Research Article Open Access
Adsorption Kinetics of Cobalt (II) Ions onto Alginate Beads from Aqueous Solutions
| Navarro AE*, Musaev H, Serrano K and Masud ME | |
| Science Department, Borough of Manhattan Community College, City University of New York, USA | |
| Corresponding Author : | Navarro AE Science Department Borough of Manhattan Community College City University of New York, USA Tel: +001212-220-8000 E-mail: anavarro@bmcc.cuny.edu |
| Received July 25, 2014; Accepted September 25, 2014; Published October 05, 2014 | |
| Citation: Navarro AE, Musaev H, Serrano K, Masud ME (2014) Adsorption Kinetics of Cobalt (II) Ions onto Alginate Beads from Aqueous Solutions. J Earth Sci Clim Change 5:223. doi: 10.4172/2157-7617.1000223 | |
| Copyright: © 2014 Navarro AE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Earth Science & Climatic Change
Abstract
Cobalt is a heavy metal that is commonly used in industrial processes and used in batteries and tanning activities. Time-dependent experiments were carried out to study the adsorption kinetics of cobalt (II) ions onto alginate beads in batch conditions at room temperature. Experiments were carried out in the absence of salt and in a 0.01 M solution of NaNO3 as a model salt to explore the effect of salinity in the adsorption. The experimental data was fitted to pseudo-first (Lagergren model) and pseudo-second order kinetics. According to the modeling, experimental data is better resembled by the pseudo-second order kinetics as shown by its correlation coefficient, indicating that less than 30 minutes are needed to reach equilibrium. Likewise, experimental data shows that salinity has a negative effect on the adsorption of cobalt (II) ions, reporting maximum adsorption capacities of 230 mg/g and 211 mg/g in the absence and in the presence of salt, respectively. It is hypothesized that electrostatic competition for the adsorption sites occurs due to the presence of Na+ ions in the solution. Finally, our results were compared to recently reported data, indicating that alginate beads have better adsorption properties than other naturally-occurring materials like algae, flower wastes and minerals like zeolite and bentonite. This study demonstrated that alginate beads are good candidates for a fast and efficient removal of cobalt (II) ions from solutions.
| Keywords |
| Adsorption; Kinetics; Cobalt (II) ions; Pseudo-first order kinetics; Pseudo-second order kinetics |
| Introduction |
| Currently, the problem of chemical contamination of natural fresh and marine waters is extremely urgent. Untreated waste water adversely affects the content of dissolved oxygen in the water, its pH, the transparency, hue, and other properties. All these problems have a negative impact on the condition of the aquatic ecosystem components, reduces productivity, and the ability to cleanse ponds [1]. |
| Use of biological and chemical methods is one of the promising areas in wastewater treatment, both household and industrial. One of these methods is biosorption of metals from solutions, which is based on the ability of microorganisms to adsorb cations of various metals, removing them from the solutions. This can be used for purification of industrial waste water from metal [2]. |
| It is known that the ability to concentrate chemical elements is a characteristic of all living organisms. Once this metal accumulation reaches a threshold, the toxic effects are manifested. In this regard, the study of the effects of heavy metals on the cells is essential to identify the mechanisms of microbial adaptation to extreme conditions of existence. |
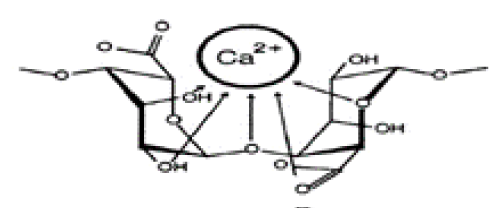
| Biosorption shares very similar fundamental basis with ion exchange in a sense that both the methods are reversible and utilize solid particles that contain ions that exchange themselves for ions in water [2]. As a more recently promoted method, the use of biosorbents has several advantages over the previous remediation processes: Lower cost, minimal production of chemical byproduct, recovery of metals following adsorption and the ability to be recovered and reused [3]. As a new and promising field of application, biosorption is attracting a large number of enterprises and are subject to numerous research and experiments. In this particular project, the biosorption kinetics of cobalt ions onto alginate gel beads was thoroughly investigated (Figure 1). |
| Alginate Gel Bead (AB) is a type of biosorbent that is constantly growing in popularity. It is made of alginate, which is a polysaccharide that is mainly obtained from brown algae [4]. Alginate is chemically composed of block-wise pattern of G-blocks (α-L-guluronic acid) and M-blocks (β-D-mannuronic acid) in a random sequence [4]. Because of alginate’s negative charge, it is viscous and can be forged into a gel. Using this property, alginate gel beads can be formed by encapsulating calcium ions, which have a very low toxicity, in alginate beads [5]. The adsorptive properties of this material reside on the presence of carboxylic acid and hydroxyl functional groups that have a high affinity towards heavy metals and other polar compounds. On the other hand, AB is highly porous and allows an extended use of the surface of the biomaterial. Therefore the pollutant is not only adsorbed on the outside of the beads, but also penetrates the beads through the microscopic channels and reaches other adsorption sites inside the bead. This technique optimizes the adsorption and makes a more efficient use of the AB. |
| Materials and Methods |
| Chemicals and solutions |
| Stock solution of Cobalt (II) ions (1000 ppm) was prepared by dissolving cobalt (II) nitrate hexahydrate, Co(NO3)2.6H2O (reagent grade, Fisher Scientific), in deionized water. The various concentrations of Cobalt (II) ion were prepared by diluting in deionized water. Once getting the needed concentrations, pH was adjusted to the desired values by using pH meter, and titrated with aliquots of 0.1 M of HCl and 0.1 M of NaOH solutions. |
| Preparation of the alginate gel beads (AB) |
| To produce alginate gel beads, sodium alginate and calcium chloride (reagent grade, Fisher Scientific) solutions had to be prepared. Alginate solution was made by dissolving 4.5 grams of sodium alginate in 150 mL of deionized water on a hot plate at 60°C under magnetic stirring. Throughout the dissolution, more deionized water was added to end up with 200 mL of alginate solution. A calcium chloride (CaCl2) solution was prepared by dissolving 33.3 g of calcium chloride in 1.5 L of deionized water with a stirring magnet. Later, the alginate solution was put into calcium chloride solution drop-wise using a peristaltic pump to produce the alginate beads. Throughout this process, a stirring magnet continuously agitated the calcium chloride to prevent the cluttering of immature alginate beads. Once all the alginate beads have been prepared, they were exhaustively rinsed with distilled water. Finally, the alginate gel beads were suspended in distilled water in a glass bottle and stored in the refrigerator at 4°C. |
| Mass ratio of the alginate gel beads |
| Prior to the adsorption experiments, the wet/dry mass ratio of alginate beads had to be obtained. To do so, the masses of the wet beads were recorded and then placed in an oven overnight at 60°C for drying. The masses of the dry beads were taken and compared to those of the wet beads. A calibration curve was constructed and a linear equation correlated the wet and dry masses. |
| Batch adsorption tests |
| Discontinuous experiments were carried out in duplicates at room temperature by combining 150 mg of dry AB with 50 mL of a cobalt (II) solution with a concentration of 100 mg/L under orbital agitation at 250 rpm for 24 h. Initial solution pH was adjusted to 5 as indicated by preliminary results. Adsorption mixture was sealed with parafilm to avoid evaporation. Upon equilibrium, the suspensions were separated by gravity. |
| Cobalt ions adsorption by AB was obtained by comparison of the initial and final cobalt concentrations after the adsorption. Cobalt concentrations were determined by UV-Vis spectrophotometry using the Zincon method at 600 nm wavelength [6]. A blue-colored complex is produced by mixing the samples solutions with an equimolar number of moles of Zincon (analytical grade, Sigma-Aldrich) in a buffer at pH 9. These colorimetric analyses were carried out using an automatized microplate reader (Synergy4, Biotek). |
| Adsorption kinetics experiments |
| Kinetics experiments were carried out in duplicates at room temperature by mixing 1.5 g of dry beads (equivalent to 30.45 g of wet beads) with 500 mL of a 100 mg/L solution of Cobalt (II) solutions. Initial pH solution was adjusted to 5 as indicated by preliminary results. In order to evaluate the effect of salinity in the adsorption of cobalt ions, an identical experiment was performed in the presence of 10 mM NaNO3. Sodium nitrate (NaNO3) salt was used as a standard salinity salt and also for its prevalence in seawater. This salt concentration (10 mM) was chosen due to the proximity in cobalt (II) ions molar concentration (100 mg/L ≈ 2 mM). The suspensions (beads in solutions), were placed on a magnetic stirrer at 250 rpm and the timer was started. A 0.5 mL sample was taken with a plastic pipette at different time intervals (in minutes) during 4 hours or until there was no change in cobalt concentration in solution. |
| The adsorption capacity (qt) was used to quantify the removal of cobalt ions from solutions for batch and kinetics experiments, and calculated by Equation 1: |
| qt = (C0 – Ct) V/m (1) |
| where C0 and Ct are the initial concentration and the concentration at time “t” of Cobalt (II), respectively. V is the volume of the suspension (0.5 L) and m is the mass of the adsorbent (1.5 g). |
| Results and Discussion |
| Batch adsorption experiments |
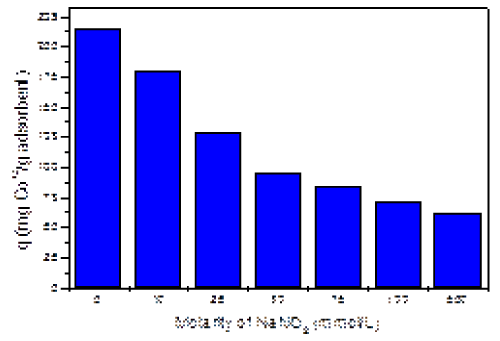
| Figure 2 shows the effect of the NaNO3 on the adsorption of cobalt ions. As indicated by the graph, the adsorption of cobalt decreases with the increase of salt concentration. This could be explained by the ionic competition between positively charged Co+2 and Na+1 ions for the adsorption sites of AB. At high salt concentration, the adsorption of cobalt is almost totally inhibited, reaching its lowest value of 61 mg/g at 500 mM of salt. This negative impact of salinity is stronger at low salt concentration due to the proximity in molar concentration between the heavy metal and NaNO3. However, at high salt molarities, this effect is attenuated; most likely due to the saturation of the adsorbent with the salt and the heavy metal in solution. These results were crucial to determine the working salt concentration for our kinetics experiments. The 10 mM salt concentration was chosen because is closer in molarity to the molar concentration of cobalt (II) ions in the solution. Moreover the changes in adsorption by the addition of 10 mM of NaNO3, caused one of the most drastic decreases in cobalt (II) adsorption, as observed in Figure 2. |
| Kinetics studies |
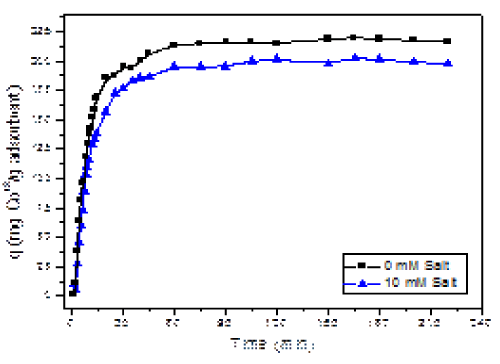
| The most important factor studied in this project was the time that is needed for the adsorbent AB to eliminate the maximum amount of Co (II) ions from the solution. The kinetics results for Cobalt (II) adsorption onto AB in the presence and absence of salt is shown in Figure 3. From our results, the maximum uptake of cobalt metal ions from solutions was achieved during the first 40 minutes at both salt concentrations. However, almost 85% of the cobalt is removed during the first 25 min of the process. The initial uptake happens very rapidly, and then slows down until it achieves the maximum adsorption. This maximum adsorption is the adsorption capacity at equilibrium (qe) and used as a comparison with other adsorbents. |
| Several mathematical theories have been used in the modeling of the adsorption kinetics of pollutants. Amongst them, the most important and commonly used are the pseudo-first order and pseudosecond order kinetics. The purpose of these models is to determine the maximum adsorption capacity at equilibrium (qe) and the adsorption kinetics rate constants (k1 or k2). The importance of the rate constants resides on the fast removal of the pollutant from the solution. A higher k means a faster removal of cobalt from the solution and a better adsorbent. Fast adsorption is attractive to industries as it minimizes operational costs, number of employees and consumed energy. The qe resembles the amount of the metal ion that can be potentially removed by a given adsorbent. For this parameter, a high qe is also associated with the efficiency of the adsorbent. The Adsorption Kinetics models are described below. |
| Pseudo-first order model |
| Lagergren’s first-order rate equation described the adsorption rate on the base of the capacity as described in Equation (2) |
| dq/dt = k1 (qe - q) (2) |
| where q is the metal concentration in solid phase at time t, and qe is the equilibrium metal concentration [7]. |
| In order to differentiate kinetics equations based on concentrations in solution from the ones based on adsorptions capacities of solids, Lagergren’s first-order rate equation has been called pseudo-first order. To apply the first-order kinetics, our equation has to be expressed as linear equation (Equation 3): |
| ln (qe – q) = ln qe – k1 t (3) |
| In order to obtain a good fitting, linear correlation should be observed in the X versus Y plots. X and Y change depending on the kinetics models that are being used. In general, pseudo-first order kinetics does not show a good linear fitting over entire contact time range [7] as observed in Figure 4 (left). When the linearization of the pseudo-first order was fitted to the experimental data, the model only adjusted to the first 25 minutes of the experiments. Only the first 25 minutes were considered for this particular modeling (Figure 4, right), resulting in a good linear correlation for the determination of kinetics parameters. |
| The pseudo-first order linear regression produced the parameters k1 and qe, which are reported in Table 1 in the presence and absence of salt. Important statistical parameters are also included to verify the statistical validity of the modeling. The results indicate a better adsorption in the absence of salt when compared to the 10 mM salt as shown by the qe values. A decrease in only 12% is observed in the presence of salt. This is a very encouraging result as it means that cobalt ions can be removed even in high salinity contaminated waters (i.e. seawater). On the other hand, the adsorption rate also decreases in the presence of salt. This is understandable as ions like Na+ and NO3- are believed to block the adsorption sites of biosorbents and inhibit (or delay) the adsorption of the target heavy metal pollutant [3,7]. Finally, good correlation coefficient (R2) and statistical significance (p-value) indicate the optimum resembling of the data by this kinetics model using a given sample population (n). |
| Pseudo-second order model |
| Pseudo-second order model was derived assuming a second order dependence of the sorption rate on available sites and described by Equation 4: |
| dq/dt = k2( qe – q)2 (4) |
| where a linearized form can be written: (Equation 5) |
| t/q = 1/k2qe 2 + 1/qe * t (5) |
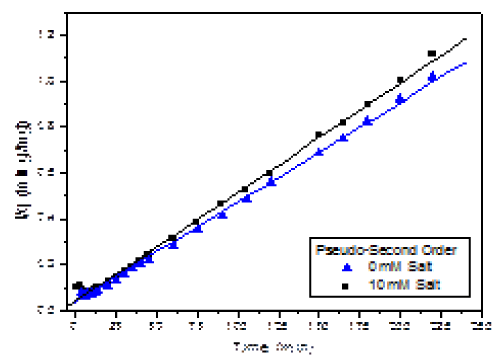
| A t versus t/q plot can be made to describe the linearity of the process (Figure 5). As shown in the graph, all data points adjusted to this model over the entire time range. |
| Pseudo-second order model is generally more appropriate than pseudo-first order model to represent kinetic data of heavy metals biosorption [4,7]. Linear regression indicates an excellent statistical validity for the pseudo-second order kinetics, as shown by the statistical parameters (Table 2). As for the fitting adsorption parameters, the results indicate a reduction on the adsorption (qe) in the presence of 10 mM salt by only 9%. Likewise, the rate constant k2 slightly increased in the presence of salt. |
| The adsorption properties of cobalt ions with AB were compared to previous studies of removal of the same heavy metal with different types of adsorbents. A summarized report is shown in Table 3. As observed, our adsorbent AB displays an outstanding cobalt removal as demonstrated by the qe values. The biomaterial red rose is the closest adsorbent with only half of the adsorption obtained with this research. As expected, inorganic materials like zeolites and other clays report very low cobalt adsorptions. These results are very positive as they demonstrate the applicability and efficiency of alginate beads in the removal of large amounts of cobalt ions from contaminated waters. Hexavalent chromium [15] and cadmium ions [16] have also been adsorbed with novel biomaterials. Yang et al. [16] have recently reported the use of α-ketoglutaric acid-modified magnetic chitosan for the removal of cadmium (II) ions, obtaining an equilibrium adsorption capacity of 72 mg/g, according to the pseudo-second order kinetics. |
| In contrast, our rate constants (k1 and k2) very low values when compared to the bibliography. However, it is important to notice that these other references report fast rate constants not on the fast adsorption kinetics, but because of the low amount of cobalt they are able to remove. In other words, for these adsorbents, it is very easy to reach equilibrium, because they do not have the potential to remove a considerable amount of the pollutant. For our adsorbent AB is takes longer to reach equilibrium because it has the potential of adsorbing up to 230 mg of cobalt ions per gram of adsorbent. |
| Conclusion |
| The potential use of alginate beads as a potential adsorbent of Cobalt (II) metal ion was studied in this project. Our results showed that 229.89 and 211.42 mg of cobalt (II) ions can be removed per gram of adsorbent in the absence and presence of salt, respectively. Kinetics modeling indicates that the adsorption follows the Pseudo-First order kinetics in the first 25 minutes of the process and fits the entire time range (until equilibrium) according to the Pseudo-Order Kinetics. These results were compared to previous studies with the same heavy metal with different adsorbents. Our adsorbent AB showed a better applicability for the removal of Cobalt (II), and can be potentially used as a good adsorbent for future research with different types of heavy metals. Future work includes the preparation of alginate beads columns to study the decontamination of cobalt-containing water in continuous process. |
| Acknowledgements |
| This works was financially supported by the BMCC Faculty Development Grant (to A.N.). The authors would also like to acknowledge the Science Department at BMCC, and LSAMP and CSTEP programs. Michael Gillespie, Helene Bach and Chiu Hong Lee are greatly thanked for their comments and support. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Atmosphere
- Atmospheric Chemistry
- Atmospheric inversions
- Biosphere
- Chemical Oceanography
- Climate Modeling
- Crystallography
- Disaster Science
- Earth Science
- Ecology
- Environmental Degradation
- Gemology
- Geochemistry
- Geochronology
- Geomicrobiology
- Geomorphology
- Geosciences
- Geostatistics
- Glaciology
- Microplastic Pollution
- Mineralogy
- Soil Erosion and Land Degradation
Recommended Journals
Article Tools
Article Usage
- Total views: 14761
- [From(publication date):
October-2014 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 10078
- PDF downloads : 4683
