Short Communication Open Access
Adverse Effects were not the Main Causes for Rotigotine Patch Withdrawal in Parkinsons Disease
Xiao Deng1,3, Bin Xiao1,3 and Eng-King Tan1,2*
1Department of Neurology, National Neuroscience Institute, Singapore
2Duke-NUS Graduate Medical School, Singapore, Singapore
3Department of Neurology, the first affiliated hospital, Guangxi Medical University, Nanning, China
- Corresponding Author:
- Dr. Tan EK
Department of Neurology, National Neuroscience Institute, Singapore
Tel: +65 6326 5003
E-mail: gnrtek@sgh.com.sg
Received date: October 01, 2015; Accepted date: October 13, 2015; Published date: October 20, 2015
Citation: Deng X, Xiao B, Tan EK (2015) Adverse Effects were not the Main Causes for Rotigotine Patch Withdrawal in Parkinson’s Disease. J Alzheimers Dis Parkinsonism 5:195. doi: 10.4172/2161-0460.1000195
Copyright: © 2015 Deng X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Rotigotine is a nonergoline dopamine agonist which can be used to treat Parkinson’s disease (PD) through a transdermal patch, the first such formulation available worldwide. There is limited information on reasons for withdrawal and potential adverse effects of rotigotine in different ethnic populations. Outpatients with PD prescribed with rotigotine patch as an add-on therapy were included and followed up for 6 months. The withdrawal reasons were recorded down in the patients who subsequently dropped out of the patch therapy. All of the patients were followed up for the adverse effects. In total, 29 patients in the withdrawal group and 18 patients in the continual group were identified during a period of 6 months. The main reasons for withdrawal were financial burden and perception from PD patients that the drug was not better compared to other current medications. The most commonly reported side effects of rotigotine patch were application site reactions. There was no significant difference with regard to the incidence of side effects between the withdrawal group and continual group (31.03%, 9/29 vs. 44.44%, 8/18, P=0.35). Taken together, our study showed that adverse effects were not the main causes of rotigotine patch withdrawal. The relatively low dosage of rotigotine patch used by our doctors may account for the perception of lack of efficiency in some patients. Our observations will provide useful experience for clinical application of the rotigotine patch in the future.
Keywords
Rotigotine; Parkinson’s disease; Adverse effects
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disorder with a life time risk of 1.5% [1]. The incidence of the disease rises significantly with age. In Singapore, nearly 1250 in 100,000 adults above 80 years old are diagnosed with PD , with a much higher incidence than 50 in 100,000 between 50 and 59 years of age [2]. The pathophysiology of PD is attributed to a loss of dopaminergic neurons in the brain which results in the symptoms of rigidity, resting tremor, bradykinesia and postural instability.
It is critical to address this devastating disease for the ageing society. Levodopa has been the gold standard therapy for PD in the past 30 years [3]. However, motor complications of PD such as motor fluctuations, dyskinesia and drug-induced involuntary movement are associated with long-term levodopa therapy [4]. Anticholinergic medications, such as benzhexol, have been used to restore the balance between dopamine and acetylcholine, by reducing the amount of acetylcholine in PD patients for over a century. Anticholinergic agents show efficacy primarily in PD patients with a predominant tremor [5]. Amantadine is an antiviral agent and also a helpful treatment for mild Parkinson’s disease. Selegiline, one of the monoamine oxidase inhibitors (MAOIs) has been approved for clinical application as an adjunctive treatment option in early stage PD by inhibiting dopamine breakdown [6]. Catechol-O-methyltransferase (COMT) inhibitors increase the effectiveness of levodopa by inhibiting its metabolism. Dopamine agonists, including pramipexole, ropinirole and rotigotine mimic the actions of dopamine in the brain [7] and they have been consistently demonstrated to have a levodopa sparing effect [8]. Rotigotine is a nonergoline dopamine agonist which can be used to treat PD through a transdermal patch. This provides a stable plasma concentration of rotigotine over 24 hours [9] and therefore effectively targets patients who have difficulty in swallowing and for those who cannot take oral medications before surgery [10]. Benefits of rotigotine treatment have been observed in both early and advanced stages of PD [11,12]. Rotigotine transdermal system at a dosage of more than 4 mg/d has been associated with a decrease in daily “off” time and significant improvements on the Unified Parkinson’s Disease Rating Scale (UPDRS) part II (activities of daily living) and UPDRS part III (motor examination) [13]. However, the concommitant safety issues may compromise its wide application [14]. Previous studies showed that the most common adverse events in PD patients who received rotigotine patch were application site reactions, the frequency of which can reach 44-46% [13,15]. Other side effects of rotigotine reported included peripheral edema, nausea, headache, dizziness and somnolence [14,16]. The relationship between the dosage of rotigotine patch and adverse effects is not clear. As rotigotine is the first patch formulation to be available worldwide and the adverse effects are not well documented in different ethnic populations, we conducted this study to address the drop-out rate and side effect profile in PD patients who have received Rotigotine.
Patients and Methods
To clarify the withdrawal reasons and adverse effects of rotigotine patch in PD patients in Singapore General Hospital (SGH), we conducted this survey by collecting data from pratice-based neurologists to assess the compliance and side effects of idiopathic PD patients who have received rotigotine transdermal patch (NEUPRO) treatment. All recruited patients met the following inclusion criteria: 1) Outpatients with the diagnosis of idiopathic PD as defined by the UK PD Brain Bank criteria seen in SGH Neurology outpatient clinic; 2) Outpatients who were prescribed with rotigotine patch as add-on therapy. Non- idiopathic Parkinsonism, PD patients with demented condition or severe chronic debilitating condition (e.g., renal failure requiring dialysis, congestive cardiac failure, diabetes mellitus with advanced complications, other CNS disorders) were excluded.
The withdrawal reasons were recorded for patients who subsequently dropped out of the patch treatment. A 6-month followup survey regarding the adverse effects of the rotigotine patch was performed on all identified patients.
Statistical analysis was performed by the software program SPSS version 21. Frequency together with proportion was reported for categorical data, while mean with Standard Deviation (SD) was reported for continuous variables. Chi-square tests were carried out to compare categorical variables between the withdrawal group and continual group. P values less than 0.05 were considered statistically significant.
Results
A total of 29 patients in the withdrawal group and 18 patients in the continual group were identified during the period of 6 months. The mean age was 65.9 ± 8.3 years in the withdrawal group and 63.1 ± 8.2 in the continual group. Patients in both groups were similar in terms of age, gender, race, average rotigotine daily dose and other medications (all P>0.05). The demographic profile of the 47 PD patients is summarized in Table 1.
| Total(n=47) | The withdrawal group (n=29) | The continual group(n=18) | |
| Age(years) | 64.8±8.3 | 65.9±8.3 | 63.1±8.2 |
| Chinese/Malay/Indian/others | 39/4/2/2 | 26/1/1/1 | 13/3/1/1 |
| Male/female (%) | 33/47 | 19/10 | 14/4 |
| Average dosage of rotigotine Patch dosage(mg/d) | 2.7 | 2.3 | 3.1 |
| L-dopa containing preparations | 42/47 | 27/29 | 15/18 |
| Dopamine agonists | 6/47 | 4/29 | 2/18 |
| Anticholinergic agents | 17/47 | 11/29 | 6/18 |
| MAO-B antagonists | 11/47 | 4/29 | 7/18 |
| Amantadine | 9/47 | 4/29 | 5/18 |
| COMT inhibitors | 0 | 0 | 0 |
Table 1: Demographic and clinical characteristics of 47 PD patients.
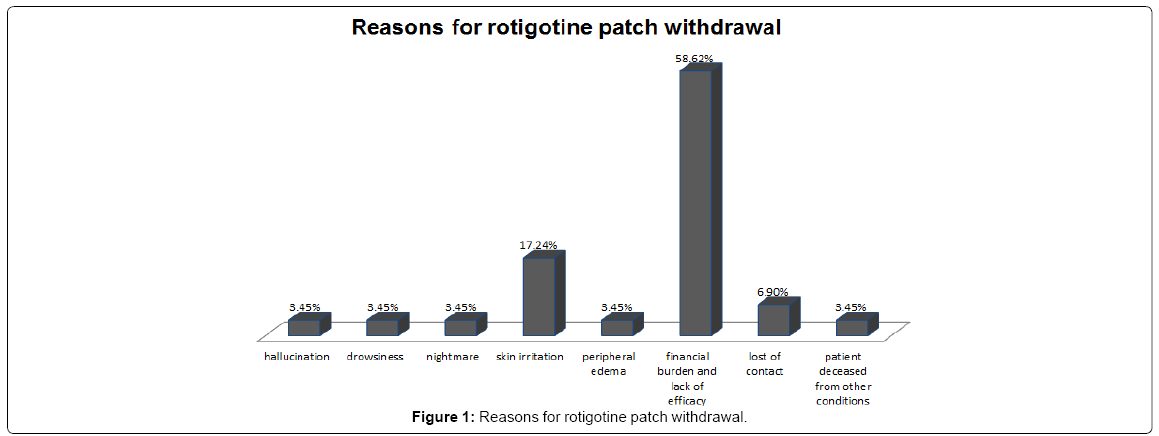
29 out of 47 patients withdrew from the rotigotine treatment. The overall dropout rate was 61.7%. The main reasons for withdrawal were financial burden and patients not feeling better compared to other current medications. These main reasons represented 58.62% of withdrawals (17/29). One patient died from an unrelated condition and two patients were uncontactable during the period, which accounted for 3.45% (1/29) and 6.90% (2/29) of withdrawals respectively. 9 patients dropped out of rotigotine patch because of adverse effects from the treatment and these made up 31.03% (9/29) of the withdrawals. 18 out of 47 patients adhered to the rotigotine treatment in the follow-up period. 8 out of these 18 patients reported adverse effects of rotigotine patch. There was no significant difference with regard to the incidence of side effects between the withdrawal group and continual group (31.03%, 9/29 vs. 44.44%, 8/18, P=0.35).
The most commonly reported side effects of rotigotine patch were application site reactions, including skin irritation/erythema and peripheral edema, which were generally mild to moderate. Incidence of skin reactions in the withdrawal group was not significantly different from the continual group (20.69%, 6/29 vs. 33.33%, 6/18, P=0.49). Other adverse effects in the withdrawal group were hallucination, drowsiness and nightmare, all with the same frequency in withdrawals (3.45% 1/29). In the continual group, one patient reported nausea while one patient experienced dizziness. However, such adverse effects occurred at the rotigotine dose of 4 mg and alleviated greatly when reduced to 2 mg (Table 2 and Figure 1).
| Number of patientsn (%) | ||
| The withdrawal group (n=29) | The continual group(n=18) | |
| Hallucination | 1(3.45%) | 0 |
| Drowsiness | 1(3.45%) | 0 |
| Nightmare | 1(3.45%) | 0 |
| Dizziness | 0 | 1(5.56%) |
| Nausea | 0 | 1(5.56%) |
| Application sites reactions (skin irritations/ peripheral edema) | 6(20.69%) | 6(33.33%) |
| Total number of side effects | 9(31.03%) | 8(44.44%) |
Table 2: Adverse effects between the withdrawal group and continual group.
Discussion
The overall withdrawal rate in our study is 61.7%, which was much higher than the 20% reported in a previous study [17]. This may be caused by variances in financial burden of patients and differences in drug adverse effects and patients’ perception of efficacy. In our study, 31.03% (9/29) of the withdrawals were due to adverse effects, suggesting moderate discrepancy in the contribution of adverse effects in the dropout of rotigotine patch between our study and published literature. In our survey, the greatest causative factor for withdrawals of rotigotine patch were financial burden and the perception from PD patients that the drug was not better compared to other current medications. Rotigotine patch is much more expensive than the oral dopamine agonists. The financial burden could be reduced if generic dopamine agonist patch is available. Importantly, significant efficacies of rotigotine patch were not reported by majority of the patients in the withdrawal group. This may be due to the low average rotigotine daily dosage of 2.3 mg. Those patients in the continual group with a higher average rotigotine daily dose of 3.1 mg were able to perceive the efficacy of the rotigotine patch, including lessening of tremors, improvement of mobility and longer “on” period. Previous studies reported significant effects were usually experienced at 4mg/d dose [13], which indicates that there is space for clinicians to increase the dose of rotigotine patch for PD patients in Singapore.
There were no significant differences in adverse events between the withdrawal goup and continual group, which indicates that side effects were not the main reasons for the withdrawal of rotigotine patch. Consistent with the previous studies [13,15], the most common adverse effect was application site reactions, which were generally mild to moderate. Incidence of application sites reactions in our survey was 25.53% (12/47), which was lower than 44-46% in the other studies [13,15]. Lower daily dosage of rotigotine patch administered to PD patients in our study may be responsible for the low incidence of application site reactions. Incidences of skin reactions in continual group were higher than withdrawal group in our study (33.33%, 6/18 vs. 20.69%, 6/29), which also supports a positive association between application site reactions and the dose of rotigotine patch.
A higher rotigotine dose may explain why other common side effects (such as nausea and dizziness) were present in the continual group but not in the withdrawal group. However, those patients in the continual group tolerated these side effects. Interestingly, some adverse effects, such as hallucination, drowsiness and nightmare were present in the withdrawal group rather than the continual group, indicating that some sensitive patients may experience side effects at the beginning of the dosage titration. In future, one may get better compliance if the sensitive patients are better informed of the side effects before they start using rotigotine patch.
In conclusion, our study showed that rotigotine is generally safe in our PD population. Adverse effects were not the main causes of rotigotine patch withdrawal. The relatively low dosage of rotigotine patch used by our doctors may account for the perception of lack of efficiency in some patients. Our observations will provide useful feedback for clinicians who plan to use the drug for PD.
Acknowledgements
We thank the National Medical Research Council for their support (PD translational clinical research programme and StaR awards).
References
- Lees AJ, Hardy J, Revesz T (2009) Parkinson's disease. Lancet 373: 2055-2066.
- Tan LC, Venketasubramanian N, Jamora RD, Heng D (2007) Incidence of Parkinson's disease in Singapore. Parkinsonism Relat Disord 13: 40-43.
- Mintzer J, Burns A (2000) Anticholinergic side-effects of drugs in elderly people. J R Soc Med 93: 457-462
- Stocchi F, Marconi S (2010) Factors associated with motor fluctuations and dyskinesia in Parkinson Disease: potential role of a new melevodopa plus carbidopa formulation (Sirio). Clin Neuropharmacol 33: 198-203.
- Pirtosek Z (2009) 'Bad guys' among the antiparkinsonian drugs. Psychiatr Danub 21: 114-118.
- Stocchi F, Vacca L, Radicati FG (2015) How to optimize the treatment of early stage Parkinson's disease. Transl Neurodegener 4: 4.
- Zagmutt FJ, Tarrants ML (2012) Indirect comparisons of adverse events and dropout rates in early Parkinson's disease trials of pramipexole, ropinirole, and rasagiline. Int J Neurosci 122: 345-353.
- Kulisevsky J, Pagonabarraga J (2010) Tolerability and safety of ropinirole versus other dopamine agonists and levodopa in the treatment of Parkinson's disease: Meta-analysis of randomized controlled trials. Drug Saf 33: 147-161.
- Elshoff JP, Braun M, Andreas JO, Middle M, Cawello W (2012) Steady-state plasma concentration profile of transdermal rotigotine: An integrated analysis of three, open-label, randomized, phase I multiple dose studies. Clin Ther 34: 966-978.
- Wullner U, Kassubek J, Odin P, Schwarz M, Naumann M, et al. (2010) Transdermal rotigotine for the perioperative management of Parkinson's disease. J Neural Transm 117: 855-859.
- Giladi N, Boroojerdi B, Korczyn AD, Burn DJ, Clarke CE, et al. (2007) Rotigotine transdermal patch in early Parkinson's disease: A randomized, double-blind, controlled study versus placebo and ropinirole. Mov Disord 22: 2398-2404.
- Poewe WH, Rascol O, Quinn N, Tolosa E, Oertel WH, et al. (2007) Efficacy of pramipexole and transdermal rotigotine in advanced Parkinson's disease: A double-blind, double-dummy, randomised controlled trial. Lancet Neurol 6: 513-520.
- Watts RL, Jankovic J, Waters C, Rajput A, Boroojerdi B, et al. (2007) Randomized, blind, controlled trial of transdermal rotigotine in early Parkinson disease. Neurology 68: 272-276.
- Oertel W, LeWitt P, Giladi N, Ghys L, Grieger F, et al. (2013) Treatment of patients with early and advanced Parkinson's disease with rotigotine transdermal system: Age-relationship to safety and tolerability. Parkinsonism Relat Disord 19: 37-42.
- LeWitt PA, Lyons KE, Pahwa R (2007) Advanced Parkinson disease treated with rotigotine transdermal system: PREFER Study. Neurology 68: 1262-1267.
- Pham DQ, Nogid A (2008) Rotigotine transdermal system for the treatment of Parkinson's disease. Clin Ther 30: 813-824.
- Trenkwalder C, Kies B, Rudzinska M, Fine J, Nikl J, et al. (2011) Rotigotine effects on early morning motor function and sleep in Parkinson's disease: a double-blind, randomized, placebo-controlled study (RECOVER). Mov Disord 26: 90-99.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 15107
- [From(publication date):
December-2015 - Jul 06, 2025] - Breakdown by view type
- HTML page views : 10534
- PDF downloads : 4573