponding 5% of body weight. This load was previously established by determining the anaerobic threshold into progressive test swimming [21], ensuring therefore the physical exercise of moderate intensity.
Parameters calorimetrics
The parameters calorimetrics were obtained in rats, fasted overnight (for 12-12 h) confined in metabolic cages (air flow=1.01/min) coupled to calorimetric (CWE, Inc, St. Paul, USA), after experimental period of 56 days. Oxygen consumption (VO2) and carbon dioxide production (VCO2) were registered by respiratory-based software program (MMX, CWE, Inc., USA) every 5 min for 20 min. The resting metabolic rate (RMR), respiratory quotient (RQ) and oxidation of carbohydrates were calculated from VO2 and VCO2, according to Strohl et al. [22].
Morfometric and nutritional parameters
After obtaining the calorimetric measurements, the animals were anaesthetized (0.1 mL/100 g body mass of 2:1 solution of 10% ketamine chloride and 2% xylazine) for measurement body length, which was used to determine Lee index (LI) [g/cm]=cube root of body weight/ length. Total energy intake (EI, kcal/day=mean food consumption per day [g] × metabolizable energy of the ration [kcal/g]) and feed efficiency (FE, g/kcal=weight gain [g]/EI) were calculated according to Kawahara et al. [23] and Santos et al. [24].
Obtaining serum samples and cardiac tissue samples
After corporal measurements, animals were anaesthetized with 10% of cetamin and 2% xilazin and euthanized by decapitation. Firstly, total blood was collected and centrifuged (1,400 g/10 min) to obtain the serum, which determined glycemia, concentration of total cholesterol, triacylglycerol and its lipoproteic fractions low-density lipoprotein (LDL-C cholesterol) and high-density lipoprotein (HDL-C cholesterol) by enzymatic colorimetric method (test LaborLab®, Brazil). Secondly, the cardiac tissue (Sample of 200 mg) was removed and washed in saline solution (9%).
Estimation of oxidative stress markers
Lipid hydroperoxide (HP) was estimated through the oxidation of ferrous ion, which in the presence of xylenol orange led to the formation of a Fe-3 – xylenol orange complex, then measured at 560 nm [25]. Superoxide dismutase [SOD, EC 1.15,1.1] activity was assayed as described elsewhere [26], through the inhibition of the reduction of nitro blue tetrazolium [NBT] in the presence of reduced nicotinamide adenine dinucleotide (NADH) and phenazine. The amount of enzyme that gave 50% inhibition of NBT reduction/mg protein was taken as one unit of enzyme activity. Glutathione peroxidase (GSH-Px, EC 1.11,1.9) activity was indirectly determined by measuring the consumption of NADPH during the reduction of oxidized glutathione (GSSG) in a reaction catalyzed by glutathione reductase, one unit of enzyme was defined as the amount required to oxidize 1 μmol GSH/min, which corresponded to 0.5 μmol NADPH oxidized/min [27].
Statistical analysis
Data normality was confirmed using the Shapiro-Wilk test. Results are presented as the mean ± standard deviation. Comparisons between groups were performed by analysis of variance (t-student test). For all the analyses, the level of significance was set at p<0.05 (Sigma Plot 12.0;USA).
Results
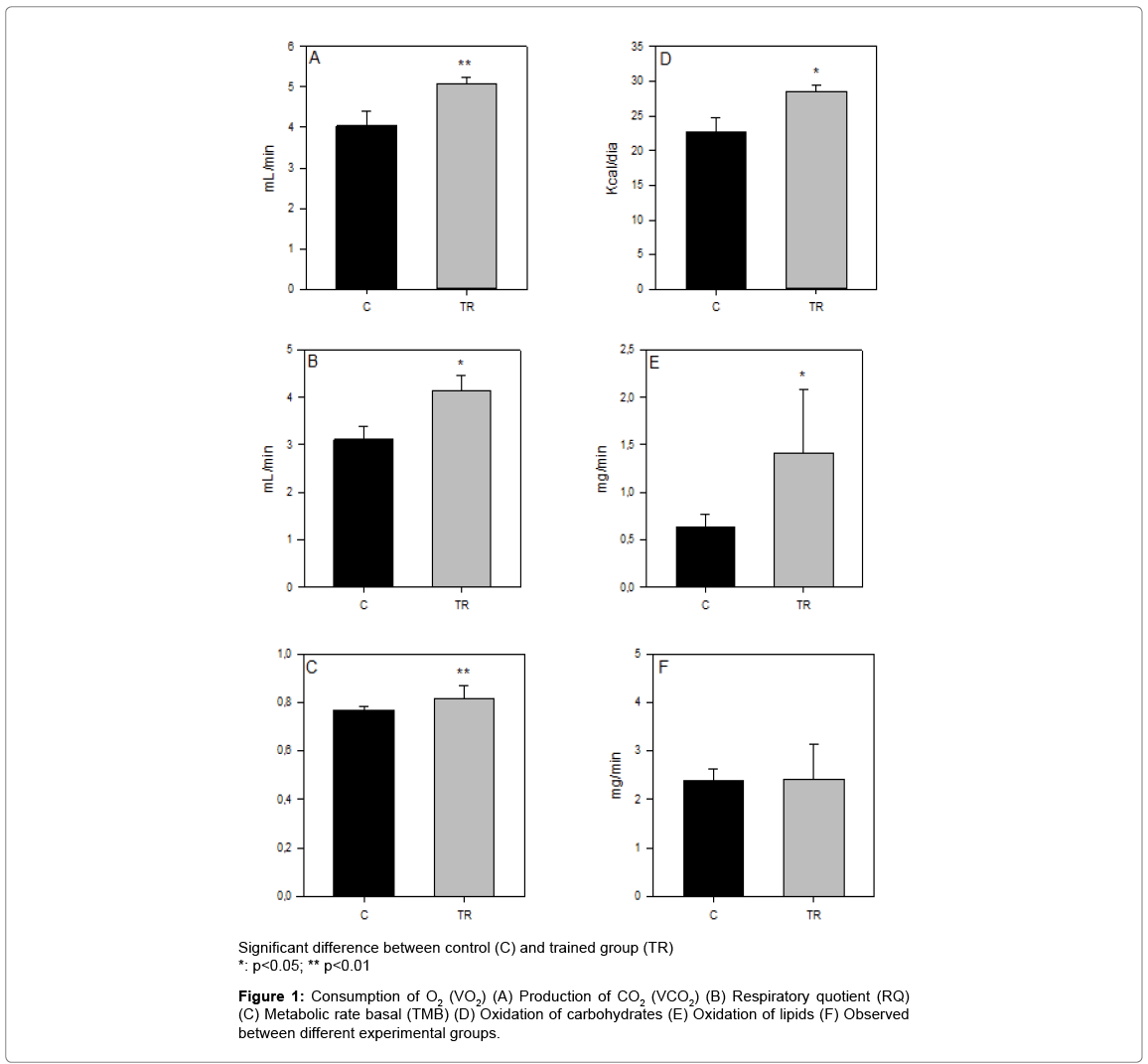
According to Table 1, the trained group (TR) gained less weight than the control group [C], but it is not enough to evidence a statistically significant value (p>0.05). The TR group showed for the Lee index, feed intake, energy intake and energy efficiency of this study higher absolute values when compared with the C group, but it is not effectively and statistically different (p>0.05). Changes in calorimetric parameters are illustrated in Figure 1. Firstly, the TR group showed extremely increase in the parameters VO2 and RQ, which evidenced statistically difference between TR and C groups (p<0.01). Secondly, the C group showed lower values for the parameters VCO2, RMR and oxidation of carbohydrates, which resulted in statistical significance (p<0.05) and thirdly the parameter oxidation of lipids showed homogeneity between TR and C groups, which means that there is not a statistical significance (p>0.05).
| Wt g | LI g/cm | FI g/day | EI kcal/day | EE g/kcal | |
|---|---|---|---|---|---|
| C | 422,55 ± 16,13 | 0,30 ± 0,01 | 21,50 ± 0,03 | 81,68 ± 0,35 | 0,74 ± 0,13 |
| TR | 415,35 ± 40,25 | 0,32 ± 0,015 | 22,16 ± 3,96 | 84,89 ± 14,94 | 0,62 ± 0,10 |
| Significance statistically between the Control group (C) and Trained group (TR) *: p<0.05 |
|||||
Table 1: Body weight (Wt), Lee index (LI), feed intake (FI), energy intake (EI) and energy efficiency (EE), observed between different experimental groups.
On the blood, this study analyzed Glucose, Total Cholesterol, LDL-C, VLDL-C, TG and HDL-C, as shown in Table 2. The TR group have shown statistical significance to the Glucose when compared with C group (p<0.05). Total Cholesterol and LDL-C statistically differed C group (p<0.01) from TR group. Serum levels of VLDL-C and TG showed statistical significance between the groups of this study (p<0.05). TR group increased the plasmatic level of HDL-C (p<0.001) when compared with C group. The trained group (TR), lipids hidroperoxide (HP) and glutathione peroxidase (GSH-Px) activity decreased significantly when compared with control group (C), while the activity of superoxide dismutase (SOD) and catalase (CAT) were not effect to promote a statistical significance (p>0.05) when comparing C and TR groups (Table 3).
| Glucose mg/dL |
Total cholesterol mg/dL |
LDL-C mg/dL |
VLDL-C mg/dL |
TG mg/dL |
HDL-C mg/dL |
|
|---|---|---|---|---|---|---|
| C | 75,98 ± 7,70 | 151,10 ± 13,76** | 87,88 ± 9,20** | 31,78 ± 7,23* | 158,8 ± 36,14* | 30,06 ± 3,82 |
| TR | 85,27 ± 8,64* | 84,13 ± 9,80 | 21,14 ± 6,10 | 22,01 ± 6,03 | 110,06 ± 30,16 | 40,98 ± 5,24** |
| Significant difference between control (C) and trained group (TR) *: p<0.05; ** p<001 |
||||||
Table 2: Plasmatic levels of glucose, total cholesterol, LDL-C, VLDL-C, TG, HDL-C, for the experimental groups.
| HP nmol/g | SOD nmol/mg | CAT µmol/g | GSH-Px nmol/mg | |
|---|---|---|---|---|
| C | 238,69 ± 16,66 | 7,62 ± 1,13 | 96,09 ± 17,72 | 46,43 ± 5,70b |
| TR | 205,63 ± 18,43* | 7,32 ± 0,95 | 108,66 ± 28,66 | 59,82 ± 8,59* |
| Significant difference between control (C) and trained group (TR) *: p<0.05 |
||||
Table 3: Cardiac tissues concentration of hidroperoxide of lipids, activities in the cardiac tissue of superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px).
Discussion
In fact, the benefits of physical exercise are already well known in the literature regarding the prevention and control of risk factors. They need, however, a more comprehensive view and a specific detail of their relation with the pathophysiology of diseases. Thus, it is essential to identify the factors and to know the effects of physical exercise on modulating cardiovascular risks, with the predominant purpose of medical conduct and the treatment of cardiopathy individuals [5,15,16].
Physical exercise can cause deviation in the homeostasis of an organism leading to reorganization of responses in different organ systems [21,28,29]. Intensity, duration and frequency of exercise have a key role in determining the metabolic responses to the effort, and may increase it or reduce it. It is recommended the intensity of physical exercise between mild to moderate, regularly performed, for the prevention of cardiovascular diseases [24,30]. Studies by Delmondes et al. [31] and Figueira et al. [32] also did not perform direct measurement of lactate and considered intense exercise as used when an overload of 5% of body weight, considering as reference the study by Gobatto et al. [21] and sorting the intense term as intense aerobic, in other words, corresponding to maximum steady state of blood lactate, which is the upper limit of heavy exercise domain.
Understanding factors related to morphometry is essential in the prevention of cardiovascular diseases because it allows better knowledge of the complications associated with overweight and diets [33]. The body weight has homogeneity between C and TR groups. Given this, it may be suggested that the influence of exercise on body weight loss is dependent on the feeding behavior, as exercised animals fed ad libitum diet showed no decrease in body weight. These results are consistent with McArdle et al. [34]. It is possible to observe that the Lee index also showed statistical difference between the groups. This result is closely related to the study by Rutledge and Adeli [35] regarding that the weight gain may be viewed not just as a result of a positive energy balance, but also as the mechanism by which the energy from energy substrates are metabolized and stored and consequently causes differences between groups.
Although it is known that the regular practice of physical exercise causes greater energy expenditure, in this study it is interesting to observe that the exercise program had no effect on feed and energy intake. However, feed efficiency has not changed between the groups. Thus, it is suggested that the intensity of moderate exercise did not affect the feeding behavior of groups.
Indirect calorimetry is a way to determine the use of energy substrates in the presence of O2 and release of CO2 by the analysis of air inhaled and exhaled by the lungs [36]. The amount of carbon dioxide produced, compared with the oxygen consumed during the oxidative processes, ranges according to the oxygen content present in oxidizable substrate. The catabolism of triglycerides in adipose tissue provides fatty acids into the blood stream and thus to oxidize them for energy tissues. Hence, the fatty acids are relatively small molecules (CH3- [CH2] n-COOH) compared with carbohydrates (C6-H12O6) and tissues require more oxygen to oxidize lipids. Moreover, complete oxidation of fatty acids for oxidative phosphorylation generates large amount of ATP, it contributes to further increase and the need for oxygen and reduce the ratio phosphate/oxygen (P/O), which reflects the number of ATP molecules produced by low oxygen atom in the mitochondrial electron transport chain [37].
The physical activity protocol used in this study normalized lipid oxidation rate due to less difference between VO2 and VCO2 observed in the TR group compared with the C group and the difference was smaller. Experimental studies have reported that aerobic physical activity increased oxygen consumption rate in patients with metabolic syndrome [38]. It is suggested that physical exercise was efficient to increase carbohydrate oxidation in TR tissues, which differ statistically from the C group.
Although lipolysis is a metabolic process stimulated by catecholamines during physical activity and therefore higher amounts of fatty acids available to be oxidized [39-41], animals of the trained group increased carbohydrate oxidation compared with the control group, suggesting greater balance in the oxidation of energy substrates. Melanson et al. [42] showed that the oxidation of lipids in individuals submitted to aerobic activity did not differ statistically from those physically inactive. The authors pointed out that such evidence could lead to the conclusion that the increased oxidation of lipids reported by some research may not represent the actual increase in the use of lipids as energy substrate. On the other hand, Bordenave et al. [43] reported that physical exercise of moderate intensity improved the ability of the organism to oxidize lipids, which was associated with the greatest oxidative capacity in skeletal muscle of patients.
The relation VCO2/VO2 was increased in trained animals, which showed rapid increase in RQ, indicating change in lipid metabolism and carbohydrate. Although the preference for oxidize carbohydrates or lipids depends on the intensity and duration of exercise, consensus seems to increase the RQ (VCO2/VO2) in the conditions proposed in this study [44]. Accordingly, Wisløff et al. [45], Wisløff et al. [46] and Kemi et al. [47] found an increase in RQ in rats submitted to moderate intensity training program.
The practice of aerobic training in animals of the TR group was not effective in reducing blood glucose to serum obtained for the C group. This result contrasts the study by Luciano et al. [48] and Pauli et al. [49], which report that exercises normalized glucose homeostasis by increasing the response of cells to insulin action such as muscle and fat. The molecular mechanism that causes the cellular internalization of glucose-mediated insulin associated with exercise training may be particularly related to the expression and activity of key regulatory proteins known as the glucose metabolism, resulting in improvement in insulin sensitivity.
Vedala et al. [50] and Vougari et al. [39] propose that regular exercise has been adopted as a strategy to reduce the harmful effects caused by poor life style and heart attacks, both associated with metabolic disorders. The basis for this strategy is evidenced mainly by the reduction of dyslipidemia, such as reduced levels of triglycerides, total cholesterol and low density lipoproteins (LDL-cholesterol) and increase high density lipoprotein (HDL-cholesterol) cholesterol [50,51]. Thus, changes in lifestyle are of particular interesting because the physical inactivity predisposes individuals to cardiovascular adverse events, most myocardial infarction index [52].
Physiological mechanisms based on exercise induce a positive lipid profile resulting from complex interactions involving hormones, enzymes and receptors. Studies suggest increase in lipoprotein lipase activity in skeletal muscle and adipose tissue while conducting moderate-to-intense physical efforts, and some post-exercise time, associated with the possible decrease in the hepatic synthesis of TG, can generate metabolic adjustments that favor lower concentrations of plasma lipids among the most physically active subjects [10,11,53].
Exercise was efficient in causing beneficial changes in lipid profile, which can also be demonstrated in human being [54].
The cholesterol serum concentration is dependent on exogenous source, from the diet, such as endogenous (synthesis) and this occurs when there is availability of acetyl-CoA molecules, a decrease in total cholesterol levels in animals if the TR group compared with the C group, which can be attributed to periods of systematic training that the animals of TR group were submitted. Such conditions also reflected in some way at low levels of LDL-cholesterol. The training protocol used in this study was able to reduce the level of total cholesterol and LDLcholesterol in serum, regardless of feeding behavior, to less than those obtained for the control group. These findings are confirmed by the study of Ishikawa et al [55] who observed decrease in the concentration of both total cholesterol and LDL-cholesterol during aerobic exercise. In addition, the literature reports that exercise decreases the likelihood of present values for these parameters considered normal according to what was previously mentioned [56].
The concentration of triglycerides and VLDL-cholesterol in C group showed a significant change compared with TR group, suggesting that hepatic synthesis of triacylglycerols and consequently of VLDLcholesterol lipoprotein, has been changed due to regular practice of physical exercise the TR animals were submitted. Kraus and Slentz reported that the triglyceride level was lower in individuals subjected to the intensity of exercise.
Approximately 90% of the triglycerides are synthesized in the liver and secreted incorporated into VLDL lipoprotein cholesterol. Beside this, Kraus and Slentz [56] reported that high levels of triglycerides and VLDL-cholesterol in the bloodstream indicates elevated hepatic lipogenesis (re-esterification of fatty acids), which can be related to training of exhaustive exercises, which can lead to excessive lipolysis. By comparison, the training protocol used in this study may explain the differences between the results obtained by these authors.
According to Otsuka et al. [6] and Ogasawara et al. [40], it is possible to relate heart disease and heart failure with triglyceride accumulation, due to the high uptake of fatty acids by the heart tissue at the expense of oxidation [56]. While the swim protocol used in this study attenuated deposit triglycerides in animals treated with diet ad libitum [TR]. These results are consistent with the literature. Bruce et al. [57] reported that exercise training is associated with fatty acid oxidation in peripheral tissues, limiting the abnormal accumulation in tissues. The triglyceride content in skeletal muscle and gastrocnemius medium in subcutaneous adipose tissue decreased in trained subjects for eight weeks compared with sedentary group [57,58].
The concentration of HDL cholesterol increased in trained rats [TR], which may indicate that moderate aerobic exercise protocol caused benefit in elevation of HDL-cholesterol. These results are consistent with Kraus and Slentz [56] and Rocco et al. [59] to report that exercise increased the HDL - cholesterol fraction and prevented the development of atherosclerosis.
Lipid accumulation in cardiac tissue may be a target of reactive oxygen species (EROS) during the lipid peroxidation process and formation of lipid hydroperoxide (HP), which has been an important biomarker of oxidative stress [53]. The result of this study evidenced a lower concentration of HP in the myocardium of the exercised animals. Considering that oxidative phosphorylation associated with mitochondrial electron transport is an important source of ROS, which may induce lipoperoxidation, the improvement in production of HP by the exercised animals may be due to a greater control in the oxidation of fatty acids and, therefore, the probability of producing EROs in the electron transport chain.
For the removal of EROs the cells depend on endogenous enzymatic antioxidant systems, represented by superoxide dismutase, which is the first line of defense against the action of ROS and catalyzes the conversion of O2 - (the first free radical formed from the partial reduction of oxygen) into H2O2, which is reduced to H2O by glutathione peroxidase and catalase [60].
While in the TR group the activity of SOD was normalized, the activities of GSH-Px and CAT were increased in comparison with the C group. These results suggest lower H2O2 production and simultaneously higher conversion of these hydrogen peroxides into H2O, indicating stabilization in the lipoperoxidation process, as verified by the values obtained for HP in these animals. Some authors related the beneficial effect of the daily aerobic training exercise, increasing the endogenous antioxidant defenses and myocardial resistance to lipoperoxidation and greater protection of the cardiac tissue against oxidative stress [53,61].
Considering that the flow of electrons increases in the mitochondria of the myocytes due to the demand of the redistribution of blood during the muscle contraction period, there is evidence that the benefit of physical activity against the oxidative stress depends basically on the intensity of the training [19,20].
Conclusion
It is important to avoid sedentariness as a life style. Based on these results, the regular practice of aerobic physical activity may promote beneficial changes in the calorimetric parameters and the lipid profile, which reduces the appearance of factors related to cardiac diseases from sedentary lifestyle.
References
- Metias EF, Aboelmaaty NM, Hussein AM, Abdallah EW, Abdelaziz A (2016) Modulation of ECG, myocardial oxidative stress markers and connexion 43 expression by ascorbic acid and ferulic acid in isoproterenol-induced myocardial infarction in rats. Biochem Physiol 5: 210.
- Camila SS, Lívia Cristina RI, Gylce EC (2012) Risk factors for coronary artery disease in young people: An integrative review of Brazilian literature. Revista de Enfermagem do Centro-Oeste Mineiro 2.
- Styskal JL, Remmen HV, Richardson A, Salmon AB (2012) Oxidative stress and diabetes: What can we learn about insulin resistance from antioxidant mutant mouse models? Free Radic Biol Med 52: 46-58
- Ceylan-Isik AF, Dong M, Zhang Y, Dong F, Turdi S, et al. (2013) Cardiomyocyte-specific deletion of endothelin receptor A rescues aging-associated cardiac hypertrophy and contractile dysfunction: Role of autophagy. Basic Res Cardiol 108: 335.
- Gray BJ, Stephens JW, Williams SP, Davies CA, Turner D, et al. (2015) Cardiorespiratory fitness is a stronger indicator of cardiometabolic risk factors and risk prediction than self-reported physical activity levels. Diab Vasc Dis Res 12: 428-435.
- Otsuka T, Takada H, Nishiyama Y, Kodani E, Saiki Y, et al. (2016) Dyslipidemia and the risk of developing hypertension in a working-age male population. J Am Heart Assoc 5: e003053.
- Stanley Jr AW, Athanasuleas CL, Buckberg G (2005) Heart failure following anterior myocardial infarction: An indication for ventricular restoration, a surgical method to reverse post-infarction remodeling. Heart Fail Rev 4: 241-254.
- Chong MF, Felding BA, Frayn KN (2007) Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr 85: 1511-1520.
- Finck BN, Han X, Courtois M, Aimond F, Nerbonne JM, et al. (2003) A critical role for PPARα-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: Modulation by dietary fat content. Proc Natl Acad Sci USA 100: 1226-1231.
- Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, et al. (2009) Major lipids, apolipo-proteins, and risk of vascular disease. JAMA 302: 1993-2000.
- Salvaro RP, Ávila Jr S (2009) Perfil Lipídico e a sua Relação com Fatores de Risco Cardiovascular em Estudantes de Nutrição. Rev SOCERJ 22: 309-317.
- Abete I, Goyenechea E, Zulet MA, Martinez JA (2011) Obesity and metabolic syndrome: Potential benefit from specific nutritional components. Nutr Metab Cardiovasc Dis 21: B1-B15.
- Nounou HA, Deif MM, Shalaby MA (2012) Effect of flaxseed supplementation and exercise training on lipid profile, oxidative stress and inflammation in rats with myocardial ischemia. Lipids Health Dis 11: 129-136.
- Dolinsky VW, Jones KE, Sidhu RS, Haykowsky M, Czubryt MP, et al. (2012) Improvements in skeletal muscle strength and cardiac function induced by resveratrol during exercise training contribute to enhanced exercise performance in rats. J Physiol 590: 2783-2799.
- Xavier HT, Izar MC, Faria Neto JR, Assad MH (2013) Sociedade Brasileira de Cardiologia. V Diretriz Brasileira de Dislipidemias e Prevenção da Aterosclerose. Arq Bras Cardiol 101: 1-20.
- Accioly MF, Padulla SAT, Carmo EM, Camargo Filho JCS, Brandão AC, et al. (2016) Efeito do treinamento físico aeróbio e do uso de estatinas sobre o perfil lipídico de animais com dislipidemia. R. bras. Ci. e Mov 24: 108-117.
- Brenner DA, Apstein CS, Saupe KW (2001) Exercise training attenuates age-associated diastolic dysfunction in rats. Circulation 104: 221-226.
- Jin H, Yang R, Li W, Lu H, Ryan AM, et al. (2000) Effects of exercise training on cardiac function, gene expression, and apoptosis in rats. Am J Physiol Heart Circ Physiol 279: H2994-H3002.
- Weston KS, Wisløff U, Coombes JS (2014) High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: A systematic review and meta-analysis. Br J Sports Med 48: 1227-1234.
- Gillen JB, Martin BJ, MacInnis MJ, Skelly LE, Tarnopolsky MA, et al. (2016) Twelve weeks of Sprint interval training improves indices of cardiometabolic health similar to traditional endurance training despite a five-fold lower exercise volume and time commitment. PLoS ONE 11: e0154075.
- Gobatto CA, Mello MAR, Sibuya CY, Azevedo JRM, Santos LA, et al. (2001) Maximal lactate steady state in rats submitted to swimming exercise. Comp Biochem Physiol 130: 21-27
- Strohl KP, Thomas AJ, Jean PSt, Schlenker EH, Koletsky RJ, et al. (1997) Ventilation and metabolism among rat strains. J Appl Physiol 82: 317-323.
- Kawahara EI, Maues NHPB, Santos KC, Barbanera PO, Braga CP, et al. (2014) Energy restriction and impact on idirect calorimetry and oxidative stress in cardiac tissue in rat. Indian J Biochem Biophys 51: 365-371.
- Santos KC, Braga CP, Barbanera PO, Seiva FRF, Fernandes Junior A, et al. (2014) Cardiac metabolism and oxidative stress biomarkers in diabetic rat treated with resveratrol. PLoS ONE 9: e102775.
- Jiang ZY, Woollard A, Wolff S (1991) Lipid hydroperoxide measurement by oxidation of Fe3+ in the presence of xylenol orange. Lipids 26: 853-856.
- Crouch RK, Gandy SC, Kisey G (1981) The inhibition of islet superoxide dismutase by diabetogenic drugs. Diabetes 30: 235-241.
- Nakamura W, Hojoda S. Hayashi K (1974) Purification and properties of rat liver glutatione peroxidase. Biochim Biophys Acta 358: 251-261
- Cunha VNC, Cunha RR, Segundo PR, Moreira SR, Simões HG (2008) Treinamento de natação na intensidade do limiar anaeróbio melhora a aptidão funcional de ratos idosos. Rev Bras Med Esporte 14: 533-538.
- Vulczak A, Monteiro MC (2008) Exercício físico e interações endócrino-imunes: revisão. Revista Eletrônica Lato Sensu, Guarapuava, Ano 3, n 1.
- Polidori MC, Mecocci P, Stahl W, Parente B, Cecchetti R, et al. (2000) Plasma levels of lipophilic antioxidants in very old patients with Type 2 diabetes. Diabetes Metab Res Rev 16: 15-19.
- Delmondes GMB, Danielly CO, Patrícia CPS, Marcelo TV, Maria SBS, et al. (2012) Efeito do treinamento físico moderado e intenso sobre os mecanismos de defesa de ratos adultos. Motriz: Revista de Educação Física 18: 699-707.
- Figueira TR, Lima MCS, Gurjão ALD, Ruas VDA, Leme JACA, et al. (2007) Efeito do treinamento aeróbio sobre o conteúdo muscular de triglicérides e glicogênio em ratos. Revista Brasileira de Ciência e Movimento, Brasília 15: 55-61.
- Pietilainen KH, Korkeila M, Bol LH, Westerterp KR, Ykijarvinen H, et al. (2010) Inaccuracies in food and physical activity diaries of obese subjects: Complementary evidence from doubly labeled water and co-twin assessments. Int J Obes 34: 437-445.
- McArdle WD, Katch FI, Katch VL (2010) Exercise physiology: Nutrition, energy and human performance, pp. 89-93.
- Rutledge AC, Adeli K (2007) Fructose and the metabolic syndrome: Pathophysiology and molecular mechanisms. Nutr Rev 65: S13-S23.
- Mourão DM, Monteiro JBR, Hermsdorff HHM, Teixeira MCL (2005) Alimentos modificados e suas implicações no metabolismo energético. Ver Nutr 18: 19-28.
- Lopaschuk GD, Ussher JR, Folmes CDL, Jaswal JS, Stanley WC (2010) Myocardial fatty acid metabolism in health and disease. Physiol Rev 90: 207-258.
- Dumortier M, Brandou F, Perez-Martin A, Fedou C, Mercier J, et al. (2003) Low intensity endurance exercise targeted for lipid oxidation improves body composition and insulin sensitivity in patients with the metabolic syndrome. Diabetes Metab 29: 509-519.
- Vougari C, Pagoni S, Vinik A, Poirier P (2013) Exercise improves cardiac autonomic function in obesity and diabetes. Metab Clin Exp 69: 609-621.
- Ogasawara J, Izawa T, Sakurai T, Takuya S, Ken S, et al. (2015) The molecular mechanism underlying continuous exercise training-induced adaptive changes of lipolysis in white adipose cells. J Obes 2015: 473430.
- Brun JF, Jean E, Ghanassia E, Flavier S, Mercier J (2007) Metabolic training: New paradigms of exercise training for metabolic diseases with exercise calorimetry targeting individuals. Ann Readapt Med Phys 50: 528-534.
- Melanson EL, Sharp TA, Seagle HM, Donahoo WT, Grunwald GK, et al. (2002) Resistance training and aerobic exercise have similar effects on 24 h nutrient oxidation. Med Sci Sports Exerc 34: 1793-1800.
- Bordenave, S, Metz L, Flavier L, Raynaud E, Brun JF, et al. (2008) Training-induced improvement in lipid oxidation in type e diabetes mellitus is related to alternations in muscle mitochondrial activity. Effect of endurance training in type e diabetes. Diabetes Metab 34: 162-168.
- Perez-Martin A, Dumortier M, Raynaud E, Brun JF, Bringer J (2001) Balance of substrate oxidation during submaximal exercise in lean and obese people. Diabetes Metab 27: 466-474.
- Wisløff U, Coombes JS, Rognmo Ø (2015) CrossTalk proposal: High intensity interval training does have a role in risk reduction or treatment of disease. J Physiol 593: 5215-5217.
- Wisløff U, Helgerud J, Kemi OJ, Ellingsen O (2001)2_max_and_cardiac_hypertrophy' title='Click here'> Intensity-controlled treadmill running in rats: VO2 max and cardiac hypertrophy. Am J Physiol Heart Circ Physiol 280: H1301-H1310.
- Kemi OJ, Haram PM, Loennechen JP, Osnes JB, Skomedal T, et al. (2005) Moderate vs. high exercise intensity: Differential effects on aerobic fitness, cardiomyocyte contractility and endothelial function. Cardiovasc Res 67: 161-72.
- Luciano E, Carneiro EM, Carvalho C R, Carvalheira J B, Peres SB, et al. (2002) Endurance training improves responsiveness to insulin and modulates insulin signal transduction through the phosphatidylinositol 3-kinase/Akt-1 pathway. Eur J Endocrinol 147: 149-157.
- Pauli JR, Ropelle ER, Silva ASR, Moraes JC, Prada PO, et al. (2010) Acute exercise reverses age-induced impairments in insulin signaling in rodent skeletal muscle. Mech Ageing Dev 131: 323-329.
- Vedala A, Wang W, Neese RA, Christiansen MP, Hellerstein MK (2006) Delayed secretory pathway contributions to VLDL-triglycerides from plasma NEFA, diet and de novo lipogenesis in humans. J Lipid Res 47: 2562-2574.
- Francescomarino S, Sciartilli A, Valerio V, Baldassarre A, Gallina S (2009) The effect of physical exercise on endothelial function. Sports Med 39: 797-812.
- Laufs U, Wassmann TC, Munzel T, Bohm M, Nickening G (2005) Physical inactivity increases oxidative stress, endothelial dysfunction and atherosclerosis. Arterioscler Thromb Vasc Biol 25: 809-814.
- Cinthia SM, Luiz DZ, Patricia MB (2016) Exercícios físicos no combate ao sobrepeso e obesidade: Intensidade versus estresse oxidativo. Ciência em Movimento-Biociências e Saúde 18: 71-85.
- Fagherazzi S, Dias RL, Bortolon F (2008) Impacto do exercício físico isolado e combinado com dieta sobre os níveis séricos de HDL, LDL, colesterol total e triglicerídeos. Rev bras med esporte 14: 381-386.
- Ishikawa YH, Suto T, Kurosawa M, Hirowatari Y, Yanai H, et al. (2010) Effects of supervised aerobic exercise training serum adiponectin and parameters of lipid glucose metabolism in subjects with moderate dyslipidemia. J Atheroscler Thromb 17: 1160-1166
- Kraus WE, Slentz CA (2009) Exercise training, lipid regulation, and insulin action: A tangled web of cause and effect. Obesity 17: S21-S26.
- Bruce CR, Thrush AB, Mertz VA, Bezaire V, Chabowski A, et al. (2006) Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. Am J Physiol Endocrinol Metab 291: 99-107.
- Petridou A, Nikolaidis MG, Matsakas A, Schulz T, Michna H, et al. (2005) Effect of exercise training on the fatty acid composition of lipid classes in rat liver, skeletal muscle and adipose tissue. Eur J Appl Physiol 94: 84-92.
- Rocco DDFM, Okuda LS, Pinto RS, Ferreira FD, Kubo SK, et al. (2011) Aerobic exercise improves reverse cholesterol transport in cholesteryl Ester transfer protein transgenic mice. Lipids 46: 617-625.
- Tsutsui H, Kinugawa S, Matsushima S (2011) Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol 301: 2181-2190.
- Viera RC, Silva CMS, Araujo MB, Garcia A, Voltarelli VA, et al. (2013) Aerobic swimming training increases the activity of antioxidant enzymes and the glycogen contente in the skeletal muscle of rats. Rev Bras Med Esporte 19: 204-208.
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 3396
- [From(publication date):
June-2017 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 2737
- PDF downloads : 659