Review Article Open Access
Advanced LC-MS/MS Techniques Dissecting Diverse Isomers of Plant Sphingolipid Species
Toshiki Ishikawa1, Daiki Yanagawa2, Maki Kawai-Yamada1 and Hiroyuki Imai2*1Graduate School of Science and Engineering, Saitama University, Japan
2Department of Biology, Graduate School of Natural Science, Konan University, Japan
- *Corresponding Author:
- Hiroyuki Imai
Department of Biology, Graduate School of Natural Science
Konan University, 8-9-1 Okamoto
Higashinada-ku, Kobe 658-8501, Japan
Tel: +81-78-435-2513
Fax: +81-78-435-2539
E-mail: imai@konan-u.ac.jp
Received date: December 26, 2013; Accepted date: January 25, 2014; Published date: January 28, 2014
Citation: Ishikawa T, Yanagawa D, Kawai-Yamada M, Imai H (2014) Advanced LC-MS/MS Techniques Dissecting Diverse Isomers of Plant Sphingolipid Species. J Anal Bioanal Tech S5:007. doi: 10.4172/2155-9872.S5-007
Copyright: © 2014 Ishikawa T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Sphingolipid is a major lipid class ubiquitously present in eukaryotes and several species of prokaryotes. Recent genetic and sphingolipidomic researches have been revealing complex structures of plant sphingolipids and their functions in various aspects of plant biological events as major membrane components as well as signaling molecules. Particularly, cis/transisomeric double bond at Δ8 position of long-chain bases (LCBs) is of great interest due to their potential impacts on stress tolerance in plants and yeast. In addition, free LCBs and their metabolism are also important for intracellular signaling pathways during various environmental stresses. In this review, we introduce two current improvements of sphingolipidomic analyses based on liquid chromatography-tandem mass spectrometry (LC-MS/MS), focusing on high-throughput quantitative analysis of complex structural isomers of plant sphingolipids.
Keywords
Plant spingolipids; LC-MS/MS
Introduction
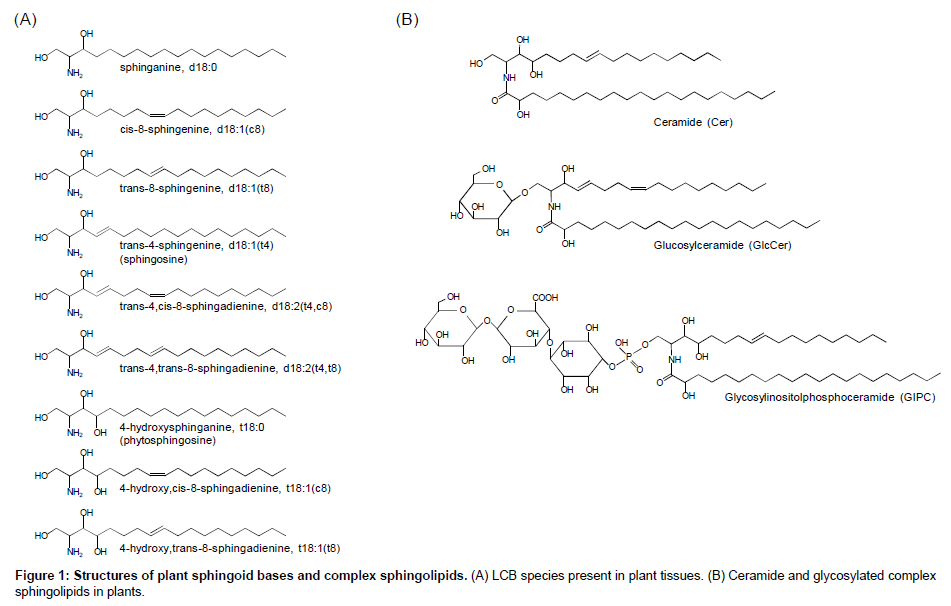
Sphingolipids is characterized to be composed of a unique fatty amino alcohol, so-called long-chain base (LCB) or sphingoid base, most of which are linked to a fatty acid via amide bond to form ceramide and more complex sphingolipids following further glycosylation and/or phosphorylation of the ceramide backbone (Figure 1). Sphingolipids are conserved ubiquitously in eukaryotes and a few species in prokaryotes. In mammals, most sphingolipids contain sphingosines with transunsaturation at the Δ4 position (Figure 1). Alternatively, 4-hydroxylated LCBs, so-called phytosphingosines, are observed in limited tissues in mammals [1-3] but known as major components in plants and fungi: in these organisms, notably, 4-unsaturated or 4-hydroxylated LCBs are often further unsaturated at the Δ8 position with cis/trans isomerism [4]. The Δ8 unsaturation is generated by sphingolipidΔ8desaturase (SLD) that is conserved widely in plants and fungi, although model yeast Saccharomyces cerevisiae and Schizosaccharomyces pombe exceptionally lack this enzyme in their genome. In addition, fungal SLDs seem to introduce a transspecific double bond, whereas plant counterparts produce both cis and trans isomers at various ratios according to plant species as well as to sphingolipid classes even in a certain species [5], implying that the stereoisomerism of LCB Δ8 unsaturation has been developed, especially in plants, along with specific biochemical/physiological functions. Recent studies suggested that LCB Δ8 unsaturation is not always essential for normal growth but plays crucial roles in some situations. Ryan et al. [6] isolated a SLD homolog from acidic soil-compatible plant Stylosanthes hamata as an Al3+tolerance-conferring gene when ectopically expressed in S. cerevisiae. The authors also demonstrated that overexpression of S. hamata SLD in Arabidopsis plants increases cis/trans ratio of tri-hydroxy species, i.e., t18:1(c8)/t18:1(t8), and enhances Al3+ tolerance. Although the authors did not address what kinds of sphingolipid classes as shown in Figure 1 are involved in the Δ8 unsaturation-mediated Al3+ tolerance in their published work, accumulated evidences implicate that glucosylceramides (GlcCers) is a possible candidate: biochemical studies have revealed that plant GlcCers generally prefer higher cis/transratio compared to other lipid classes, e.g. free ceramides and glycosyl inositol phosphoceramdes (GIPCs) [7-10]. In fact, Arabidopsis sld mutant lacking Δ8 unsaturation of LCB moieties showed drastic decreases in GlcCer species but neither in Cer nor GIPC [11]. S. cerevisiae and S. pombelack not only LCB desaturases but also GlcCer synthase, which might indicate evolutionary relationship between LCB unsaturation and GlcCer synthesis. In addition, loss of endogenous GlcCer synthase leads to higher sensitivity to Al3+ toxicity in Kluyveromyceslactis (Imai et al. unpublished data). These insights suggest that sphingolipid Δ8 unsaturation is closely associated with GlcCer synthesis, including cis isomers preferably in some cases, which has a crucial role in Al3+ tolerance based on conserved mechanisms in plants and fungi. Another physiological function of Δ8 unsaturation is relation to cold tolerance, which was first proposed from comparative analysis of LCB composition in GlcCer in several grapevine species showing different cold sensitivity [12]. A recent reverse genetic study on Arabidopsis sld1mutants supported implication of Δ8 unsaturation of GlcCer LCB moieties in cold tolerance [11]. Δ8 unsaturation-deficient sld1mutants showed not only lower GlcCer contents but also elevated sensitivity to cold stress. The authors clearly demonstrated the altered sphingolipid profiles by LC-MS/MS-based sphingolipidomic analysis, which has become an essential tool for sphingolipid studies in plants established by several groups [9,13,14]. For example, in the established LC-MS/MS analyses using a reverse-phase C18 column, sphingolipid species are separated according to their structural identities, i.e., 1) structures of polar head group; 2) carbon length, n-9 double bond and 2-hydroxylation of fatty acyl moieties; 3) C4-hydroxylation and Δ4/Δ8 double bonds of LCB moieties [9]. Combined with the chromatographic separation and MS/MS-based detection systems, more than hundred species of Arabidopsis sphingolipids can be analyzed, which has contributed to overall profiling of metabolic alterations in free LCBs, ceramides and complex glycosphingolipids in several Arabidopsis mutants lacking sphingolipid-related genes [11,15-18]. In addition, more recent studies utilizing high-resolution MS by LC-coupled linear ion trap (LTQ)/ orbitrap or matrix-assisted laser decomposition/ionization-time of flight (MALDI-TOF) system have extended our knowledge to various structures of sugar chains and ceramide moieties of GIPCs present in a wide variety of plant species [19-21]. However, despite these recent developments of MS-based profiling methods for plant sphingolipids, they are yet insufficient to individually analyze cis/trans isomers of sphingolipids that are indistinguishable by MS detection and difficult to be separated by usual HPLC/UPLC methods. Thus, quantitative analysis of cis/trans isomers in the plant various sphingolipid classes so far requires chemical decomposition of their ceramide backbones into fatty acid and LCB constituents, resulting in loss of their intact structural information. In addition, traditional time-consuming chromatographic fractionations are necessary prior to the component analyses, which is a bottle-neck for high-throughput sphingolipidomic studies.
In this review, we introduce two improved LC-MS/MS methods with a particular focus on cis/trans isomers in plant sphingolipidome. One is for GlcCers, whose Δ8 isomers are of great interest in association with plant stress tolerance as described above. Another method is to analyze free LCBs after chemical derivatization, which enables highthroughput measurement with fine separation and highly sensitive quantification of all the plant endogenous LCBs present as free forms at pmol to sub-nmol per g fresh weight (FW) orders.
Separation of cis/transisomers in glucosylceramides
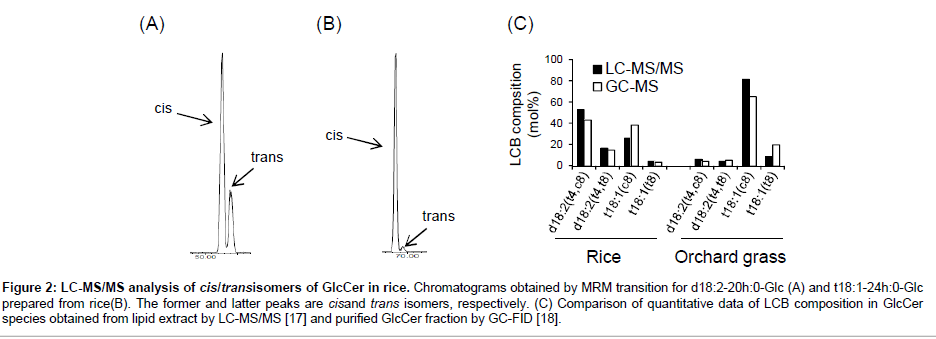
As mentioned above, plant GlcCers have potential interests in functional impacts arisen from cis/trans stereochemistry at the Δ8 position of LCB acyl moieties. There are many previous efforts to determine structural diversity of LCB species in GlcCers from various plant sources employing traditional chromatographic fractionations and LCB analysis followed by chemical degradation of the ceramide moieties. Recent advances in LC-MS-based (sphingo) lipidomics can profile intact GlcCer species without pre-fractionation and chemical degradation [13,22,23], although these methods were unable to separate cis/trans isomers. On the other hand, we recently have developed LCMS/ MS analysis to characterize plant GlcCer species containing Δ8 cis/trans isomers of LCB moieties, essentially described in a previous report [24]. Complete baseline resolution of cis/trans isomers of plant GlcCers was achieved by tandemly connecting two SUPERIOREX ODS columns (500 mm in total length): this column is characterized by high carbon content (24%), which is a prerequisite for efficient separation of cis/trans isomers of complex sphingolipids. In addition, we established chromatographic GlcCer isomers using a simple solvent gradient with only water and methanol supplemented with formic acid (Figure 2A and 2B). The back pressure of the tandemed SUPERIOREX ODS column is sufficiently low (~11 MPa) even in the application of this mobile phase composed of methanol. This method provides detailed quantitative compositions of Δ8 cis/trans-isomeric dihydroxy and trihydroxy LCBs (mainly d18:2 and t18:1) with retaining intact structures of the ceramide backbone with mono-glucosylation. In addition, since this LC-MS/MS method employed species-selectable MRM transitions, precursor ions [M+H]+ to product ions corresponding to respective LCB moieties, extra handlings to purify sphingolipid classes are not usually required prior to analyses. As reported previously, this method actuallyachieved quantitative profiles of GlcCer species with cis/trans isomers from rice and orchard grass [24]. As shown in Figure 2C, LCB compositions obtained by the LC-MS/MS method are largely consistent with the ones obtained by GC-MS analyses following traditional fractionation and chemical degradation of ceramide moieties [24,25]. Utilizing MS/MS detection also has a great advantage for high sensitivity, minimizing sample volume and detecting even minor species. Furthermore, molecular determination of GlcCer species containing various length of fatty acid-derived acyl chain is easily characterized by their equallyinterval elution times according to carbon numbers [24,26]. Thus, this method is hopeful to be extended to various GlcCer sources from plants and other organisms that possess species-specific composition of fatty acid and LCB moieties, facilitating our knowledge of cis/trans isomer distribution and their physiological functions in future studies.
Figure 2: LC-MS/MS analysis of cis/transisomers of GlcCer in rice. Chromatograms obtained by MRM transition for d18:2-20h:0-Glc (A) and t18:1-24h:0-Glc prepared from rice(B). The former and latter peaks are cisand trans isomers, respectively. (C) Comparison of quantitative data of LCB composition in GlcCer species obtained from lipid extract by LC-MS/MS [17] and purified GlcCer fraction by GC-FID [18].
Profiling of All LCB Species Present as Free Forms in Plant Tissues
In mammalian cells, sphingomyelin present in the outer leaflet of the plasma membrane undergoes breakdown into ceramide and sphingosine by sphingomyelinase and ceramidase, which triggers complex signaling pathways for apoptotic cell death under stress conditions [27,28]. Although very little is known whether free LCBs such as mammalian sphingosine also exist and act as a signaling molecule in plant cells, treatment of plant leaves with fumonisins or AAL toxin, known as ceramide synthase inhibitors produced by several species of plant-pathogenic fungi, leads to accumulation of free LCBs, mainly t18:0 and d18:0, in plant tissues as well as mammalian cells [29]. Exogenous addition of free LCB species also induces phototoxic symptoms similar to those caused by the fungal toxins [30], indicating that disruption of ceramide metabolism and resultant accumulation of free LCBs lead to the disease symptoms. In addition, phosphorylation of free LCBs is involved in disease resistance accompanying hypersensitive cell death [31], in response to chilling [32] and in regulation of stomatal aperture under drought stress [33-36]. These evidences suggest that free LCBs and their phosphorylation play roles in signaling pathways in plants as well known in animals. However, detailed quantitative composition of plant free LCBs is unclear because of their very low amounts and structural complexity compared to mammalian LCBs. Recent advances in LC-MS/MS analysis have evidenced that LCB species with basal acyl modifications observed in complex ceramides are also present as free bases in plant extracts [11,13,15,16]. Modifications of LCBs, i.e., hydroxylation and desaturation, are assumed to occur on ceramides rather than free bases. However, the evidence that free LCBs with such modified species are present in Arabidopsis tissues suggest that free LCBs present in plant cells are released via deacylation of ceramides and/or complex sphingolipids by ceramidase as well as those derived from sphingomyelin in mammalian cells. Alternatively, plant LCBmodifying enzymes might have activity on free LCBs. Determination of more detailed compositions including cis/trans isomers will progress our understandings on metabolic pathways and physiological functions of free LCBs in plants.
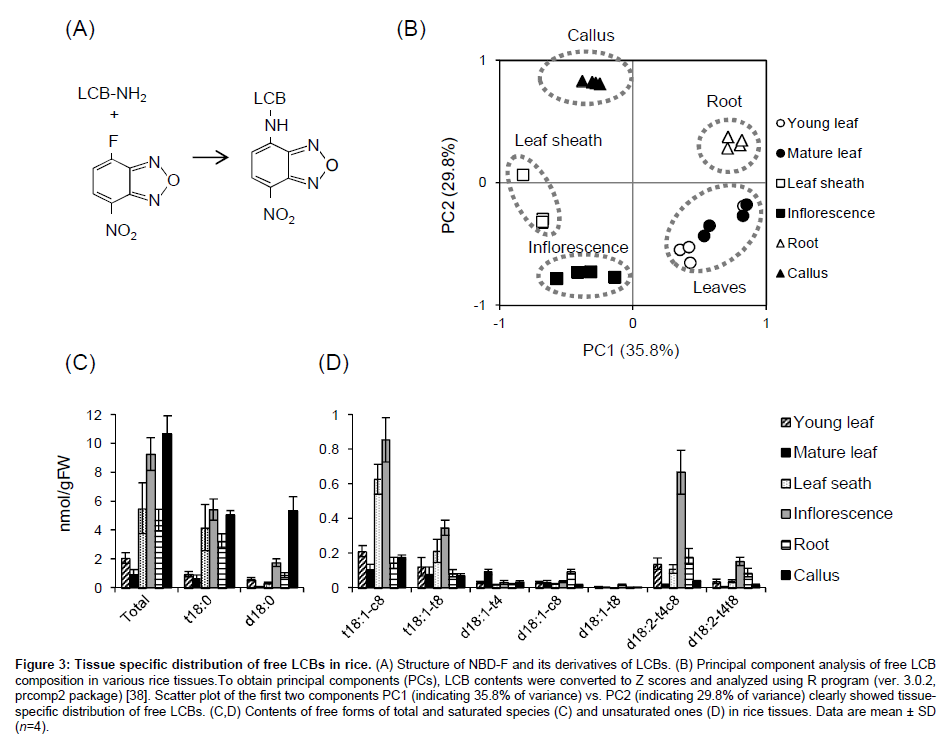
We recently established chemical derivatization of free LCBs with 4-fluoro-7-nitrobenzofurazan (NBD-F, Figure 3A) [37]. Although this reagent is usually used for fluorescence derivatization of amino acids and their metabolites, we applied it to derivatization of the amino group of LCB following LC-MS/MS analysis. One of general advantages of NBD-F is high stability of its derivatives, whereas o-phthalaldehyde, which has been more often used for fluorescence derivatization of LCBs and amino acids, commonly forms unstable derivatives. We have confirmed NBD derivatives of free LCBs are stable even at room temperature during storage for more than one week [37]. In addition, the derivatization provides much better separation and sensitivity of all the plant endogenous LCBs compared to their intact free bases [32]. Using a gradient composed of water and mixture of methanol/ acetonitrile, three d18:1 isomers d18:1(t4), d18:1(c8) and d18:1(t8) were resolved sufficiently, and other cis/trans isomers, i.e., t18:1(c8)/ t18:1(t8) and d18:2(t4,c8)/d18:2(t4,t8), were also separated absolutely. Furthermore, high sensitivity and selectivity of the NBD derivatives in LC-MS/MS system enables us to analyze free LCB composition in plant materials without extra purifications such as chromatography or solid-phase extraction. Figure 3B-3D shows results from application of this new method to profiling free LCBs in various tissues of rice by the protocol published elsewhere [37]. Principal component analysis clearly showed that young and mature leaves are similar but other tissues have distinct compositions of free LCBs (Figure 3B). Total free LCB contents in young and mature leaves were comparable levels reported in Arabidopsis [14], whereas other tissues, i.e., leaf sheath, inflorescence, root and callus culture accumulated up to 10-fold higher (Figure 3C). The accumulated species were predominantly t18:0, and also comparable d18:0 in callus. Unsaturated species were much lower but actually present in these tissues at pmol/gFW levels (Figure 3D). Consistent with GlcCers, free LCBs also preferred cis isomers than trans ones at Δ8 positions both of trihydroxy and dihydroxy species, suggesting that molecular mechanism producing the isomers in GlcCers and free LCBs are likely common in rice. In addition, leaf sheath and inflorescence contained much higher t18:1 species and inflorescence also accumulated d18:2 species. Though more efforts for the molecular origins and consequences of the differentially accumulated free LCB species in various tissues should be made, our improved method will contribute to further approaches for their functions in future studies.
Figure 3: Tissue specific distribution of free LCBs in rice. (A) Structure of NBD-F and its derivatives of LCBs. (B) Principal component analysis of free LCB composition in various rice tissues.To obtain principal components (PCs), LCB contents were converted to Z scores and analyzed using R program (ver. 3.0.2, prcomp2 package) [38]. Scatter plot of the first two components PC1 (indicating 35.8% of variance) vs. PC2 (indicating 29.8% of variance) clearly showed tissuespecific distribution of free LCBs. (C,D) Contents of free forms of total and saturated species (C) and unsaturated ones (D) in rice tissues. Data are mean ± SD (n=4).
Conclusion
In this article we reviewed current methodological improvement focusing on plant-specific structural isomers of sphingolipids. LCMS/ MS-based approaches combined with the special HPLC system (tandemed high carbon column for GlcCer separation) or optimized chemical derivatization (NBD derivatization and negative ESIMS/ MS detection for free LCB profiling) have succeeded to profile plant-specific sphingolipid isomers using lipid mixtures from tissues without class fractionations used in traditional methods. In addition, higher sensitivity of the new methods can reduce amounts of analytes required for measurements. Taken together, these methods achieved high-throughput profiling of isomers of GlcCers and free LCBs, which effectively facilitate studies requiring numerous numbers of analytes, such as screening mutant/transgenic plants and for assessment of effects of various stress treatments.
References
- Smith EL, McKibbin JM, Karlsson KA, Pascher I, Samuelsson BE (1975) Characterization by mass spectrometry of blood group A active glycolipids from human and dog small intestins. Biochemistry 14: 2120-2124.
- Bouhours JF, Glickman RM (1977) Rat intestinal glycolipids. III. Fatty acids and long chain bases of glycolipids from villus and crypt cells. Biochim Biophys Acta 487: 51-60.
- Sekine M, Suzuki M, Inagaki F, Suzuki A, Yamakawa T (1987) A new extended globoglycolipid carrying the stage specific embryonic antigen-1 (SSEA-1) determinant in mouse kidney. J Biochem 101: 553-562.
- Pata MO, Hannun YA, Ng CK (2010) Plant sphingolipids: decoding the enigma of the Sphinx. New Phytol 185: 611-630.
- Takakuwa N, Kinoshita M, Oda Y, Ohnishi M (2002) Isolation and characterization of the genes encoding delta(8)-sphingolipiddesaturase from Saccharomyces kluyveri and Kluyveromyceslactis. Curr Microbiol 45: 459-461.
- Ryan PR, Liu Q, Sperling P, Dong B, Franke S, et al. (2007) A higher plant delta8 sphingolipiddesaturase with a preference for (Z)-isomer formation confers aluminum tolerance to yeast and plants. Plant Physiol 144: 1968-1977.
- Imai H, Ohnishi M, Hotsubo K, Kojima M (1997) Sphingoid base composition of cerebrosides from plant leaves. Biosci Biotech Biochem 61: 351-353.
- Sperling P, Franke S, Lüthje S, Heinz E (2005) Are glucocerebrosides the predominant sphingolipids in plant plasma membranes? Plant Physiol Biochem 43: 1031-1038.
- Markham JE, Li J, Cahoon EB, Jaworski JG (2006) Separation and identification of major plant sphingolipid classes from leaves. J Biol Chem 281: 22684-22694.
- Minamioka H, Imai H (2009) Sphingoid long-chain base composition of glucosylceramides in Fabaceae: a phylogenetic interpretation of Fabeae. J Plant Res 122: 415-419.
- Chen M, Markham JE, Cahoon EB (2012) Sphingolipid Δ8 unsaturation is important for glucosylceramide biosynthesis and low-temperature performance in Arabidopsis. Plant J 69: 769-781.
- Kawaguchi M, Imai H, Naoe M, Yasui Y, Ohnishi M (2000) Cerebrosides in grapevine leaves: distinct composition of sphingoid bases among the grapevine species having different tolerances to freezing temperature. Biosci Biotechnol Biochem 64: 1271-1273.
- Markham JE, Jaworski JG (2007) Rapid measurement of sphingolipids from Arabidopsis thaliana by reversed-phase high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 21: 1304-1314.
- Markham JE, Lynch DV, Napier JA, Dunn TM, Cahoon EB (2013) Plant sphingolipids: function follows form. Curr Opin Plant Biol 16: 350-357.
- Chen M, Markham JE, Dietrich CR, Jaworski JG, Cahoon EB (2008) Sphingolipid long-chain base hydroxylation is important for growth and regulation of sphingolipid content and composition in Arabidopsis. Plant Cell 20: 1862-1878.
- Wang W, Yang X, Tangchaiburana S, Ndeh R, Markham JE, et al. (2008) An inositolphosphorylceramide synthase is involved in regulation of plant programmed cell death associated with defense in Arabidopsis. Plant Cell 20: 3163-3179.
- Michaelson LV, Zäuner S, Markham JE, Haslam RP, Desikan R, et al. (2009) Functional characterization of a higher plant sphingolipid Delta4-desaturase: defining the role of sphingosine and sphingosine-1-phosphate in Arabidopsis. Plant Physiol 149: 487-498.
- Kimberlin AN, Majumder S, Han G, Chen M, Cahoon RE, et al. (2013) Arabidopsis 56-amino Acid serine palmitoyltransferase-interacting proteins stimulate sphingolipid synthesis, are essential, and affect mycotoxin sensitivity. Plant Cell 25: 4627-4639.
- Buré C, Cacas JL, Wang F, Gaudin K, Domergue F, et al. (2011) Fast screening of highly glycosylated plant sphingolipids by tandem mass spectrometry. Rapid Commun Mass Spectrom 25: 3131-3145.
- Blaas N, Humpf HU (2013) Structural profiling and quantitation of glycosyl inositol phosphoceramides in plants with Fourier transform mass spectrometry. J Agric Food Chem 61: 4257-4269.
- Cacas JL, Buré C, Furt F, Maalouf JP, Badoc A, et al. (2013) Biochemical survey of the polar head of plant glycosylinositolphosphoceramides unravels broad diversity. Phytochemistry 96: 191-200.
- Sugawara T, Aida K, Duan J, Hirata T (2010) Analysis of glucosylceramides from various sources by liquid chromatography-ion trap mass spectrometry. J Oleo Sci 59: 387-394.
- Okazaki Y, Kamide Y, Hirai MY, Saito K (2013) Plant lipidomics based on hydrophilic interaction chromatography coupled to ion trap time-of-flight mass spectrometry. Metabolomics 9: 121-131.
- Imai H, Hattori H, Watanabe M (2012) An improved method for analysis of glucosylceramide species having cis-8 and trans-8 isomers of sphingoid bases by LC-MS/MS. Lipids 47: 1221-1229.
- Watanabe M, Imai H (2011) Characterization of glucosylceramides in leaves of the grass family (Poaceae): Pooideae has unsaturated hydroxy fatty acids. BiosciBiotechnolBiochem 75: 1838-1841.
- Cahoon EB, Lynch DV (1991) Analysis of Glucocerebrosides of Rye (Secalecereale L. cv Puma) Leaf and Plasma Membrane. Plant Physiol 95: 58-68.
- Ohanian J, Ohanian V (2001) Sphingolipids in mammalian cell signalling. Cell Mol Life Sci 58: 2053-2068.
- Cuvillier O (2002) Sphingosine in apoptosis signaling. BiochimBiophysActa 1585: 153-162.
- Abbas HK, Tanaka T, Duke SO, Porter JK, Wray EM, et al. (1994) Fumonisin- and AAL-Toxin-Induced Disruption of Sphingolipid Metabolism with Accumulation of Free Sphingoid Bases. Plant Physiol 106: 1085-1093.
- Tanaka T, Abbas HK, Duke SO (1993) Structure-dependent phytotoxicity of fumonisins and related compounds in a duckweed bioassay. Phytochemistry 33: 779-785.
- Zhang H, Huang L, Li X, Ouyang Z, Yu Y, et al. (2013) Overexpression of a rice long-chain base kinase gene OsLCBK1 in tobacco improves oxidative stress tolerance. Plant Biotech 30: 9-16.
- Dutilleul C, Benhassaine-Kesri G, Demandre C, Rézé N, Launay A, et al. (2012) Phytosphingosine-phosphate is a signal for AtMPK6 activation and Arabidopsis response to chilling. New Phytol 194: 181-191.
- Ng CK, Carr K, McAinsh MR, Powell B, Hetherington AM (2001) Drought-induced guard cell signal transduction involves sphingosine-1-phosphate. Nature 410: 596-599.
- Coursol S, Fan LM, Le Stunff H, Spiegel S, Gilroy S, et al. (2003) Sphingolipidsignalling in Arabidopsis guard cells involves heterotrimeric G proteins. Nature 423: 651-654.
- Coursol S, Le Stunff H, Lynch DV, Gilroy S, Assmann SM, et al. (2005) Arabidopsis sphingosine kinase and the effects of phytosphingosine-1-phosphate on stomatal aperture. Plant Physiol 137: 724-737.
- Nakagawa N, Kato M, Takahashi Y, Shimazaki K, Tamura K, et al. (2012) Degradation of long-chain base 1-phosphate (LCBP) in Arabidopsis: functional characterization of LCBP phosphatase involved in the dehydration stress response. J Plant Res 125: 439-449.
- Ishikawa T, Imai H, Maki KY (2013) Development of an LC-MS/MS Method for the Analysis of Free Sphingoid Bases Using 4-Fluoro-7-nitrobenzofurazan (NBD-F). Lipids.
- Dean CB, Nielsen JD (2007) Generalized linear mixed models: a review and some extensions. Lifetime Data Anal 13: 497-512.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15978
- [From(publication date):
specialissue-2014 - Jul 18, 2025] - Breakdown by view type
- HTML page views : 11305
- PDF downloads : 4673