Editorial Open Access
Advance Diagnosis of Drug Resistance in Cancer: Towards Point-of-Care Electronic Nanodevice
Pranjal Chandra*
Amity Institute of Biotechnology, Amity University Uttar Pradesh Sector-125, Noida- 201303, Uttar Pradesh, India
- *Corresponding Author:
- Pranjal Chandra
Amity Institute of Biotechnology
Amity University, Uttar Pradesh
Noida 201303, India
Tel: +91-120-4392644
Fax: +91-120-4392295
E-mail: pchandra1@amity.edu
Received date: April 28, 2014; Accepted date: April 30, 2015; Published date: May 07, 2015
Citation: Chandra P (2015) Advance Diagnosis of Drug Resistance in Cancer: Towards Point-of-Care Electronic Nanodevice. J Anal Bioanal Tech 6:e120. doi: 10.4172/2155-9872.1000e120
Copyright: © 2015 Chandra P. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Editorial
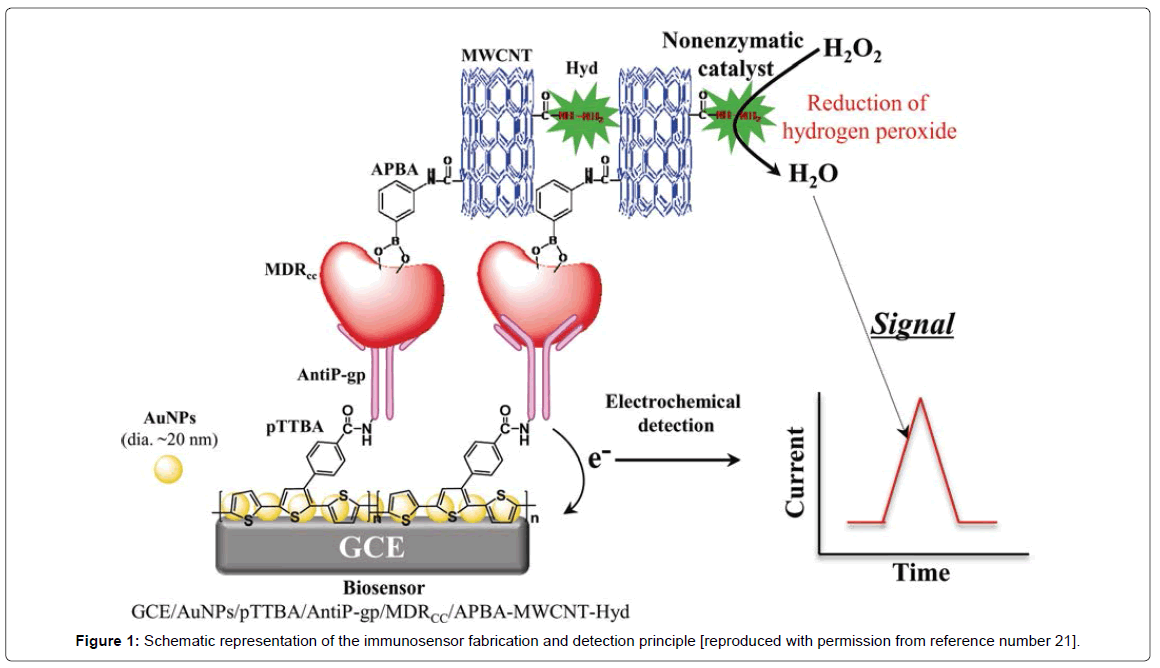
The development of multidrug resistance (MDR) in cancer cells is the main reason of cancer chemotherapy failure. Numerous mechanisms have been found which are responsible in the development of MDR in cancer cells [1]. The most important and well-studied factor responsible for is MDR, is a cell membrane transporter “permeability glycoprotein, (P-gp)” which is encoded by the MDR1 gene. P-gp possesses two membrane-spanning domains and two nucleotide-binding which work as energy dependent pump and reduces the transport of drug through the cell membrane. This results in low concentration of these drugs within the cells than the clinically relevant therapeutic levels [2,3]. This leads in the development of resistance in cancer cells towards drugs. In medical science it is extremely important to detect the onset of resistance in cancer cells which will be very helpful to design new therapeutic strategies to cure cancer patients. In the view of such an important clinical condition, numerous methods have been developed for the highly sensitive detection of these cancer cells. These methods include; polymerase chain reaction (PCR) [4], immunohistochemistry [5], flow cytometry [6], and microarray [7]. While these methods have been applied for the detection of drug resistant cancer cells (DRCC), but they are less sensitive, need extremely trained professionals, and lacks the ability to be miniaturized for the onsite medical detection. In recent years, electrochemical nanosensors are found to be the most promising approach to resolve the issues related to sensitivity, rapidity, selectivity, and ability to be miniaturized [8-17]. Thus, in biosensorbased detection technologies have been also attempted for the detection of DRCC. In this regard in mid-2000, Du et al., developed an electrochemical immunosensor for the detection of P-gp expressed on the K562/ADM l leukemic cells [18]. The detection signal was obtained due to the immunoreaction between P-gp monoclonal antibody and P-gp expressed cancer cells followed by the binding of secondary Alkaline Phosphatase (AP) conjugated antibody which catalyzes the reaction of 1-naphthyl phosphate to generate amperometric signals. The detection limit of this sensor was 1.0 × 104 cells/ml. In another study, an indirect nanosensor based method was developed to detect DRCC [19]. The main concept in this work was to monitor the uptake of anticancer drug by the cells. A carbon nanotube-glassy carbon nanosensor was developed and the uptake of anticancer drug, daunorubicin was monitored based on supramolecular interactions. The uptake of daunorubicin was monitored in terms of its direct electron transfer process. The method was very effective and was able to discriminate between DRCC and drug sensitive cancer cells. In another strategy, Zhang et al., developed a label free electrochemical nanosensor for detection of drug resistant leukemia K562/ADM cells based on P-gp expressions on the cell surface [20]. The detection was based on a nanosensor which was developed by immobilizing P-gp antibody onto a conducting polymer and gold nanoparticles composite. The interaction of the P-gp expressing leukemia cells with the sensor was monitored by electrochemical impedance spectroscopy, which is considered to be a label free method for bio-molecular detection. The sensor was very effective to detect DRCC up to 80 cells/ml, however, it was not applied to detect these cells in biological samples such as; blood and/or serum samples to evaluate its real clinical value. The latest and most sensitive sensor to detect DRCC has been developed by Chandra et al., and has ability to detect DRCC in biological matrix very effectively [21]. The overall sensor fabrication and its detection mechanism has been illustrated in Figure 1. The sensor was designed by immobilizing the P-gp antibody on highly conducting gold nanoparticles – conducting polymer composite developed in Yoon-Bo Shim's laboratory at Pusan National University, South Korea. A sandwiched type sensing format was adopted for the detection of DRCC where P-gp antibody served as a detector probe and aminophenol boronic acid attached with carbon nanotube and hydrazine served as a reported probe. The analytical signal was obtained due to the powerful electrocatalytic activity of hydrazine towards hydrogen peroxide reduction. An exponential range for the DRCC detection using this novel sensor was achieved between 50 and 100,000 cells/ml with the detection limit of 23 ± 2 cells/ml, which is the lowest value compared to any other DRCC sensor reported till date. The designed sensor was highly selective and was able detect the target cells in presence of drug sensitive and noncancerous cells and other chemical molecules present in the real sample environment. The clinical value of the sensor was examined by detecting DRCC in serum samples, and the results were very promising indicating its real biomedical value.
These studies clearly show that there are huge interest to develop highly sensitive point-of-care diagnostic methods to diagnose drug resistance in cancer cells. More research in this area will surely help the clinicians to design the appropriate therapeutic strategy which will reduce the pain of cancer patients and finally can be able to save their life. Future studies should be directed towards integrating these electronic nanosensors with microfluidic systems to develop a pointof- care nanodevice to address more precise issues related to drug resistance in cancer.
Acknowledgements
Dr. Pranjal Chandra thanks to Amity Institute of Biotechnology, Amity University Uttar Pradesh, Noida, India for providing the necessary research facility.
References
- Krishna R, Mayer LD (2000) Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs.Eur J Pharm Sci 11: 265-283.
- Bellamy WT (1996) P-glycoproteins and multidrug resistance.Annu Rev PharmacolToxicol 36: 161-183.
- Gottesman MM , Fojo T, Bates SE (2002) Multidrug resistance in cancer: role of ATP-dependent transporters.Nat Rev Cancer 2: 48-58.
- Murphy LD, Herzog CE, Rudick JB, Fojo AT, Bates SE (1990) Use of the polymerase chain reaction in the quantitation of mdr-1 gene expression.Biochemistry 29: 10351-10356.
- Chan HS,Thorner PS, Haddad G, Ling V (1990) Immunohistochemical detection of P-glycoprotein: prognostic correlation in soft tissue sarcoma of childhood.J ClinOncol 8: 689-704.
- Ludescher C,Thaler J, Drach D, Drach J, Spitaler M, et al. (1992) Detection of activity of P-glycoprotein in human tumour samples using rhodamine 123.Br J Haematol 82: 161-168.
- Gillet J P, Efferth T, Steinbach D, Hamels J, de Longueville F, et al. (2004) Microarray-based detection of multidrug resistance in human tumor cells by expression profiling of ATP-binding cassette transporter genes. Cancer Res 64: 8987-8993.
- Chandra P, Noh HB, Shim YB (2013) Cancer cell detection based on the interaction between an anticancer drug and cell membrane components.ChemCommun (Camb) 49: 1900-1902.
- Chandra P, Noh HB, Won MS, Shim YB (2011) Detection of daunomycin using phosphatidylserine and aptamer co-immobilized on Au nanoparticles deposited conducting polymer.BiosensBioelectron 26: 4442-4449.
- Chandra P,Zaidi SA, Noh HB, Shim YB (2011) Separation and simultaneous detection of anticancer drugs in a microfluidic device with an amperometric biosensor.BiosensBioelectron 28: 326-332.
- Koh WC, Chandra P, Kim DM, Shim YB (2011) Electropolymerized self-assembled layer on gold nanoparticles: detection of inducible nitric oxide synthase in neuronal cell culture.Anal Chem 83: 6177-6183.
- Zhu Y, Chandra P, Shim YB (2013) Ultrasensitive and selective electrochemical diagnosis of breast cancer based on a hydrazine-Au nanoparticle-aptamerbioconjugate.Anal Chem 85: 1058-1064.
- Zhu Y, Chandra P, Song KM, Ban C, Shim YB (2012) Label-free detection of kanamycin based on the aptamer-functionalized conducting polymer/gold nanocomposite.BiosensBioelectron 36: 29-34.
- Chandra P,Koh WC, Noh HB, Shim YB (2012) In vitro monitoring of i-NOS concentrations with an immunosensor: the inhibitory effect of endocrine disruptors on i-NOS release.BiosensBioelectron 32: 278-282.
- Yadav SK, Agrawal B, Chandra P, Goyal RN (2014) In vitro chloramphenicol detection in a Haemophilusinfluena model using an aptamer-polymer based electrochemical biosensor. BiosensBioelectron 55:337-342.
- Chandra P, Son NX, Noh H-B, Goyal RN, Shim Y-B (2013) Investigation on the downregulation of dopamine by acetaminophen administration based on their simultaneous determination in urine. BiosensBioelectron 39: 139-144.
- Noh HB, Chandra P, Moon JO, Shim YB (2012) In vivo detection of glutathione disulfide and oxidative stress monitoring using a biosensor.Biomaterials 33: 2600-2607.
- Du D, Ju H, Zhang X, Chen J, Cai J, Chen H (2005) Electrochemical immunoassay of membrane P-glycoprotein by immobilization of cells on gold nanoparticles modified on a methoxysilyl-terminated butyrylchitosan matrix. Biochemistry 30: 11539-11545.
- Zhang H, Jiang H, Sun F, Wang H, Zhao J, et al. (2011) Rapid diagnosis of multidrug resistance in cancer by electrochemical sensor based on carbon nanotubes-drug supramolecularnanocomposites. Biosens. Bioelectron. 26: 3361-3366.
- Zhang S, Zhang L, Zhang X, Yang P, Cai J (2014) An efficient nanomaterial-based electrochemical biosensor for sensitive recognition of drug-resistant leukemia cells.Analyst 139: 3629-3635.
- Chandra P, Noh HB2, Pallela R2, Shim YB3 (2015) Ultrasensitive detection of drug resistant cancer cells in biological matrixes using an amperometricnanobiosensor.BiosensBioelectron 70: 418-425.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14586
- [From(publication date):
June-2015 - Mar 29, 2025] - Breakdown by view type
- HTML page views : 10013
- PDF downloads : 4573