Research Article Open Access
Adjustment of Matrix-Assisted Laser Desorption/Ionization for Glycolipids
Yusuke Suzuki1*, Akira Okamoto1, Anila Mathew1,2 and Yasunori Kushi1
1College of Science and Technology, Nihon University, Tokyo, Japan
2Anila Mathew’s current address is Bio-Nano Electronics Research Centre, Toyo University, 2100, Kujirai, Kawagoe, Saitama 350-8585, Japan
- *Corresponding Author:
- Yusuke Suzuki
Department of Materials and Applied Chemistry
College of Science and Technology
Nihon University, Chiyoda-ku, Japan
Tel: +81-3-3259-0793
Fax: +81-3-3293-7572
E-mail: suzuki.yuusuke@nihon-u.ac.jp
Received date: July 15, 2015; Accepted date: July 24, 2015; Published date: July 31, 2015
Citation: Suzuki Y, Okamoto A, Mathew A, Kushi Y (2015) Adjustment of Matrix- Assisted Laser Desorption/Ionization for Glycolipids. J Anal Bioanal Tech 6:262 doi:10.4172/2155-9872.1000262
Copyright: © 2015 Suzuki Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Gangliosides isolated from biological sources are usually detected as sodium/potassium adduct ions and these ions are easily fragmented by dissociation of labile glycosidic bonds in the positive ion mode Mass Spectrometry (MS). A large number of conditions for fragment suppression of these acidic glycolipids by using non-acidic matrices or changing metal additions have been demonstrated. Compared to sodium/potassium adduct ions, the cesium adduct ions suppress the fragmentation of these acidic glycolipids. On the contrary, lithium adduct ions induce the fragmentation, but generate more informative fragment ions of glycolipids than other alkali metal adduct ions in post-source decay, MS/MS as well as MS spectra. To suppress the fragmentation of labile glycosidic bond and generate more informative fragmentation, we have examined and established the optimal condition for the detection of [M+Li]+ of gangliosides (GM3 and GM2) using the matrix-assisted laser desorption/ionization time-of-flight MS by adjusting matrix and alkali metal salt combinations and concentrations.
Keywords
Ganglioside; MALDI-TOF MS; Alkali metal adduct ion; MS/MS
Abbreviations
MALDI-TOF MS: Matrix-Assisted Laser Desorption/Ionization Time-Of-Flight Mass Spectrometry; GSL: Glycosphingolipid; DHB: 2,5-Dihydroxybenzoic Acid
Introduction
Gangliosides are sialic acid-containing glycosphingolipids, which are components of plasma membrane. It is well known that gangliosides/glycophospholipids play important roles in a wide variety of cellular functions, including cell-cell interactions, cell growth and differentiation, and signaling [1-3]. A detailed structural characterization is required to resolve their functions. A number of analytical methods are available for the characterization of the gangliosides/glycophospholipids. Matrix-Assisted Laser Desorption/ Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS) is one of the indispensable tools for the rapid structural characterization [4,5]. In the positive ion mode MS spectra of biological samples, sodium/potassium adduct (Na+/K+) ions are usually detected and the labile glycosidic/phosphate bonds are easily fragmented [5]. Since the detection of molecular ions is required for the determination of whole molecular weight and simple interpretation of MS pattern, the suppression of the acidic glycolipid fragments are important and necessary. Furthermore, informative Na+/K+ fragments are often hardly obtained by further mass analysis, such as Post-Source Decay (PSD) or MS/MS. Therefore, the detailed structural information of glycolipids requires fragment acceleration. As described above, an understanding of the reciprocal events between fragment suppression and acceleration is necessary for characterization of glycolipids.
In the MS spectra, the stabilities and properties of ions are drastically changed by alkali metal species, and the cationization is easily changed by the addition of alkali metal salts to the sample. Lithium salts accelerate fragmentation of the labile compound, and hence the relative intensities of molecular ions are decreased in the MS spectra. However, lithium adduct (Li+) ions of these gangliosides or phospholipids generate more informative fragment ions than other alkali metal ions in the MS/MS [6-10]. On the other hand, cesium salts suppress fragmentation of the labile compound, and the relative intensities of molecular ions are increased in the MS spectra. However, cesium adduct (Cs+) ions do not generate sufficient fragment ions for characterization of the acidic glycolipids in MS/MS compared to other alkali metal ions [11]. As described above, molecular ions could be obtained by changing the molecular ions from Na+/K+ to Cs+ adducts for MS analysis, and detailed fragment ions could be obtained by changing molecular ions to Li+ adducts for MS/MS analysis. Li+ and Cs+ have compatible properties, and combining the advantageous properties of lithium and cesium salts helps in controlling the ionizing condition of these glycolipids.
To suppress the fragmentation of labile compounds, the use of various kinds of matrices are also reported including non-acidic and liquid matrices for MALDI-TOF MS analysis [12-17]. So far, 2,5-Dihydroxybenzoic Acid (DHB) is the most widely used matrix for glycoconjugate analysis. Compared to the other common matrices such as α-cyano-4-hydroxycinnamic acid (CHCA) and sinapinic acid, DHB allows softer desorption. Under 337 nm radiation, non-acidic matrices such as 2-amino-3-hydroxypyridine, norharman, 6-aza-2- thiothymine, and 2'4'6'-trihydroxyacetophenone, were used for the fragment suppression of acid-labile compounds like synthetic polymer, acidic oligosaccharide, glycopeptides, and other glycoconjugates [18]. Furthermore, liquid matrix of DHB with butylamine (DHBB) suppressed neuraminic acid fragmentation of monosialylmonofucosyl- lacto-N-neohexaose, ganglioside, and 3'-sialyllactose [16]. Liquid matrix of 2-(4-hydroxyphenylazo)benzoic acid (HABA) with 1,1,3,3-tetramethylguanidine and spermine were used for heparin sulfate [17]. Recently, Liang et al., showed that frozen solution of aqueous acetonitrile containing oligosaccharides and DHB generates more oligosaccharide ions and less fragments from PSD spectra [19].
As described above, a number of trials for regulating the fragment pattern of labile compounds including gangliosides using alkalimetal addition and non-acidic/liquid matrix have been reported. The fragment patterns were regulated by the species and concentrations of matrices and alkali-metal salts. In this study, we established an optimum condition for the analysis of GM3 and GM2 gangliosides using alkalimetal salts and non-acidic matrices.
Materials and Methods
GM3 (Neu5Ac 2-3Gal 1-4Glc 1-1'Cer) prepared from bovine brain was purchased from HyTest, Ltd. (Turku, Finland). Lithium chloride and cesium chloride were purchased from Nacalai tesque, Inc. (Kyoto, Japan). 2,5-Dihydroxy benzoic acid was purchased from Bruker Daltonics (Leipzig, Germany).
MALDI-TOF MS analysis of ganglioside GM3
MALDI-TOF MS was performed on a Voyager RP-PRO MALDITOF mass spectrometer (Life Tech., California, USA) equipped with a 337 nm nitrogen laser. MS spectra was calibrated externally using a peptide calibration standard mixture containing bradykinin ([M+H]+, 757.40), angiotensin II ([M+H]+, 1045.64), and human adrenocorticotrophic hormone (ACTH, fragments 18-39) ([M+H]+, 2465.20) as 10 pmol/μL solutions. The ganglioside GM3 were dissolved in chloroform/methanol (C/M, 1:1, v/v) as 10 mg/mL solutions. The matrices used in this study were dissolved in water at concentrations of 10 mg/mL. The alkali metal salts were also dissolved in water at various concentrations. The matrix, alkali metal salts, and GM3 or GM2 were mixed in a glass tube and placed on the target plate for crystallization. Crystallization was accelerated by a gentle stream of cold air.
Results and Discussion
Confirmation of MALDI-TOF MS spectrum of ganglioside GM3
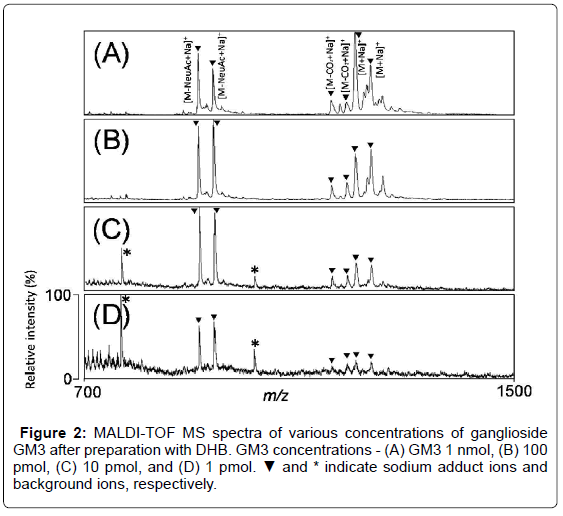
Figure 1 shows the structures of matrices used in this study. To determine the optimal condition for ganglioside analysis, various concentrations of GM3 (1 nmol, 100 pmol, 10 pmol, and 1 pmol) in DHB matrix were analyzed by MALDI-TOF MS in positive ion mode (Figure 2A-2D). In the MS spectra, the molecular ions were detected at m/z 1205 and 1233, and the ions derived from loss of sialic acid from GM3 were detected as sodium adducts at m/z 913 and 941. The ions derived by the loss of carbon dioxide from GM3 were also detected as sodium adducts at m/z 1161 and 1189. On the other hand, the background peaks at m/z 768 and 1017 were detected, and poor signal to noise ratios were observed in the lower concentrations (1 and 10 pmol) of GM3 were observed (Figure 2C and 2D). Therefore, further experiments were carried out using 100 pmol of GM3.
Effect of alkali metal salt on MALDI-TOF MS pattern of GM3
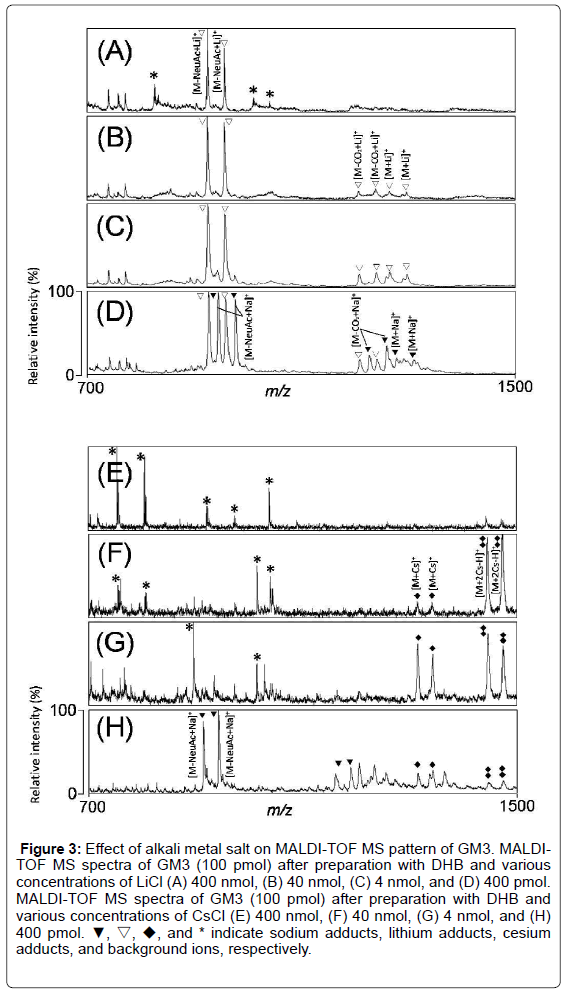
To confirm the effect of alkali metal salts on MALDI-TOF MS pattern of GM3, we analyzed MALDI-TOF MS spectra of GM3 after preparation with DHB and various concentrations of lithium salts (400 nmol, 40 nmol, 4 nmol, and 400 pmol) (Figure 3A-3D). In the MS spectra, the molecular ions were detected at m/z 1194 and 1221 (Figure 3B and 3C), and the ions derived from loss of sialic acid from GM3 were detected as lithium adducts at m/z 897 and 925 (Figure 3A-3D). The ions derived by the loss of carbon dioxide from GM3 were also detected at m/z 1144 and 1172 as lithium adducts. Furthermore, the sodium adduct ions were detected at m/z 912, 940, 1160, and 1188 at lower concentration of LiCl (400 pmol) (Figure 3D).
Figure 3: Effect of alkali metal salt on MALDI-TOF MS pattern of GM3. MALDITOF MS spectra of GM3 (100 pmol) after preparation with DHB and various concentrations of LiCl (A) 400 nmol, (B) 40 nmol, (C) 4 nmol, and (D) 400 pmol. MALDI-TOF MS spectra of GM3 (100 pmol) after preparation with DHB and various concentrations of CsCl (E) 400 nmol, (F) 40 nmol, (G) 4 nmol, and (H) 400 pmol. ▼, ▽, ◆, and * indicate sodium adducts, lithium adducts, cesium adducts, and background ions, respectively.
Next, we analyzed MALDI-TOF MS spectra of GM3 after preparation with DHB and various concentrations of cesium salts (400 nmol, 40 nmol, 4 nmol, and 400 pmol) (Figure 3E-3H). In the MS spectra, the molecular ions were detected as cesium adducts at m/z 1315 and 1343 and as double cesium adducts at m/z 1446 and 1474 (Figure 3F and 3G). The sodium adduct ions were detected at m/z 912, 940, 1160, 1188, 1205, and 1233 at lower concentration of CsCl (400 pmol) (Figure 3H). The ratios of cationization changed from sodium to single and/or double cesium adducts in a CsCl concentrationdependent manner (Figure 3E-3H). Although the detection of GM3 derived ions were suppressed at high concentration of CsCl (400 nmol), the ions derived from loss of sialic acid or carbon dioxide from GM3 were almost suppressed at high concentration of CsCl (Figure 3E-3G).
These results indicate that lithium ions induce and cesium ions suppress the fragmentation of gangliosides, compared to sodium/ potassium adduct ions which are usually detected in MALDI-TOF MS, as reported previously. Therefore, we concluded that the optimal concentration of these salts were few nmols for changing cationization.
Effect of matrices on MALDI-TOF MS pattern of GM3
Non-acidic/liquid matrices are well known to suppress fragmentation of gangliosides [11-17, 20]. We analyzed MALDI-TOF MS spectra of GM3 after preparation with various matrices having both acidic and non-acidic properties (Figure 4). Although GM3 derived ions were detected in all MALDI-TOF MS spectra, the ratio of molecular ions to N-acetylneuraminic acid (NeuAc)-dissociated ions was higher after preparation with Norharman, than with other matrices. Therefore, we compared the MS spectra of GM3 after preparation with DHB and Norharman for further analyses.
Combinational effect of matrices and alkali metal salts on MALDI-TOF MS pattern of GM3
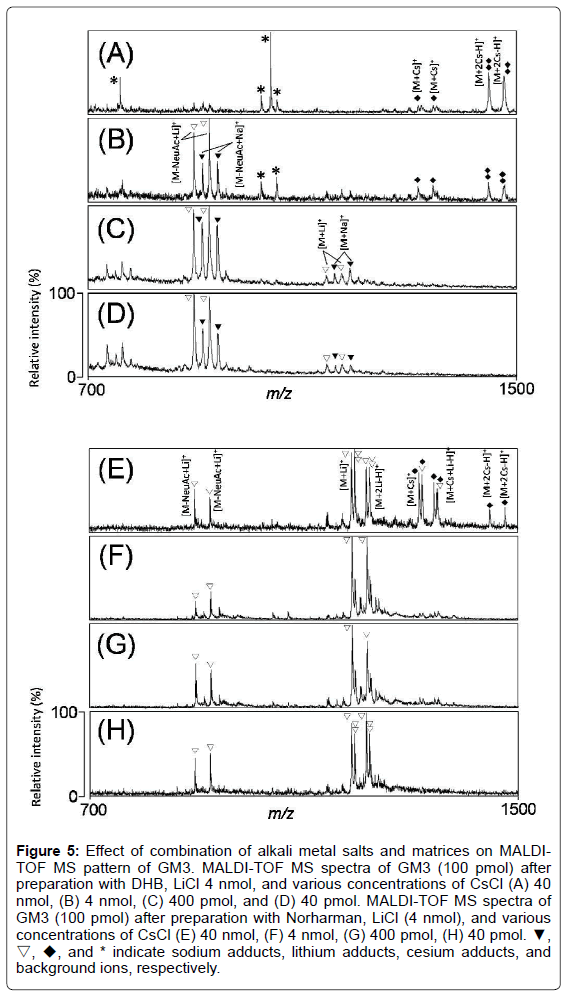
We analyzed MALDI-TOF MS spectra of GM3 after preparation with DHB and 4 nmol of LiCl, and various concentrations of CsCl. The GM3 derived ions were detected as single or double cesium adducts as well as sodium adducts under high concentration of CsCl (400 nmol) (Figure 5A). On the other hand, the GM3 derived ions were detected as sodium and lithium adducts under low concentrations of CsCl (40, 4 nmol, or 400 pmol) (Figure 5B-5D). After preparation with DHB, LiCl, and CsCl, fragmentation of GM3 was not suppressed, and the lithiated molecular ions of GM3 ([M+Li]+) could not be detected (Figure 5A- 5D).
Figure 5: Effect of combination of alkali metal salts and matrices on MALDITOF MS pattern of GM3. MALDI-TOF MS spectra of GM3 (100 pmol) after preparation with DHB, LiCl 4 nmol, and various concentrations of CsCl (A) 40 nmol, (B) 4 nmol, (C) 400 pmol, and (D) 40 pmol. MALDI-TOF MS spectra of GM3 (100 pmol) after preparation with Norharman, LiCl (4 nmol), and various concentrations of CsCl (E) 40 nmol, (F) 4 nmol, (G) 400 pmol, (H) 40 pmol. ▼, ▽, ◆, and * indicate sodium adducts, lithium adducts, cesium adducts, and background ions, respectively.
Norharman do not contain any acidic functional group, and is a more non-acidic matrix than DHB. Therefore, Norharman can be used for acid-labile samples and fragment suppression of sialic acid. We analyzed MALDI-TOF MS spectra of GM3 after preparation with Norharman and 4 nmol of LiCl, and various concentrations of CsCl. Although single and double cesiated molecular ions of GM3 were detected under high concentration of CsCl (400 nmol) (Figure 5E), lithiated molecular ions of GM3 were detected (Figure 5F-5H). Furthermore, NeuAc-dissociated ions were remarkably suppressed (Figure 5E-5H). These results indicated that the optimal analytical conditions for gangliosides using Norharman, LiCl, and CsCl inhibit dissociation of sialic acid, while allowing the detection of lithiated molecular ions of GM3.
MALDI-TOF MS analysis of GM2 using DHB combinational conditions of Norharman, LiCl, and CsCl
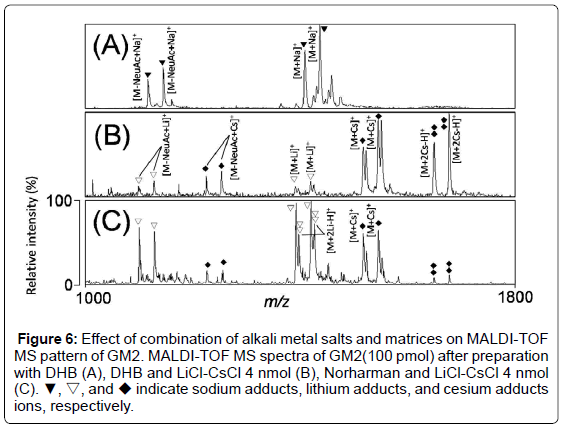
To confirm whether the established method can be applied for other ganglioside analyses, we analyzed MALDI-TOF MS spectra of GM2, which has a more complicated structure than GM3. In the MS spectrum with DHB, molecular ions of GM2 were detected at m/z 1408 and 1436, and the ions derived from loss of sialic acid from GM2 were detected at m/z 1115 and 1143 as sodium adducts (Figure 6A). Next, we analyzed MALDI-TOF MS spectra of GM2 after preparation with DHB and 4 nmol of LiCl-CsCl. The molecular ions of GM2 were detected as single or double cesium adducts at m/z 1517 and 1545, or at m/z 1649 and 1677 (Figure 6B). However, the low relative ratio of [M+Li]+ derived from GM2 was observed (Figure 6B). We analyzed MALDITOF MS spectra of GM2 after preparation with Norharman and 4 nmol of LiCl-CsCl. Although [M+Cs]+ or loss of sialic acid of GM2 as lithium adducts were detected, [M+Li]+ were detected as the major peaks (Figure 6C).
Figure 6: Effect of combination of alkali metal salts and matrices on MALDI-TOF MS pattern of GM2. MALDI-TOF MS spectra of GM2(100 pmol) after preparation with DHB (A), DHB and LiCl-CsCl 4 nmol (B), Norharman and LiCl-CsCl 4 nmol (C). ▼, ▽, and ◆ indicate sodium adducts, lithium adducts, and cesium adducts ions, respectively.
These results indicated that, our approach of adjusting the matrix, and alkali metal salt combinations and concentrations are powerful tools for changing the alkali metal cation species in gangliosides during analysis by MALDI-TOF MS.
Alkali metal cations were investigated for their effectiveness in glycolipid analysis by MALDI-TOF MS [5-8]. The fragmentation yields for oligosaccharides or glycolipids were found to be related to alkali metal ion size and electrostatic charge density. Li adducts increase fragmentations of labile glycosidic bonds as well as ceramide moieties of glycolipids [5,8,10,21]. Cs adducts decrease fragmentations of glycolipids, therefore molecular ions can be detected in the MS spectra [11,18]. Although the fragmentation of sialic acid is not efficiently suppressed, the molecular ions of GM3 were changed from Na+ to Cs+ after preparation with Li/Cs alkali metal salts and DHB. Finally, the conditions for fragment suppression of sialic acid and detection of [M+Li]+ ions were established by the combinations of these alkali salts and Norharman.
Recently, new types of mass spectrometries, such as MALDI ionmobility MS, have been developed and they have become powerful tools for the ganglioside analyses [22]. Our established method is quite simple and easily adjustable; therefore it is also thought to be useful for the analysis of other glycolipids as well as gangliosides by regardless of mass types. Furthermore, 2,6-dihydroxyacetophenone (DHAP) has been reported as a new matrix for acidic glycolipid analysis [23]. In our preliminary data, we confirmed the fragmentation suppression of ganglioside GM3 using DHAP more effectively than Norharman, but the cesium adducted molecular ions were detected as the major peaks under the established condition. In the future, we will examine the applicability of this established method in the analysis of other acidic glycolipids, and will confirm the optimal condition of DHAP preparation and effectiveness of liquid matrices on the glycolipid analysis using this method.
In this study, we established the optimal condition for the detection of [M+Li]+ of gangliosides (GM3 and GM2) using the MALDITOF MS by adjusting matrix and alkali metal salt combinations and concentrations. This is the first study reporting a quite simple method for the detection of lithiated molecular ion of ganglioside in the positive ion MS. In the near future, we would expect that more detailed information of ganglioside is determined by MS/MS with selection of [M+Li]+ . Furthermore, the optimization of ionization and Cs/Li salt combinations is quite simple and easily adjustable method, as shown in this manuscript. Therefore, we also expect that the method would be useful to ganglioside as well as other glycolipid analyses even using other mass spectrometries.
Acknowledgement
This work was supported by Nihon University College of Science and Technology Grant-in Aid for Fundamental Science Research (YS), and partly supported by Grant-in-Aid for Young Scientists (B) No. 24750164 from MEXT Japan (YS). The authors declare no conflicts of interest.
References
- Murate M, Hayakawa T, Ishii K, Inadome H, Greimel P, et al. (2010) Phosphatidylglucoside forms specific lipid domains on the outer leaflet of the plasma membrane. Biochemistry 49: 4732-4739.
- Yu RK, Suzuki Y, Yanagisawa M (2010) Membrane glycolipids in stem cells. FEBS Lett 584: 1694-1699.
- Nozaki H,Itonori S, Sugita M, Nakamura K, Ohba K, et al. (2010) Invariant Valpha14 natural killer T cell activation by edible mushroom acidic glycosphingolipids. Biol Pharm Bull 33: 580-584.
- Jackson SN,Colsch B, Egan T, Lewis EK, Schultz JA, et al. (2011) Gangliosides' analysis by MALDI-ion mobility MS. Analyst 136: 463-466.
- Suzuki Y, Suzuki M, Ito E, Goto-Inoue N, Miseki K, et al. (2006) Convenient structural analysis of glycosphingolipids using MALDI-QIT-TOF mass spectrometry with increased laser power and cooling gas flow. J Biochem 139: 771-777.
- Arigi E, Singh S, Kahlili AH, Winter HC, Goldstein IJ, et al. (2007) Characterization of neutral and acidic glycosphingolipids from the lectin-producing mushroom, Polyporussquamosus. Glycobiology 17: 754-766.
- Calvano CD, De Ceglie C, Aresta A, Facchini LA, Zambonin CG (2013) MALDI-TOF mass spectrometric determination of intact phospholipids as markers of illegal bovine milk adulteration of high-quality milk. Anal BioanalChem 405: 1641-1649.
- Hsu FF, Turk J (2001) Structural determination of glycosphingolipids as lithiated adducts by electrospray ionization mass spectrometry using low-energy collisional-activated dissociation on a triple stage quadrupole instrument. J Am Soc Mass Spectrom 12: 61-79.
- Hsu FF, Turk J (2010) Electrospray ionization multiple-stage linear ion-trap mass spectrometry for structural elucidation of triacylglycerols: assignment of fatty acyl groups on the glycerol backbone and location of double bonds. J Am Soc Mass Spectrom21: 657-669.
- Hsu FF, Turk J, Stewart ME, Downing DT (2002) Structural studies on ceramides as lithiated adducts by low energy collisional-activated dissociation tandem mass spectrometry with electrospray ionization. J Am Soc Mass Spectrom 13: 680-695.
- Griffiths RL, Bunch J (2012) A survey of useful salt additives in matrix-assisted laser desorption/ionization mass spectrometry and tandem mass spectrometry of lipids: introducing nitrates for improved analysis. Rapid Commun Mass Spectrom 26: 1557-1566.
- Giménez E,Benavente F, Barbosa J, Sanz-Nebot V (2010) Ionic liquid matrices for MALDI-TOF-MS analysis of intact glycoproteins. Anal BioanalChem 398: 357-365.
- Laremore TN,Murugesan S, Park TJ, Avci FY, Zagorevski DV, et al. (2006) Matrix-assisted laser desorption/ionization mass spectrometric analysis of uncomplexed highly sulfated oligosaccharides using ionic liquid matrices. Anal Chem 78: 1774-1779.
- Laremore TN, Zhang F, Linhardt RJ (2007) Ionic liquid matrix for direct UV-MALDI-TOF-MS analysis of dermatan sulfate and chondroitin sulfate oligosaccharides. Anal Chem 79: 1604-1610.
- Li YL, Gross ML (2004) Ionic-liquid matrices for quantitative analysis by MALDI-TOF mass spectrometry. J Am Soc Mass Spectrom 15: 1833-1837.
- Mank M, Stahl B, Boehm G (2004) 2,5-Dihydroxybenzoic acid butylamine and other ionic liquid matrixes for enhanced MALDI-MS analysis of biomolecules. Anal Chem 76: 2938-2950.
- Przybylski C,Gonnet F, Bonnaffé D, Hersant Y, Lortat-Jacob H, et al. (2010) HABA-based ionic liquid matrices for UV-MALDI-MS analysis of heparin and heparan sulfate oligosaccharides. Glycobiology 20: 224-234.
- Rashidzadeh H, Wang Y, Guo B (2000) Matrix effects on selectivities of poly(ethylene glycol)s for alkali metal ion complexation in matrix-assisted laser desorption/ionization. Rapid Commun Mass Spectrom 14: 439-443.
- Liang CW, Chang PJ, Lin YJ, Lee YT, Ni CK (2012) High ion yields of carbohydrates from frozen solution by UV-MALDI. Anal Chem 84: 3493-3499.
- Bonnel D, Franck J, Mériaux C, Salzet M, Fournier I (2013) Ionic matrices pre-spotted matrix-assisted laser desorption/ionization plates for patient maker following in course of treatment, drug titration, and MALDI mass spectrometry imaging. Anal Biochem 434: 187-198.
- Levery SB, Toledo MS, Doong RL, Straus AH, Takahashi HK (2000) Comparative analysis of ceramide structural modification found in fungal cerebrosides by electrospray tandem mass spectrometry with low energy collision-induced dissociation of Li+ adduct ions. Rapid Commun Mass Spectrom 14: 551-563.
- Laremore TN,Linhardt RJ (2007) Improved matrix-assisted laser desorption/ionization mass spectrometric detection of glycosaminoglycan disaccharides as cesium salts. Rapid Commun Mass Spectrom 21: 1315-1320.
- Colsch B, Woods AS (2010) Localization and imaging of sialylatedglycosphingolipids in brain tissue sections by MALDI mass spectrometry. Glycobiology 20: 661-667.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15234
- [From(publication date):
October-2015 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 10624
- PDF downloads : 4610