Mini Review Open Access
Advances in Cell Culture: More than a Century after Cultivating Cells
Aline G Souza1*, Izabella C C Ferreira1, Karina Marangoni1,2, Victor A F Bastos1 and Vivian A Goulart11Laboratory of Nanobiotechnology, Institute of Genetics and Biochemistry, Federal University of Uberlândia, MG, Brazil
2Laboratory of Cancer Molecular Genetics, Faculty of Medical Sciences, University of Campinas, SP, Brazil
- Corresponding Author:
- Aline Gomes de Souza
Laboratory of Nanobiotechnology
Institute of Genetics and Biochemistry
Federal University of Uberlândia (UFU)
38400-902, Uberlândia-MG, Brazil
Tel: +55 34 3225-8440
E-mail: alingosouza@yahoo.com.br
Received date: March 23, 2016; Accepted date: April 06, 2016; Published date: April 13, 2016
Citation: Souza AG, Ferreir ICC, Marangoni K, Bastos VAF, Goulart VA (2016) Advances in Cell Culture: More than a Century after Cultivating Cells. J Biotechnol Biomater 6:221. doi:10.4172/2155-952X.1000221
Copyright: © 2016 Souza AG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
To elucidate and understand complex physiological mechanisms, in vivo research is the gold standard. However, in 1907, Harrison started the in vitro cell culture as we know today, opening a path for new assays and techniques. This was a major advance in the scientific field. The possibility to monitor cell growth, differentiation and response to any number of stimuli was a leap for drug trials and screening. More than 100 years has passed, and various cell cultures techniques were developed and perfected. Diverse culture mediums and culture conditions were elaborated to attend the scientist needs. Among those advances, three-dimensional cell culture was a major breakthrough that enables a better representation of the in vivo microenvironmental characteristics. With those continuous advances in cell culture, in vitro assays are getting more reliable providing results that better represent in vivo responses.
Background
The cell culture technique is widely explored in the research fields. It aims to comprehend the cellular behavior and mechanisms in various diseases, such as cancer. However, cell culture studies and assay are only possible because in 1907, Harisson, a pioneer in cell culture, was determined to study and understand the origin of nerve fibers. For that, he developed a method to keep live cells in the laboratory, initiating the in vitrocell culture procedures [1,2].
After the results obtained by Harrison, Alexis Carrel also focused his studies in the in vitro culture and conservation of animal cells for extended periods of time [1]. From those studies, other researches such as Holtfreter, Moscona and Leighton, dedicated themselves to the improvement of in vitro cell culture techniques. Since them, massive advances were made in this field [3-5].
Initially, the monolayer cell culturewas fundamental for cellular research. However, the tissue specific architecture, cell-cell interactions, mechanical and biochemical signals are lost in the simplified conditions of the monolayer culture (2D) [2,6].
Since each tissue and cell type have specific needs and behavior, different culture techniques and mediums were developed, to fulfill the cells every need. Culture medium has a direct impact in the development, nutrition and differentiation of cells. They can be altered or supplemented to proper nourish the cells, however, it is of fundamental importance to consider that tissues and organs are threedimensional structures, continuously perfused by the blood stream [7]. That said, a significant difference is evident between the cellular functions and behavior in a monolayer culture bathed by culture medium, and a three-dimensional complex structure nurtured by the blood stream [6,7].
In this way, aiming to mimic the cellular characteristics of an in vivo environment, new methodologies have been proposed and developed, enabling three-dimensional (3D) cell culture in vitro.
3D Cell Culture
The 3D cell culture represents an important advance in cellular biology studies, since relevant biological aspects of the in vivo structures, can now be better represented in vitro.
Although this formidable technique has receive more attention in the scientific community in the last years, was Carrel in 1912 that observed the direct relation between cells proliferationand growth medium availability, when he noticed that the central region of his cell colonies presented a high incidence of necrosis. To solve this issue, Carrel used silk treads as a scaffold for cells growth and development, describing what could be the first three-dimensional cell culture method, more than 100 years ago [1,8].
Based on Carrel’s idea, other platforms were developed aiming to improve 3D cell culture techniques. Alternative methods for 3D cell culture can present the use of scaffolds, similar to the experiment conducted by Carrel, while others aim to use scaffold-free methods, preventing the cell adhesion to the surface, and enabling the formation of cellular spheroids. Zhao et al. [8] proposed the use of alginate hydrogel for the culture of bovine embryos cells, to observe their development in a 3D system. Andersen et al. [9] also presented this system as a formidable tool for 3D cell culture; since alginate is a linear anionic polysaccharide, with the capability of polymerize in a reversible way in the presence of calcium or others divalent cations. Thereof, this polysaccharide, when polymerized is nontoxic, biocompatible and can be injected in animal models without harm.
Matrigel is another well explored system for the formation of three-dimensional structures. Sodunke et al. [10] presented two methodologies to create bulk micropatterns of matrigel, microtransfer molding (microTM) and dry lift-off technique. This scaffold was used to culture normal and cancer epithelial mammary cells in 3D. The results showed that both cells were able to develop with phenotypes similar to those observed in 3D cell cultures conducted by other techniques. Furthermore, normal cells developed as predicted, with an organized structure, while cancer cells presented a disorganized growth with invasive behaviour. Dolega et al. [11] proposed a valuable model for cell culture in matrigel, based on the use of matrigel beads with microfluidic flow control. Prostatic and breast cells were encapsulate on the beads, and the acinus development was monitored. With this technique, each bead function as one single 3D cell culture compartment, for single cells to develop in one acinus per bead, facilitating the cell population control and allowing the isolation and analysis of singles beads and cells.
Another study based on scaffold technique was reported by Recha- Sancho and Semino [12], that used heparin based self-assembling peptide scaffold (RAD16-I) as a 3D culture model to recover cartilage phenotype of de-differentiated human articular chondrocytes. The reestablishment of chondrogenic phenotype included: change in cell morphology to a more elongated shape, synthesis of proteoglycans (PGs) by cells, formation of a compacted structure with improved mechanical properties and up-regulation of specific extracellular matrix (ECM) proteins of mature cartilage.
There are also scaffold-free models that use, for example, constant rotation systems, inhibiting the cells adhesion and promoting the formation of cellular spheroids. Cha et al. [13] presented a simple method for 3D mesenchymal stem cells culture, using a rotation platform. In this system, the cells were maintained in constant suspension with mild rotation, and were capable of forming cellular spheroids, maintaining their viability, differentiation capabilities and normal proliferation rates.
Other important advance in 3D cell culture is the use of the magnetic levitation method (MLM), proposed by Haisler et al. [14]. This method use iron oxide (Fe2O3) and gold (Au) nanoparticles covered with polylysine, and a magnetic apparatus to enable the formation of a 3D structure. By this technique the cell remain suspended in an air-liquid interface and are clustered together in a dense 3D spheroid structure, by the application of an external magnetic field, provided by the magnetic apparatus. Besides enabling the synthesis of extracellular matrix components, this structural organization is able of mimicking the cellular microenvironment in a more reliable way [14,15].
The MLM has been of great importance for the 3D cell culture studies, because it allows the use of 3D cultured cell in other assays, such as western blotting, immunofluorescence, qPCR, immunohistochemistry, cellular invasion, differentiation, co-culture, and even for the selection of biological targets against specific cells [16,17]. Molina et al. [18] demonstrated a cellular invasion assay with human glioblastoma and normal astrocytes cultures, to investigate the invasion mechanisms of the former. Tseng et al. [19] used MLM to create co-culture models, applying sequential assembly in various 3D cell cultures. Souza et al. [17] applied the MLM in the culture of prostate tumor cell linage, PC-3, to select specific RNA aptamers, since the authors claim that the 3D cell culture enable better expression and exposure of specific membrane surface proteins, besides mimicking the in vivo tumor microenvironment.
Miyamoto et al. [20] used a different 3D culture technique, he used a Tapered Stencil for Cluster Culture (TASCL) device, to create liver spheroids in vitro in order to study drug discovery. The TASCL device consists of microwells and has an overall size of 10 mm × 10 mm. It was created using polydimethylsiloxane and can be placed on a six-well cell culture. The cell suspension added to the device, sediment and forms a cluster in the microwells. Another different 3D culture device also used to perform drug testing was described by Patra et al. [21]. Spheroids of human hepatocellular carcinoma cells (HepG2) were formed, cultured, and treated with anti-cancer drugs inside a microfluidic device composed of two polydimethylsiloxane (PDMS) layers.
There are also methodological approaches that link scaffold and scaffolds-free techniques. Laundos et al. [22] applied a rotary orbital hydrodynamic culture system to single-cell suspensions of embryonic stem (ES)-derived neural stem/progenitor cells (ES-NSPCs), to obtain homogeneously-sized neurospheres. Then, these rotary neurospheres were cultured in a 3D fibrin hydrogel, resulting in an increased percentage of neuronal cells.
As we can see, the cell type and the 3D culture method, impacts on cell organization and formation of different 3D structures.
2D versus 3D Cell Culture
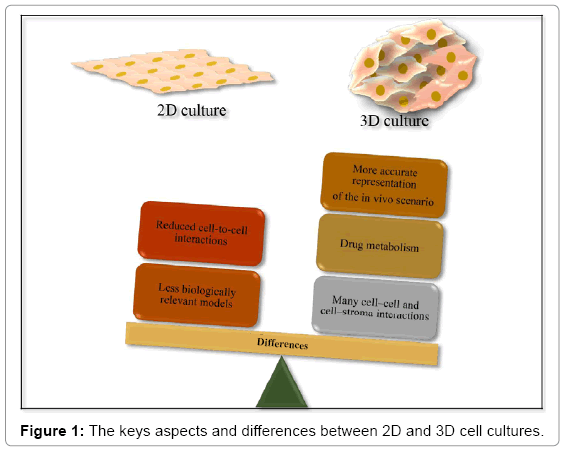
2D cell culture fail to mimic in vivo state, as the monolayer morphology allows that only a portion of the cell membrane contacts with neighboring cells, and cells are not allowed to pile on top of one another. Because oxygen, nutrient or waste gradients and true extracellular matrix (ECM) are absent, the microenvironment is nonphysiologically uniform. Other limitations associated with 2D models have been identified; such as the loss of tissue-specific architecture, cellto- cell interactions and mechanical and biochemical signals [23,24].
In contrast, the microenvironment generated by 3D cell culture exhibits unique biochemical and morphological features more representative of the in vivo state, once it permits cells to grow or interact with its surroundings, resulting in relevant cell-cell and cell- ECM interactions. Such interactions are considered essential for multiple cellular processes, including differentiation and proliferation [23,24]. Because of multiple variables in the environment surroundings cells in a 3D model, respond differently to stimuli when compared to 2D cultures [25].
The majority of developing targets identified from in vitro systems fail in clinical trials due to either unacceptable toxicity or limited efficacy in humans. This demonstrates that the traditional 2D cell systems are ineffective in predicating clinical responses. On the other hand, the 3D models tend to have better drug predicative value [23], once they create an artificial environment that mimic more accurately the in vivo cells [24]. Furthermore, the use of 3D models for in vitro analysis, increase the relevance and efficiency of in vitro studies and reduce the dependence on in vivo studies [26], which can reduce the cost and time to identify new drug candidates [27]. Figure 1 represents the main aspects and differences related to cell culture in 2D and 3D.
3D Cell Culture and Cancer Researches
Once the 3D cell culture simulates physiological in vivo conditions, of a heterogeneous microenvironment, it can contribute to the understanding of tumor cell growth and survival, therapy resistance and identification of novel cancer targets [28,29]. Thereby, this method is currently being used in a wide range of cancer researches.
The geometry and pathophysiological gradients exhibited by multicellular tumor spheroid (MCTS) are comparable to in vivo tumors. These multi-cellular 3D models can simulate more closely the tumor microenvironment structure, reconstruct a tissue-like cytoarchitecture, and exhibit growth, differentiation, and therapy responses similar to those observed in vivo [23,27].
Wang et al. [27] developed a 3D MCTS-CCA system constructed by culturing multi-cellular tumor spheroid (MCTS) in the chitosan/ collagen/alginate (CCA) fibrous scaffold for anticancer drug screening. MCF-7 cells cultured in this 3D system showed increased proliferation rate, a rise of total viable cells, drug-resistance and metabolism closer to the tumor in vivo when compared with the 2D cultured cells. Furthermore, MCTS showed the characteristic of epithelial mesenchymal transition (EMT) which is used by carcinoma cells to facilitate metastatic spread.
A 3D system that stimulates the outgrowth of morphologically complex and hormone-responsive mammary tissues was described by Sokol et al. [30]. Primary human breast epithelial cells isolated from patient reduction mammoplasty tissues were seeded in 3D hydrogels scaffolds, and rapidly self-organized and expanded to form mature mammary tissues. These tissues contained luminal, basal, and stem cells in the correct topological orientation and also exhibited the ductal and lobular morphologies observed in the human breast. The expanded tissues also responded to hormones, they became hollow when treated with estrogen and progesterone, and with further addition of prolactin produced lipid droplets.
Fitzgerald et al. [31] investigated metastatic prostate cancer cell culture on different collagen-based scaffolds in order to develop 3D bone metastases model and to assess its potential for delivery gene therapeutics that target these metastases. Two prostate cancer cell lines (PC3 and LNCaP) were cultured on three different collagenbased scaffolds (collagen and composites of collagen containing either glycosaminoglycan or nanohydroxyapatite). Both prostate cancer cell lines actively infiltrated and proliferated on the scaffolds, and their grown displayed increased resistance to docetaxel treatment. However, nanoparticles containing siRNA achieved cellular uptake and silenced the expression of the endogenous GAPDH gene in the 3D model.
Conclusions
In more than a century of the development of the first in vitro animal cell culture, the scientific field has had substantial advances towards the improvement of cell cultures techniques. Amongst those advances, the 3D cell culture is a cornerstone, since it opens new doors and expectations for the investigations of various diseases were morphological, and functional properties are of great importance. In this way, the cell culture advances are pointing to new approaches in the scientific research, combining information’s already gathered by monolayer cell culture and elaborating more sophisticate methods, each time more accurate and similar to in vivo models.
References
- Do Amaral JB, Machado-Santelli GM (2011)Acultura de célulasem 3 dimensões e a suaaplicaçãoemestudosrelacionados a formação do lumen. Naturalia 34: 1-20.
- Breslin S,O'Driscoll L (2013) Three-dimensional cell culture: the missing link in drug discovery.Drug Discov Today 18: 240-249.
- Carrel A (1912) On the permanent life of tissues outside of the organism.J Exp Med 15: 516-528.
- Moscona A (1957) The development in vitro of chimeric aggregates of dissociated embryonic chick and mouse cells.ProcNatlAcadSci U S A 43: 184-194.
- Leighton J (1951) A sponge matrix method for tissue culture; formation of organized aggregates of cells in vitro.J Natl Cancer Inst 12: 545-561.
- Leea JB, Sonb SH, Parkb MC, Kima TH, Kima MG, et al. (2015) A novel in vitro permeability assay using three-dimensional cell culture system. JBiotechnol205: 93-100.
- Li Z, Cui Z (2014) Three-dimensional perfused cell culture.BiotechnolAdv 32: 243-254.
- Zhao S, Liu ZX, Gao H, Wu Y, Fang Y, et al. (2015)A three-dimensional culture system using alginate hydrogel prolongs hatched cattle embryo development invitro.Theriogenology 84: 184-192.
- Andersen T, Auk-Emblem P, Dornish M (2015) 3D Cell Culture in Alginate Hydrogels. Microarrays 4: 133–161.
- Sodunke TR, Turner KK, Caldwell SA, McBride KW, Reginato MJ, et al. (2007) Micropatterns of Matrigel for three-dimensional epithelial cultures.Biomaterials 28: 4006-4016.
- Dolega ME,Abeille F,Picollet-D'hahan N,Gidrol X (2015) Controlled 3D culture in Matrigelmicrobeads to analyze clonal acinar development.Biomaterials 52: 347-357.
- Recha-Sancho L, Semino CE (2016) Heparin based self-assembling peptide scaffold reestablish chondrogenic phenotype of expanded de-differentiated human chondrocytes. J Biomed Mater Res A.
- Cha HM, Kim SM, Choi YS, Kim DI (2015) Scaffold-free three-dimensional culture systems for mass production of periosteum-derived progenitor cells.J BiosciBioeng 120: 218-222.
- Haisler WL,Timm DM, Gage JA, Tseng H, Killian TC, et al. (2013) Three-dimensional cell culturing by magnetic levitation.Nat Protoc 8: 1940-1949.
- Souza GR, Molina JR, Raphael RM, Ozawa MG, Stark DJ, et al. (2010) Three-dimensional tissue culture based on magnetic cell levitation.Nat Nanotechnol 5: 291-296.
- Haisler WL,Timm DM, Gage JA, Tseng H, Killian TC, et al. (2013) Three-dimensional cell culturing by magnetic levitation.Nat Protoc 8: 1940-1949.
- Souza AG,Marangoni K, Fujimura PT,Alves PT,Silva MJ, et al. (2016) 3D Cell-SELEX: Development of RNA aptamers as molecular probes for PC-3 tumor cell line.Exp Cell Res 341: 147-156.
- Molina JR, Hayashi Y, Stephens C, Georgescu MM (2010) Invasive glioblastoma cells acquire stemness and increased Akt activation.Neoplasia 12: 453-463.
- Tseng H, Gage JA, Raphael RM, Moore RH, Killian TC, et al. (2013) Assembly of a three-dimensional multitype bronchiole coculture model using magnetic levitation. Tissue Eng Part C Methods 19: 665-675.
- Miyamoto Y,Ikeuchi M, Noguchi H,Yagi T, Hayashi S (2015) Spheroid Formation and Evaluation of Hepatic Cells in a Three-Dimensional Culture Device.Cell Med 8: 47-56.
- Patra B,Peng CC, Liao WH, Lee CH, et al. (2016) Drug testing and flow cytometry analysis on a large number of uniform sized tumor spheroids using a microfluidic device.Sci Rep 6: 21061.
- Laundos TL, Silva J, Assuncao M, Quelhas P, Monteiro C, et al. (2016) Rotary orbital suspension culture of embryonic stem cell-derived neural stem/progenitor cells: impact of hydrodynamic culture on aggregate yield, morphology and cell phenotype. J Tissue EngRegen Med.
- Ryan SL, Baird AM, et al. (2016) Drug Discovery Approaches Utilizing Three-Dimensional Cell Culture.Assay Drug DevTechnol 14: 19-28.
- Antoni D,Burckel H,Josset E, Noel G, et al. (2015) Three-dimensional cell culture: a breakthrough in vivo.Int J MolSci 16: 5517-5527.
- Worthington P,Pochan DJ,Langhans SA (2015) Peptide Hydrogels - Versatile Matrices for 3D Cell Culture in Cancer Medicine.Front Oncol 5: 92.
- Casey A, Gargotti M, Bonnier F, Byrne HJ (2016)Chemotherapeutic efficiency of drugs in vitro: Comparison of doxorubicin exposure in 3D and 2D culture matrices.ToxicolInVitro 33: 99–104.
- Wang JZ, Zhu YX, Ma HC, Chen SN, Chao JY, et al. (2016) Developing multi-cellular tumor spheroid model (MCTS) in the chitosan/collagen/alginate (CCA) fibrous scaffold for anticancer drug screening. Mater SciEng C Mater BiolAppl 62: 215–225.
- Singh M, Close DA, Mukundan S, Johnston PA, Sant S (2015) Production of Uniform 3D Microtumors in Hydrogel Microwell Arrays for Measurement of Viability, Morphology, and Signaling Pathway Activation. Assay Drug DevTechnol 13: 570-583.
- Eke I,Hehlgans S,Sandfort V,Cordes N (2016) 3D matrix-based cell cultures: Automated analysis of tumor cell survival and proliferation.Int J Oncol 48: 313-321.
- Sokol ES, Miller DH, Breggia A, Spencer KC, Arendt LM, et al. (2016) Growth of human breast tissues from patient cells in 3D hydrogel scaffolds. Breast Cancer Res 18: 19.
- Fitzgerald KA, Guo J, Tierney EG, Curtin CM, Malhotra M, et al. (2015) The use of collagen-based scaffolds to simulate prostate cancer bone metastases with potential for evaluating delivery of nanoparticulate gene therapeutics. Biomaterials 66: 53-66.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 15560
- [From(publication date):
June-2016 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 14364
- PDF downloads : 1196