Adsorption of Streptomyces Roseiscleroticus Glucose Isomerase on Chitosan Beads
Received: 01-Feb-2022 / Manuscript No. jbtbm-22-53061 / Editor assigned: 02-Feb-2022 / PreQC No. jbtbm-22-53061(PQ) / Reviewed: 24-Feb-2022 / QC No. jbtbm-22-53061 / Revised: 01-Mar-2022 / Manuscript No. jbtbm-22-53061 (R) / Published Date: 08-Mar-2022 DOI: 10.4172/2155-952X.1000267
Abstract
This study was aimed at immobilizing Streptomyces roseiscleroticus glucose isomerase by adsorption on chitosan beads obtained from shells of water snail and investigating the properties of the adsorbed enzyme with respect to pH, temperature and reusability. The major challenge in the utilization of soluble enzymes relates to their cost of production, recovery and possible reutilization which can be solved by enzyme immobilization. Several methods of enzyme immobilization are abounding. They include, adsorption, entrapment, cross linking and covalent binding to an insoluble solid support. One major advantage of enzyme immobilization by adsorption is the little or no damage to the enzyme and the reversibility of the reaction process. Chitosan was produced by the deactivation of chitin obtained from shells of water snail. The extracted chitosan was subjected to Fourier transform infrared spectroscopy (FTIR) analysis. The FTIR spectrum of the extracted chitosan showed characteristic absorption peaks within 3641 and 3028 cm-1. The extracted chitosan was used as an immobilization support in the form of chitosan beads. Adsorption of the crude and purified glucose isomerase on chitosan beads was achieved at pH 6.0 after 120 min with an immobilization efficiency of 72.68% and 75.31% respectively. The purified adsorbed glucose isomerase had an optimum pH and temperature of 8.5 and 70°C, respectively. Studies on the reusability or storage stability of the purified adsorbed glucose isomerase on chitosan beads showed that, 66%, 52% and 30% of the adsorbed enzyme activity were retained after 24, 48 and 72 hrs. Respectively. Immobilized glucose isomerase is of huge importance in the food processing industries because of its usefulness in the production of sugar syrups and other sugar substitutes.
Keywords
Streptomyces roseiscleroticus; Glucose isomerase; Chitosan; Adsorption; pH
Introduction
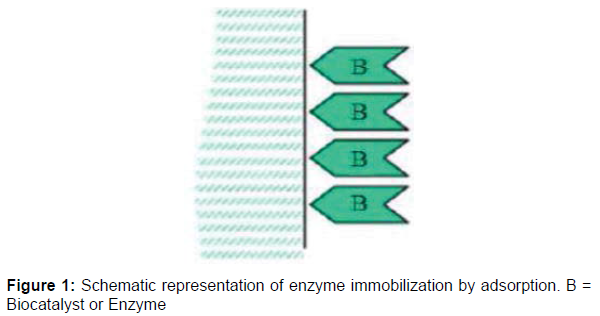
Glucose isomerase is an intracellular enzyme that catalyzes the reversible isomerization of D-glucose to D- fructose. This enzyme has attracted great attention because of its usefulness in the food processing industries for the production of sugar syrups and other sugar substitutes [1]. This has made glucose isomerase applicable in a number of food processing industries and biotechnological processes and has prompted the search for its reliable availability. The use of immobilized enzymes is currently a routine process for the production of many useful industrial products. Enzymes are naturally robust and efficient as they can be used for the production of many different molecules that have a wide range of applications. Enzyme immobilization onto inert material is currently a very active area of research because immobilized enzymes have been shown to be recoverable, reusable and more stable than soluble ones. These characteristics are often desired features of enzymes for commercial utilization. Enzyme immobilization remains a means to circumvent the challenges of soluble enzymes. Immobilized enzymes are currently the subject of considerable interest because of their advantages over soluble enzymes. With the ever increasing demands of biotechnology industries, there is a need to improve the productivity, reaction stability, reusability and shelf life of enzymes. Various methods of enzyme immobilization of have been reported. These methods include adsorption onto an insoluble carrier. Entrapment in a polymeric matrix, cross linking with a functional reagent and covalent linking to an insoluble solid material. However, the most used methods are based on physical immobilization (adsorption or physical entrapment) and chemical immobilization (covalent binding and cross linking). The choice of immobilization technique is very important as it prevent the loss of enzyme activity by not changing the chemical nature or reactive groups in the binding site of enzyme. Enzyme immobilization by adsorption is the simplest method of immobilization. This method is based on weak forces (e.g., ionic bonds, hydrogen bonds, van der Waals forces, hydrophobic bonding or salt linkages) of interaction between the enzyme and immobilization support while still enabling an efficient binding process. One major advantage of enzyme immobilization by adsorption is the little or no damage to the enzyme or protein as well as the reversibility of the reaction process (Figures 1and 2).
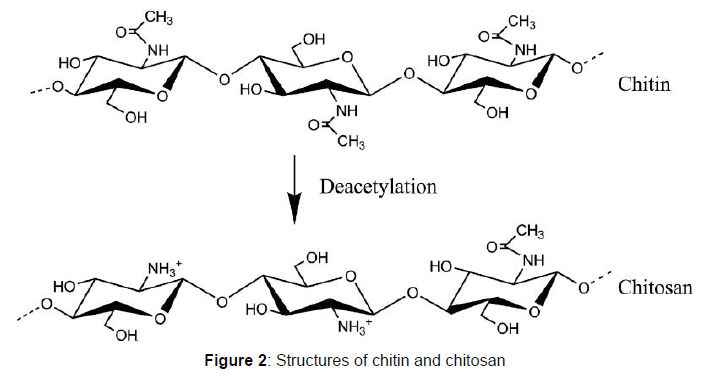
The unique properties of chitosan arises due to the avalanche of free amino (-NH2) and hydroxyl (-OH) groups on the chitosan chains which often serves as reaction sites or centers for chitosan reactions. The distinguishing characteristics of chitosan also include its strong biocompatibility, hydrophilicity, high affinity and biodegradability. These properties of chitosan enables chitosan to bind to a number of materials such as proteins, biomasses, cholesterols, fats, metal ions and even cells. Immobilized glucose isomerase is very useful in the food processing industries because of it is utilized in the production of sugar syrups and other sugar substitutes. This research therefore focuses on utilizing chitosan as a support material for the adsorption of Streptomyces roseiscleroticus glucose isomerase and also, investigating the properties of the adsorbed enzyme with respect to pH, temperature and reusability.
Materials and Methods
Chemicals and reagents
All chemicals used in this research were of analytical grade and were obtained directly from the manufacturers. Major chemicals such as carbazole, cysteine hydrochloric acid, Bovin serum albumin (BSA), tetra trimethyl ammonium bromide (TTAB) and Folin- ciocalteau reagent were obtained from Sigma Aldrich, Germany.
Glucose isomerase production and purification
Glucose isomerase was produced from Streptomyces roseiscleroticus and partially purified using the methods as described
Glucose isomerase assay
Glucose isomerase assay was carried out as described. An aliquot (0.1 ml) of enzyme solution was mixed with the reaction mixture. The reaction mixture contained 0.5 ml of 0.1 M potassium phosphate buffer pH 8.0, 0.2 ml of 0.05 M MgSO4.7H2O and 0.2 ml of 1 M glucose solution. After incubation in water bath at 60⁰C for 30 min, 1 ml of 0.5 M perchloric acid was added to stop the reaction. Fructose formed was determined by the method of Dische and Borenfreund (1951) in which 0.2 ml of 1.5% cysteine hydrochloride, 6 ml of 70% H2SO4 and 0.2 ml of 0.12% alcoholic carbazole were added to the reaction mixture. The purple colour developed was measured spectro photo metrically at 560 nm [2]. The absorbance was converted to glucose isomerase (GI) activity using a fructose standard curve. One unit of GI activity (U) is defined as the amount of enzyme that produced one μmol of D-fructose per min under the assay conditions.
Protein determination
Protein content of the enzyme was determined by the method of Lowry, using Bovine Serum Albumin (BSA) as standard.
Extraction of chitosan from shells of water snail
Chitosan was extracted from shells of water snail by the deacetylation of chitin using the methods as described.
Fourier transforms infrared spectroscopy (FTIR) of the extracted chitosan
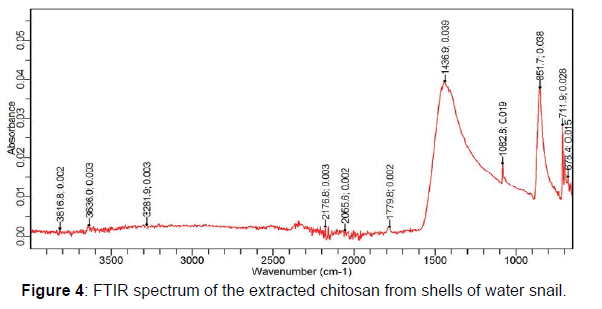
The extracted chitosan was pulverized into fine powder and subjected to Fourier transform infrared spectroscopy analysis for their unique characteristic functional groups. The FTIR spectra were recorded using an Agilent Carry 630; Agilent Technologies, FTIR spectrometer with a frequency range of 4000 - 625 cm-1.
Preparation of the chitosan beads
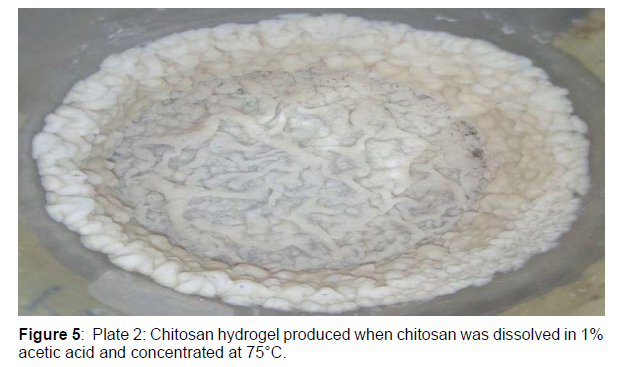
The chitosan beads were prepared according to method as described. A known weight of chitosan (2.5 g) was dissolved in 100 ml of 1% acetic acid in a conical flask, concentrated at 75°C until a chitosan hydrogel is formed and then allowed to cool for 2 h. The chitosan hydro gel formed was introduced as droplets into 100 ml of 30% sodium hydroxide (NaOH) solution using a sterilized string to precipitate the chitosan beads. The chitosan beads formed was washed thoroughly with distilled water to neutrality. After washing, the produced chitosan beads were allowed to dry. The bead yield of the chitosan beads formed was determined
Chitosan bead yield (%) = Weight of chitosan bead formed/Weight of the formed hydrogel ×100
Adsorption of glucose isomerase on chitosan beads
Glucose isomerase was immobilized by adsorption on chitosan beads as described. Dried chitosan beads were introduced into a test tube (15cm by 1cm) and 10 ml of the enzyme solution (glucose isomerase) was introduced to the packed chitosan beads in the test tube. The packed chitosan beads containing the enzyme were allowed to stand for 2 hr at 37 ⁰C. Thereafter, the unbound protein was decanted off from the packed chitosan beads in the test tube. Glucose isomerase activity of the bound and unbound enzyme was assayed, respectively. Percentage immobilization efficiency of the bound enzyme on the chitosan bead was determined.
Determination of the percentage immobilization efficiency of the adsorbed glucose isomerase The immobilization efficiency of glucose isomerase immobilization to the chitosan beads was calculated as described using the formula.
Immobilization efficiency (%) = Specific activity of immobilized enzyme / Specific activity of free enzyme×100
Assay for the adsorbed glucose isomerase
The immobilized glucose isomerase was assayed as described, except that the free enzyme solution was replaced with 0.5 g of the glucose isomerase immobilized chitosan beads. For each assay of the immobilized glucose isomerase, the immobilized glucose isomerase chitosan beads were put into fresh reaction mixture (0.5 ml of 0.1 M potassium phosphate buffer pH 7.5, 0.2 ml of 0.05 M MgSO4.7H2O and 0.2 ml of 1 M glucose solution) and incubated in a water bath at 60°C for 30 min. After incubation, the reaction mixture was decanted and separated from the glucose isomerase immobilized chitosan beads. The chitosan beads were removed and washed with phosphate buffer (0.1 M, pH 7.5) to remove any residual substrate within the chitosan beads. To the reaction mixture, 1 ml of 0.5 M perchloric acid was added to stop the reaction. Fructose formed was determined by the method of Dische and Borenfreund (1951) in which 0.2 ml of 1.5% cysteine hydrochloride, 6 ml of 70% H2SO4 and 0.2 ml of 0.12% alcoholic carbazole were added to the reaction mixture. The purple colour developed was measured spectrophotometrically at 560 nm. The absorbance was converted to glucose isomerase (GI) activity using a fructose standard curve. One unit of GI activity (U) is defined as the amount of enzyme that produced one μmol of D-fructose per min under the assay conditions [3].
Determination of the total bound protein of the adsorbed glucose isomerase
The total bound protein concentration of the immobilized enzyme was determined according to using Bovine serum albumin (BSA) as a standard. For each determination, a known weight (0.5 g) of the glucose isomerase immobilized chitosan beads was mixed with 0.9 ml of distilled water. A volume of 5 ml of solution E (as described below) was added to the immobilized chitosan beads and allowed to stand at room temperature for 10 min. Then, 0.5 ml of solution C (dilute Folin- Ciocalteau phenol reagent) was added, mixed rapidly and allowed to stand for 30 mins. After standing, the beads were removed and washed with phosphate buffer (0.1 M, pH 7.5) for subsequent reuse. To the reaction mixture, the absorbance was read at 750 nm using UV spectrophotometer. Absorbance values were converted to protein concentrations by extrapolation from the protein standard curve.
• Solution A: An alkaline sodium carbonate (Na2CO3) was prepared by dissolving 2g of Na2CO3 in 100ml of 0.1M NaOH (0.4g of sodium hydroxide pellets were dissolved in 100ml of distilled water).
• Solution B: A copper tetraoxosulphate IV - sodium potassium tartarate solution was prepared by dissolving 0.5g of CuSO4 in 1g of sodium potassium tartarate, all in 100ml of distilled water. It was prepared fresh by mixing stock solution, and so was done whenever required.
• Solution C: Folin-Ciocalteau phenol reagent was made by diluting the commercial reagent with distilled water in a ratio of 1:1 on the day of use.
• Solution D: Standard protein (Bovine Serum Albumin, BSA) solution
• Solution E: Freshly prepared alkaline solution was made by mixing 50ml of solutions A and 1ml of solution B.
pH optimization of immobilization by adsorption
The optimization of the immobilization pH of adsorption was determined as described by the method of dried chitosan beads were introduced into a test tube (15cm by 1cm) and a volume (10 ml) of the enzyme solution was introduced to the packed chitosan beads in the test tube and adjusted to pH 5, 6 and 7 respectively. Immobilization by adsorption and the assay for the adsorbed enzyme at the various pH was carried out as described above.
Time optimization of immobilization by adsorption
The optimization of the immobilization contact time of adsorption was determined as described. Dried chitosan beads (0.5 g) were introduced into a test tube (15cm by 1cm). Enzyme solution (10 ml) was introduced to the chitosan beads and allowed to stand for 30, 60, 120 and 150 min, respectively at the predetermined adsorption pH. Immobilization by adsorption and the assay for the adsorbed enzyme at the various contact time was carried out as described above.
Characterization of the adsorbed glucose isomerase on chitosan beads
Effect of pH on the activity of the adsorbed glucose isomerase The effect of pH on the activity of the adsorbed glucose isomerase was determined as described by incubating the glucose isomerase adsorbed chitosan beads in various buffers of pH ranging from pH 3.0 - 9.0 at 0.5 interval, that is, 20 mM sodium acetate buffer (pH 3.5 - 5.0), 20 mM phosphate buffer (pH 6.0 - 7.0) and 20 mM Tris-HCl buffer (pH 8.0 – 9.0) together with the reaction mixture (0.2 ml of 0.05 M MgSO4.7H2O and 0.2 ml of 1 M glucose solution) in a water bath at 60 ⁰C for 30 min [4] . After incubation, the reaction mixture was decanted and separated from the chitosan beads. The chitosan beads were removed and washed to remove any residual substrate within the beads. To the reaction mixture, 1 ml of 0.5 M perchloric acid was added to stop the reaction. Fructose formed was determined by the method of Dische and Borenfreund (1951) in which 0.2 ml of 1.5% cysteine hydrochloride, 6 ml of 70% H2SO4 and 0.2 ml of 0.12% alcoholic carbazole were added to the reaction mixture. The purple colour developed was measured spectrophotometrically at 560 nm. The absorbance was converted to glucose isomerase (GI) activity using a fructose standard curve. One unit of GI activity (U) is defined as the amount of enzyme that produced one μmol of D-fructose per min under the assay conditions.
Effect of temperature on the activity of the adsorbed glucose isomerase
The effect temperature on the activity of the adsorbed glucose isomerase was determined as described by incubating the glucose isomerase adsorbed chitosan beads together with the reaction mixture at the pre-determined optimum pH at various temperatures ranging from 30 – 90°C at 10°C interval for 30 min. After incubation, the reaction mixture was decanted and separated from the chitosan beads. The chitosan beads were removed and washed to remove any residual substrate within the chitosan beads. To the reaction mixture, 1 ml of 0.5 M perchloric acid was added to stop the reaction. Fructose formed was determined by the method of Dische and Borenfreund (1951) in which 0.2 ml of 1.5% cysteine hydrochloride, 6 ml of 70% H2SO4 and 0.2 ml of 0.12% alcoholic carbazole were added to the reaction mixture. The purple colour developed was measured spectrophotometrically at 560 nm. The absorbance was converted to glucose isomerase (GI) activity using a fructose standard curve. One unit of GI activity (U) is defined as the amount of enzyme that produced one μmol of D-fructose per min under the assay conditions.
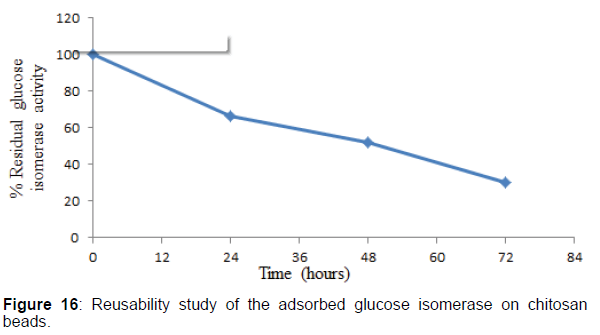
Reusability studies of the adsorbed glucose isomerase on chitosan beads
The reusability of the adsorbed glucose isomerase on chitosan beads was tested as described by the methods of Zhu. The reusability study of the adsorbed glucose isomerase was monitored at 24 hr interval for 72 hr after ten cycle or ten repeated reuse. After each assay, the chitosan beads were removed from the reaction mixture, washed severally with 0.1 M phosphate buffer, pH 7.5, to remove any residual substrate within the chitosan beads. For each reaction, the chitosan beads were introduced into fresh reaction medium and the enzyme activity assayed at the optimum conditions of pH and temperature. The enzyme activity of the adsorbed glucose isomerase was assayed and expressed as a percentage of its residual activity in comparison to the initial enzyme activity of the adsorbed enzyme.
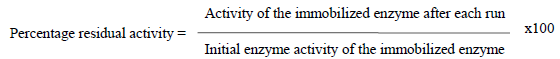
Results and Discussions
A chitosan yield of 44.69% was obtained after the deacetylation of chitin from the shells of water snail. Chitosan yields have been reported to be relatively low after de acetylation. This might be due to the depolymerization of the chitosan polymer, loss of sample mass or weight from excessive removal of acetyl groups during the deacetylation process and loss of chitosan particles during washing. However, chitosan yield obtained from this study shows a relative quantity and availability of chitosan from the shells of water snail. This could be attributed to the snail shell species (Achatina maginata) used for the chitosan production. The Fourier transform infrared spectroscopy (FTIR) analysis of the extracted chitosan showed characteristic absorption peaks within 3641 and 3028 cm-1 this is indicative of the stretching vibrations of the hydroxyl (- OH) and amine (-NH2) groups of chitosan. Also, absorption bands at 1436 and 1408 cm-1 in the extracted chitosan indicates the vibrations of carbonyl group [5]. Extra absorption bands in the regions of 1438-1067 cm-1 (from the extracted chitosan) are suggestive of the presence of the characteristic amide band II (N-H bendings) primary amine groups present on chitosan polymers (Figures 3 and 4).
Prior to the immobilization, chitosan hydrogel was produced from chitosan. Chitosan hydrogels are three dimensional macromolecular network of polymer chain that are hydrophilic and exists as colloidal gels. They are also described as cross-linked or uncross-linked macromolecular network that swells in solvent medium (e.g. water, organic acid, inorganic acid or biological fluids. The swelling behavior of chitosan hydrogels is dependent on the pH and temperature of the solvent medium making them a highly water soluble polyelectrolyte.
Chitosan hydrogels, like other hydrogels, contain much water. Part of this water is tightly bound to the polymer while and the rest is present as free water. Hydrogel are also called aqua gel. Furthermore, the chitosan hydrogel was transformed into chitosan beads. The bead yield of the chitosan hydrogel transformed into chitosan beads was 64.1%( Figure 5).
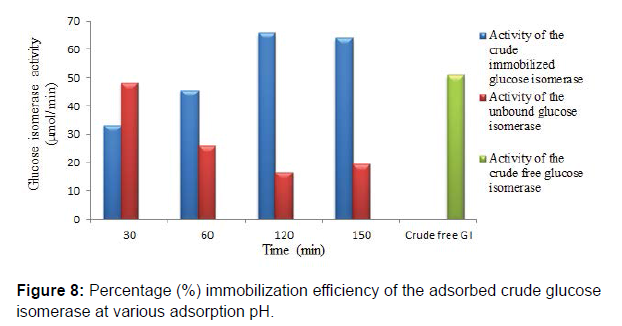
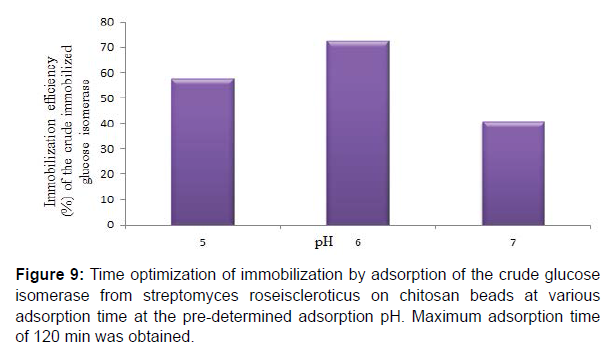
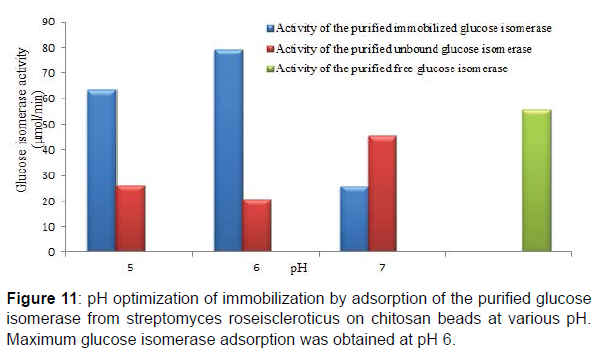
Chitosan beads prepared by precipitation in sodium hydroxide solution have been reported to be suitable and ideal for enzyme immobilization. They are also reported to possess efficient biocompatibility, strong affinity, high mechanical strength, rigidity and broad surface area for enzyme immobilization. Studies on glucose isomerase immobilization by adsorption showed that the adsorbed crude glucose isomerase had a maximum glucose isomerase activity at pH 6.0 after 2 hr with immobilization efficiency of 57.64, 72.68 and 40.97% at pH 5.0, 6.0 and 7.0, respectively. Enzyme immobilization by adsorption on a support polymer is very largely dependent on the ionic strength and adsorption pH (Figures 6-8).
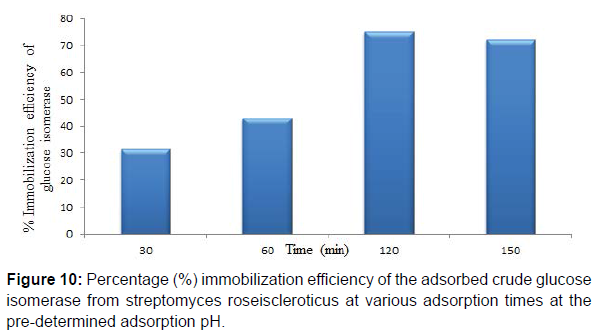
Maximum adsorption time of 120 min at the predetermined adsorption pH was obtained for the adsorbed crude glucose isomerase. Also immobilization efficiencies of 31.38, 42.93, 75.17 and 72.26% at 30, 60, 120 and 150 min adsorption time, respectively for the adsorbed crude glucose isomerase was obtained. Enzyme immobilization by adsorption on a support polymer is also very largely dependent on the and adsorption time, surface area, porosity and physical characteristics of both the enzyme and the support, such as the number and type of nucleophilic and reactive groups present on the enzyme and the immobilization support surfaces, respectively (Figure 9,10). Reported that different adsorption pH result in a difference in charge distribution on the surface of the immobilization support and the enzyme. Therefore affecting the binding between the enzyme and the immobilization support. Adsorption on chitosan is only possible at pH solutions lower or below its pKa value. This supports the maximum glucose isomerase adsorption on chitosan beads at pH 6.0 obtained in this study.
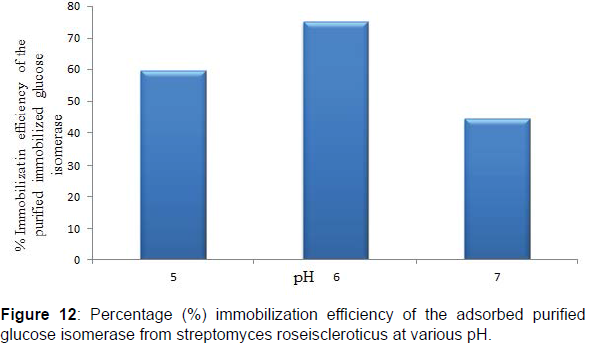
Chitosan has as its major functional groups free amino (-NH2) and hydroxyl (-OH) moieties with a pKa value of 6.3 and increasingly protonated, this results in the abundance and availability of nucleophilic centers and radicals on chitosan molecules that are utilized in the binding of proteins, enzymes, biomasses and other biocatalysts onto chitosan (Figures 11 and 12). Similarly, at pH solutions beyond or higher than its pKa the amino groups of chitosan becomes highly deprotonated thus a great reduction in the availability of nucleophiles and nucleophilic centers required for chitosan Studies on the adsorption of the purified glucose isomerase on chitosan beads showed that maximum glucose isomerase activity was achieved at pH 6.0 after 2 hr with an immobilization efficiencies of 59.53, 75.31 and 44.30% at pH 5.0, 6.0 and 7.0, respectively. Enzyme immobilization efficiency above 50% are indicative of a strong interaction of binding between the reactive groups present on the enzyme and on the immobilization support surface (Figures 13 and 14).
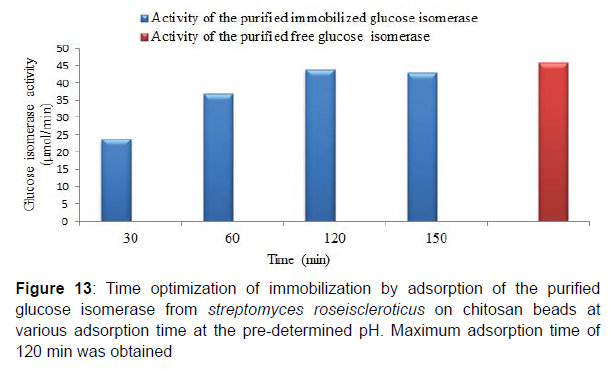
Similarly, maximum adsorption time for the purified adsorbed glucose isomerase was obtained at 120 min at the predetermined adsorption pH . The immobilization efficiency of the purified adsorbed glucose isomerase at various adsorption times was 51.41, 80.46, 95.1 and 93.6% at 30, 60, 120 and 150 minutes respectively. Enzyme immobilization efficiency has also been reported to be influenced greatly by the duration or time of immobilization and by the temperature of immobilization. Reported that enzyme immobilization efficiency gradually increases with a prolonged adsorption time as the immobilization efficiency remains constant after the optimum adsorption time. Similarly, prolonged adsorption time enables the penetration of the enzyme into the pore spaces of the immobilization support and hence, promotes the number and strength of bonds formed. Enzyme adsorption time during enzyme immobilization is very crucial in order to establish and attain multipoint interaction between the enzyme and support groups. Also, immobilization at moderate temperature (e.g. room temperature, 37 ⁰C), is reported to increase the vibration of the enzyme and immobilization support thus, facilitating greater interaction and formation of enzyme-support linkages. Large number and strength of the enzyme-support linkages formed during the adsorption time often results in a high immobilization efficiency. Similarly moderate temperature during immobilization creates poor solvency as a result of the reduction or elimination of steric repulsion, thereby promoting protein polymer interaction and higher immobilization efficiency [6].
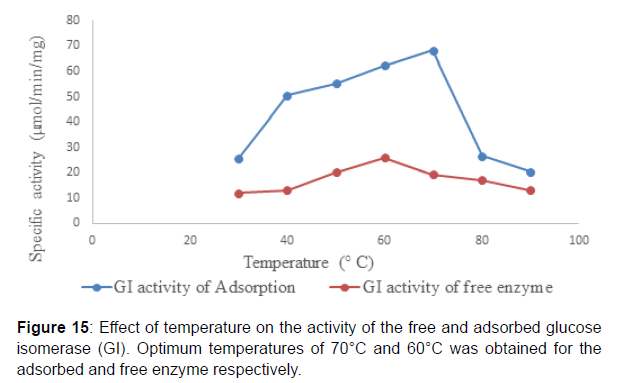
Studies on the effect of pH on the activity of the adsorbed glucose isomerase revealed that, an optimum pH 8.5 and 7.5 was obtained for the adsorbed and free enzyme respectively reported an optimum pH of 8.0 for immobilized glucose isomerase onto surface-modified chitosan gel beads and an optimum pH of 7.5 for the free glucose isomerase (Figure 15). The shift or adjustment in pH optimum after enzyme immobilization has been reported for most enzymes) Reported that the pH of the microenvironment of the immobilization support compared to the reaction mixture or bulk solution is primarily dependent on charges on the support or carrier and it is influenced greatly by the partitioning of the hydrogen ions in and out of the enzyme matrix. This in turn affects enzyme activity and substrate binding . Similarly, reported that immobilized enzymes are often discovered to be more active at extremes of pH, an indication that immobilization broadened the pH activity profile of the enzyme. Studies on the effect of temperature on the activity of the adsorbed glucose isomerase showed that, an optimum temperature of 70⁰C and 60⁰C was obtained for both the adsorbed and free enzyme respectively. This is similar to the findings of who reported in their work that the optimum temperature range for the free and immobilized glucose isomerase onto surface-modified chitosan gel beads was found to be about 60 and 80⁰C respectively( Figure 16 ).
The adsorption of glucose isomerase on chitosan beads led to the increase in the optimum temperature of the enzyme from 60 to 70⁰C. This could be suggestive of changes in conformational flexibility the adsorbed enzyme may require a higher activation energy with which to reorganize its conformation to that necessary for catalysis.
Studies on the reusability stability of the purified adsorbed glucose isomerase on chitosan beads were investigated. The reusability studies showed that 66% of the adsorbed glucose isomerase activity was retained after 24 hr prior to ten times repeated reuse. After 24 hr., the adsorbed glucose isomerase activity decreased smoothly during the continuous operation process, with the adsorbed glucose isomerase retaining 52 and 30% after 48 and 72 hr respectively respectively. The gradual decrease in the adsorbed glucose isomerase activity during the repeated use is a common phenomenon with enzyme immobilization, particularly, immobilization by adsorption. Also, the gradual decrease in the adsorbed glucose isomerase activity during its repeated use could be attributed to enzyme desorption and leaching. Enzymes desorption and leaching is a major limitation in enzyme immobilization by adsorption. In addition, the gradual decrease in the adsorbed glucose isomerase activity during each repeated and continuous use could be suggestive of the release of the enzyme from the chitosan beads or matrix, which totally results in the reduction of the amount of enzyme bound on the chitosan beads.
Conclusion
Chitosan beads obtained from chitosan proofed to be an effective immobilization support for glucose isomerase immobilization. The adsorbed glucose isomerase showed good immobilization efficiency and reusability over time. These properties are essential credentials in the utilization of a biocatalyst for industrial processes. Immobilized glucose isomerase is of huge importance in the food processing industries because of its usefulness in the production of sugar syrups and other sugar substitutes.
Declarations
The authors declare no conflict of interest.
Funding statement
This research work did not receive any grant from any funding agencies.
Acknowledgements
Enormous gratitude to Professor Ferdinand Chimera Chilaka and Professor Sabinus Oscar Onyebuchi Eze of the Department of Biochemistry, Faculty of Biological Sciences, University of Nigeria, Nsukka (U.N.N.) for the provision of all the chemicals and reagents used for this research.
References
- Arasaratnam V, Galaev IY and Mattiasson B (2000) Reversibility soluble biocatalyst: Optimization of trypsin coupling to Eudargit S-100 and biocatalyst activity in soluble and precipitated forms. Enzyme and Microbial Technology 27(3):254-263.
- Ahmad R and Sardar M (2015) Enzyme immobilization: an overview on nanoparticles as immobilization matrix. Analytical Biochemistry 4(2): 1-8.
- Ahmed SA, El-Shayeb NM, Hashem AM and Abdel-Fattah AF (2013) Biochemical studies on immobilized fungal β-glucosidase. Brazilian Journal of Chemical Engineering 30: 747 – 758.
- Akakuru OU and Isiuku BO (2017) Chitosan hydrogels and their glutaraldehyde-crosslinked counterparts as potential drug release and tissue engineering systems - synthesis, characterization, swelling kinetics and mechanism. Journal of Physical Chemistry and Biophysics 7(3):1-7.
- Alessandra B and Simona S (2019) Industrial applications of immobilized enzymes - A review. Molecular Catalysis 479: 1 -20.
- Bernal C, Rodríguez K and Martinez R (2018) Integrating enzyme immobilization and protein engineering: an alternative path for the development of novel and improved industrial biocatalysts. Biotechnological Advances 36: 1470–1480.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Chinyelum OP, Onyebuchi ESO, Chiemeka CF (2022) Adsorption of Streptomyces Roseiscleroticus Glucose Isomerase on Chitosan Beads. J Biotechnol Biomater, 12: 267. DOI: 10.4172/2155-952X.1000267
Copyright: © 2022 Chinyelum OP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2194
- [From(publication date): 0-2022 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 1698
- PDF downloads: 496