Adult Laryngeal Hemangioma: A Rare Case Report
Received: 12-May-2015 / Accepted Date: 11-Jul-2015 / Published Date: 17-Jul-2015 DOI: 10.4172/2161-119X.1000201
Abstract
In contrast to infantile laryngeal hemangiomas, adult laryngeal hemangiomas are very rare and differ from infantile ones in location of the lesion and symptoms. We report a rare adult laryngeal hemangioma located in supraglottis causing hoarseness. After excision by direct microlaryngoscopy, patient had improved voice and no recurrence in 4 months.
Keywords: Adult laryngeal hemangioma, Hemangioma, Laryngeal hemangioma
249808Introduction
Hemangiomas of the larynx are classified into adult and infantile forms. The incidence of the laryngeal hemangioma in infants is 4-5%, however incidence in adults is unknown due to the scarcity of case reports [1]. The tumor is self-limited and resolves in approximately half of pediatric patients by 5 years of age and resolves further with age. In contrast, adult hemangioma does not regress spontaneously [2]. Infantile hemangiomas are more frequent in girls and occur usually in subglottic region but, in adults they are more frequent in males and often occur in supraglottic and glottic regions [3,4]. Also clinical symptoms differ due to location of the lesion; hoarseness, dysphagia, dysphonia and shortness of breath are the most common symptoms in adults, whereas fluctuating respiratory distress and stridor are main symptoms in infantile hemangiomas [5]. We present a case of a laryngeal hemangioma in an adult male, who was treated surgically without any complications.
Case Report
A 43 year old male presented to our clinic with complaint of hoarseness for seven years. There was no dysphagia or respiratory distress. In addition, he had hemoptysis from time to time. The patient was a cigarette smoker for the past 15 years (1 pack a day). He had no other known diseases.
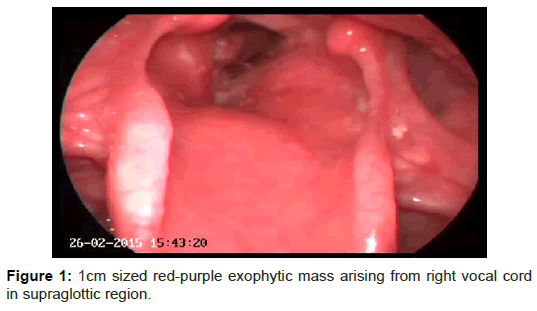
General examination was normal except indirect laryngoscopy in which about 1 cm sized red-purple exophytic mass was seen arising from right vocal cord in supraglottic region (Figure 1). Vocal cords were mobile and the mass was not narrowing the airway.
Direct laringoscopy was performed under general anesthesia and the mass was excised using cautery under microscope. The tumor was grasped with forceps to expose it’s stump extending to subglottic region. By each grasping, excessive hemorrhage was seen and small cotton ball soaked with epinephrine and cauterization were used without injuring the mucosa. All the tumor was excised and hemorrhage was taken under control and no complications was seen. The patient was discharged from the hospital following day and followed up regulary.
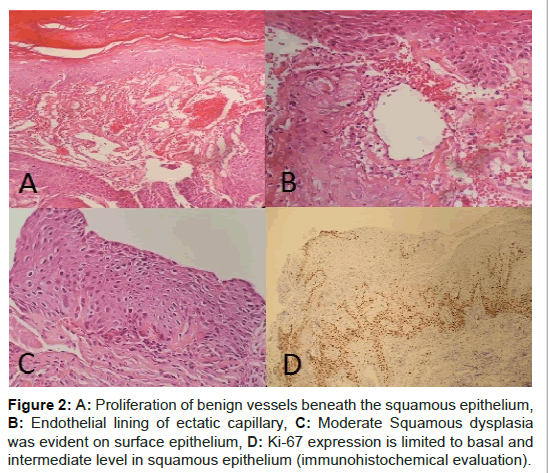
The specimen submitted to pathology was red colored tissue fragments which the largest was 1 cm in diameter. Histological evaluation showed that tissue fragments were covered with squamous epithelium with occasional ulceration (Figure 2A). In some areas tissue was covered with fibrin. Aggregates of packed, thin-walled vascular capillaries, usually with endothelial lining were present in subepithelial areas. Blood-filled vessels were separated by scant connective tissue (Figure 2B). Lumina were thrombosed and organized in some vessels.
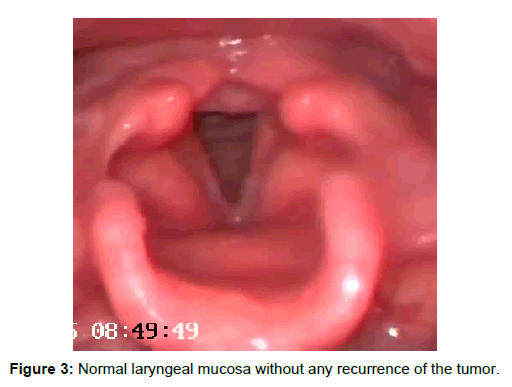
Hemosiderin pigment due to vessel rupture was also observed. In addition to hemangioma, moderate degree of squamous dysplasia was observed in one tissue fragment (Figure 2C). In this area, Ki- 67 expression was observed in midepithelium where as a strong p16 expression was not evident (Figure 2D). The patient had improved voice and no complaints after 4 months and no recurrence was seen in the larynx postoperatively (Figure 3).
Discussion
MacKenzie, in 1871, was first to describe laryngeal hemangioma [6]. Later in 1921, Sweetser differentiated subglottic hemagioma of infancy from glottic and supraglottic hemangiomas of adulthood [7]. Adult hemangiomas are rare and are more often of cavernous form [8]. Cavernous hemagiomas differ from capillary hemangiomas, because its vascular channels are larger, less well circumscribed and usually deeper in submucosal tissues [4]. Our patient had capillary hemangioma.
The etiologic factors are thought to be vocal abuse, cigarette smoking and laryngeal trauma [9]. Although most common symptom of infantile hemangioma is respiratory distress, this is almost never the case in adults [9]. Hoarseness, cough, hemoptysis, dyspnea are more common among adults [5]. In our case, the main complaint of our patient was hoarseness and hemoptysis and he was a cigarette smoker without any history of trauma or intubation or vocal abuse.
Figure 2: A: Proliferation of benign vessels beneath the squamous epithelium, B: Endothelial lining of ectatic capillary, C: Moderate Squamous dysplasia was evident on surface epithelium, D: Ki-67 expression is limited to basal and intermediate level in squamous epithelium (immunohistochemical evaluation).
Laryngeal hemangiomas are diagnosed primarily by history and laryngologic examination. The phonation sign of Menzel, which consists of increased firmness, erection and deeping color of the growth during phonation, may be of diagnostic value [9]. Doppler ultrasound, computed tomography, technetium imaging, and plain radiographs can help in determining the dimensions and extent of the lesion [10].
In differential diagnosis of laryngeal hemangioma, branchial cleft cyts, dermoid cysts and laryngeal webs should be considered. Large branchial cleft cysts may prolapse into laryngeal cavity and result in illusion of laryngeal cysts. Squamous cell carcinoma may be associated with laryngeal cysts, usually located in supraglottic areas. Histological examination usually reveals the nature of lesion.
Factors influencing choice of therapy are patient’s age, type, size and localization of the tumor. In 90% of pediatric cases, hemangiomas occur by first year of life [11] They generally undergo expansion in the first 5 months of life, followed by regression of the lesion [12,13]. If spontaneous regression does not occur, a persistent hemangioma usually respond to propronolol [14]. Large hemangiomas are treated either surgically or by radiotherapy. Adult hemangiomas are not very progressive tumors. Small hemangiomas can be managed conservatively. Large hemangiomas can be treated by systemic steroids, injection of corticosteroids or ethanol, surgical excision, cryosurgery, radiation therapy and CO2 laser excision [4,15]. Sharifi et al. treated a case of supraglottic hemangioma with Argon plasma coagulation which is extensively used for endobronchial tumors, benign and malignant obstructions of the tracheobronchial tree. The patient showed no recurrence after 7 months [16]. In large lesions, even tracheotomy may be required [4]. Removal of pediatric hemagioma with microdebrider is believed to be minimally invasive, safe, simple and effective method especially when the lesion causes tracheal stenosis >50% with recurrent infection and acute laryngeal obstruction [17]. Wang et al. stated that ultrasonic scalpel is also a safe and effective way to remove laryngeal hemangiomas [18]. Transoral robotic resection has also been proposed in treatment of larngeal hemangioma allowing less damage to surrounding mucosal tissue and enabling easy control of bleeding [19]. Excision under microlaryngoscopy is a common surgical technique as we preferred in our patient with no complications or recurrence.
References
- Kilcline C, Frieden IJ (2008) Infantilehemangiomas: how common are they? A systematic review of the medical literature. PediatrDermatol25:168-169.
- Mulliken JB, Fishman SJ, Burrows PE (2000)Vascularanomalities. CurrProblSurg 37:517-584.
- Berkes B, Sente M (1998) Adult laryngeal hemangioma. Med pregl51:547-550.
- Iriz A, Durmaz E, Akmansu SH, Dagli M, Albayrak L, et al. (2009) Vocal Cord Hemangioma; A Rare Localization in Adults. Turk J Med Sci39:305-307.
- Akhtar S, Shamim AA, Ghaffar S, Ahmed MS, Ikram M (2012) Adult laryngeal haemangioma: a rare entity. J Pak Med Assoc62:173-174.
- MacKenzie M (1871) Essay on Growth of the larynx. Lindsay and Blakeston, Philadephia.
- Yilmaz MD, Aktepe F, Altuntas A (2004) Cavernous hemangiomas of the left vocal cord. Eur Arch Otolaryngol216:310-311.
- Kimmelman CP, Sugar JO, Lowry LD (1979) Resident's page. Pathologic quiz case 2. Hemangioma of the vocal cord. Arch Otolarygol105:500-502.
- Stal S, Hamilton S, Spira M (1986)Hemangiomas, lymphangiomas, and vascular malformations of the head and neck. OtolaryngolClin North Am 19:769-796.
- Kawakami M, Hayashi I, Yohimura K, Ichihara K, Nishikawas S, et al. (2006) Adult giant hemangioma of the larynx. AurisNasus Larynx 33:479-482.
- Enjolras O, Mulliken JB (1993)The current management of vascular birthmarks. PediatrDermatol10: 311-312.
- Chang LC, Haggestrom AN, Drolet BA, Baselga E, Chamlin SL, et al.(2008) Growth characteristics of infantile hemangiomas, implications for management. Pediatrics122:360-367.
- Celiksoy MH, Paksu MS, Atmaca S, Sancak R, Hancioglu G (2014)Management of subglottic hemangioma with propranolol. Am J Otolaryngol 35:414-416.
- Lin YS, Ho HS (2010) Adult Laryngeal Hemangioma. Tzu Chi Medical Journal 22:237-240.
- Sharifi A, Nazemieh M, Moghadaszadeh M (2014) SupraglotticHemangioma as a Rare Cause of Recurrent Hemoptysis: A New Treatment Modality with Argon Plasma Coagulation (APC). Tanaffos13:50-52.
- Huang Q, Lyu J, Zhang Z, Jiao Y, Wu H (2014) Treatment of infantile subglottic hemangioma by microdebrider. ZhonghuaEr Bi Yan HouTou Jing WaiKeZaZh 49:457-461.
- Wang X, Zhao X, Zhu W (2015) Resection of a laryngeal hemangioma in an adult using an ultrasonic scalpel: A case report. OncolLett6: 2477–2480.
- Wang WH, Tsai KY (2015)Transoral robotic resection of an adult laryngeal haemangioma and review of the literature. J LaryngolOtol 6:614-618.
Citation: Karatayli-Ozgursoy S, Basaran M, Umudum H, Akmansu SH (2015) Adult Laryngeal Hemangioma: A Rare Case Report. Otolaryngology 5:201. DOI: 10.4172/2161-119X.1000201
Copyright: © 2015 Karatayli-Ozgursoy S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 20620
- [From(publication date): 7-2015 - Apr 11, 2025]
- Breakdown by view type
- HTML page views: 15891
- PDF downloads: 4729