Research Article Open Access
Adiponectin and Resistin Levels in Umbilical Serum of Term Neonatesand Relation to Birth Weight
Saad Mohamed1, Ahmad El-Askary2* and Alaa Megahed31Department of Pediatrics, Al-Azhar University, Egypt
2Department of Medical Biochemistry, Al-Azhar University, Egypt & College of Applied Medical Sciences, Taif University, KSA
3Departments of Obstetrics and Gynecology, Al-Azhar University, Egypt
- *Corresponding Author:
- Ahmad El-Askary
Department of Medical Biochemistry
Al-Azhar University
Egypt & College of Applied Medical Sciences
Taif University, KSA
Tel: 00201010011697
E-mail: ahmadelaskary3@gmail.com
Received Date: April 29, 2017; Accepted Date: May 29, 2017; Published Date: June 2, 2017
Citation: Mohamed S, El-Askary A, Megahed A (2017) Adiponectin and Resistin Levels in Umbilical Serum of Term Neonates and Relation to Birth Weight. Neonat Pediatr Med 3: 127. doi:10.4172/2572-4983.1000127
Copyright: © 2017 Mohamed S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Neonatal and Pediatric Medicine
Abstract
Background: Optimal birth weight is an important factor for the future health of the newborns. Aberrations in fetal growth are associated with adverse health effects both in early life and in late childhood and adulthood. Fetal growth is controlled by both maternal hormones and nutrition. Adipokines, including resistin and adiponectin are known regulators of energy metabolism; although their role in the regulation of fetal growth still poorly understood. Objective: The aim of the present study was to evaluate the relationship between adiponectin and resistin with abnormalities of neonatal birth weight and to identify the correlation between these proteins and various maternal and neonatal factors. Patients and Methods: Comparative controlled study included 120 full term newborns recruited from Al-Azhar University Hospital (New Damietta), during the period from January 2016 to February 2017. Included newborns were divided into 3 groups; group 1) 40 small for gestational age (SGA) newborns, 2) 40 large for gestational age (LGA) newborns, and group 3) 40 apparently healthy appropriate for gestational age (AGA) newborns, were selected randomly. Serum umbilical cord adiponectin and resistin were measured by ELISA. Results: There was no significant difference between groups as regard to maternal age (P: 0.797), parity (P: 0.77), gestational age (P: 0.528) and BMI (P: 0.091). Umbilical cord resistin and adiponectin were significantly lower among LGA group (resistin: 16.9 ± 1.92 ng/ml; adiponectin: 6.74 ± 2.23 ᶙg/ml), and significantly elevated among SGA group (resistin: 23.03 ± 3.97 ng/ml; adiponectin: 14.92 ± 3.19 ᶙg/ml) than AGA group (resistin: 17.98 ± 1.89 ng/ml; adiponectin: 11.04 ± 1.91 ᶙg/ml; P
Keywords
Resistin; Adiponectin; Cord blood; Neonates; Small for gestational age; Insulin; Birth weight; Fetal macrosomia
Introduction
Optimal fetal growth is considered a corner stone in determining life expectancy, while growth aberrations may have implications for disease risk across the lifespan [1]. Abnormal fetal growth, both restriction and overgrowth, is associated with fetal, infant, and child mortality and morbidity [2,3].
Fetal growth restriction increases the risks of intrauterine demise, neonatal morbidity, and neonatal death [4]. Furthermore, growthrestricted fetuses are predisposed to the development of cognitive delay in childhood and diseases in adulthood [5].
Fetal macrosomia is associated with increased mortality, nerve injuries, fractures and birth asphyxia [6]. In the long term, infants who are LGA are at higher risk of cardiovascular and metabolic complications in adulthood [7].
Intrauterine growth depends mainly on trans-placental supply of nutrients and the hormonal environment of the fetus [8]. Fetal hormones control the passage of nutrients between fetal oxidative metabolism, mass accumulation and tissue differentiation. It also controls fetal growth through its action on the placenta [9,10].
Adiponectin and resistin are adipocyte-secreted hormones with suggested roles in energy and glucose homeostasis. They also implicated in the pathophysiology of obesity-related insulin resistance [11]. However, their specific role during fetal and neonatal periods is under-investigated [12].
Adiponectin is a protein consisting of 247 amino acids, including the N-terminal hypervariable region and a C-terminal like globular domain [13]. Several studies have demonstrated that adiponectin plays a role in regulating fetal growth by modulating placental nutrient transport [14-16]. It prevents insulin-stimulated amino acid uptake in cultured primary human trophoblast cells by modulating insulin receptor substrate phosphorylation [17]. Furthermore, adiponectin expression in the placenta is related to maternal blood glucose across the range from normal to pathological [18], indicating a role in regulating placental transport and growth of the fetus in relation to maternal nutrient status [19].
Resistin is a recently discovered protein that is secreted by adipocytes and can exert pleiotropic biological effects through endocrine, paracrine and autocrine mechanisms [20]. It is also secreted from human placenta and is supposed to act as energy source in fetal metabolism [21]. Resistin was detected with high levels in cord blood during gestation consisting with a regulatory action on tissue differentiation and fetal growth [22].
The factors controlling intrauterine growth are important in early life programming of health in later life [23]; in addition, the mechanisms of fetal weight regulation during pregnancy remain poorly understood [9]. Therefore, we conducted this comparative controlled study to highlight the possible role of adipokines in regulating fetal growth, which might help us better understanding the pathophysiology of fetal growth aberrations and the associated morbidities.
At present, little information is available with respect to adiponectin and resistin in newborn birth weight. Thus, the aim of this work is to study the relationship between adiponectin and resistin with the occurrence of fetal macrosomia and fetal growth restriction and to identify the correlation between these proteins and various maternal and neonatal factors.
Materials and Methods
Design and setting
The present study included 120 full term (gestational age ≥37 weeks) newborns recruited from obstetric department at Al-Azhar University Hospital (New Damietta), during the period from January 2016 to February 2017. Included newborns were divided into 3 groups; group 1) 40 newborns were small for gestational age (SGA), having birth weight below the 10th percentile for gestational age, group 2) 40 newborns were large for gestational age (LGA), with birth weight less than the 90th percentile for gestational age, and group 3) 40 apparently healthy appropriate for gestational age (AGA) newborns, were selected randomly with birth weight between 10th and 90th percentiles.
Exclusion criteria included neonates with major congenital malformations, presence of symptoms or signs suggestive of congenital infection (microcephaly, hepatosplenomegaly, cataract, etc.) and suspected chromosomal disorders. Other exclusion criteria were: maternal diabetes, history of maternal treatment with corticosteroids in recent pregnancy, documented chorioamnionitis, and premature rupture of membranes more than 18 hours before delivery.
Maternal age, parity and mode of delivery were reported. Maternal BMI was calculated by dividing the weight in kg by the square height in meters. Gestational age was determined based on the last menstrual period, the first trimester ultrasound examination and Ballard scoring. Neonatal anthropometric measurements (weight, length head circumference) were evaluated by an expert physician.
Blood samples (5 ml of cord blood) were collected from the umbilical vein after delivery of the infant. Blood samples were centrifuged and aliquoted, and frozen at -80°C until analysis. The adiponectin in the serum was determined by a commercially available ELISA (R&D Systems, Wiesbaden, Germany). The measurement of resistin in cord blood was performed by Bender Med systems (Vienna, Austria) and by using primary and secondary antibodies by standard ELISA method.
The study was approved by the local ethical committee. Written informed consent was obtained from parents.
Statistical Analysis
Data were analyzed using the SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). Quantitative data were expressed as the mean ± standard deviation (SD). For comparisons of data, the Student’s t test was used to compare between two means and one way analysis of variance (ANOVA) to compare more than two means.
Qualitative data were presented as relative frequency and percent distribution. For comparison between groups, Chi square test was used. Pearson correlation coefficient was performed to correlate different variables. For all tests, significance was considered if p<0.05.
Results
General characters of studied cases
There was no significant difference between groups as regard gestational age (P: 0.3), maternal age (P: 0.75), parity (P: 0.48), and BMI (P: 0.09). Also, no significant difference between groups as regard mode of delivery (P: 0.82), neonatal gender (P: 0.37). Birth weight, length and head circumference are demonstrated in Table 1.
| Variable | SGA (n=40) | AGA (n=40) | LGA (n=40) | P value | |||
|---|---|---|---|---|---|---|---|
| All groups | LGA vs. AGA | SGA vs. AGA | |||||
| Maternal age | 25.75 ± 3.1 | 26.23 ± 3.16 | 26 ± 3.17 | 0.797 | 0.5 | 0.75 | |
| Parity | Primipara | 15 (37.5%) | 12 (30%) | 13 (32.5%) | 0.77 | 0.81 | 0.48 |
| Multipara | 25 (62.5%) | 28 (70%) | 27 (67.5%) | ||||
| Maternal BMI | 27.32 ± 3.66 | 27.66 ± 4.17 | 29.2 ± 4.41 | 0.091 | 0.7 | 0.11 | |
| Gestational age (weeks) | 38.05 ± 0.99 | 38.25 ± 1.15 | 38 ± 0.98 | 0.528 | 0.41 | 0.3 | |
| Sex | Male | 18 (45%) | 22 55%) | 23 (57.5%) | 0.49 | 0.82 | 0.37 |
| Female | 22 (55%) | 18 (45%) | 17 (42.5%) | ||||
| Mode of delivery | NVD | 16 (40%) | 17 (42.5%) | 12 (30%) | 0.47 | 0.24 | 0.82 |
| CS | 24 (60%) | 23 (57.5%) | 28 (70%) | ||||
| Birth weight (grams) | 2045 ± 239 | 3118 ± 364 | 3673 ± 153 | <0.001 | <0.001 | <0.001 | |
| Length | 44.28 ± 1.83 | 48.59 ± 2.04 | 50.49 ± 1. 32 | <0.001 | <0.001 | <0.001 | |
| Head circumference | 30.98 ± 0.92 | 33.38 ± 1.08 | 34.56 ± 0.77 | <0.001 | <0.001 | <0.001 | |
Table 1: Characteristics of mothers and neonates in studied groups.
Umbilical serum adiponectin and resistin levels in studied groups
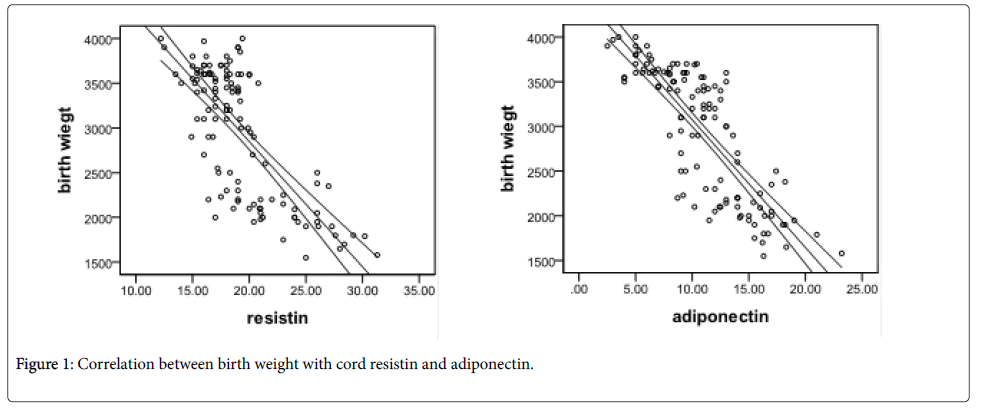
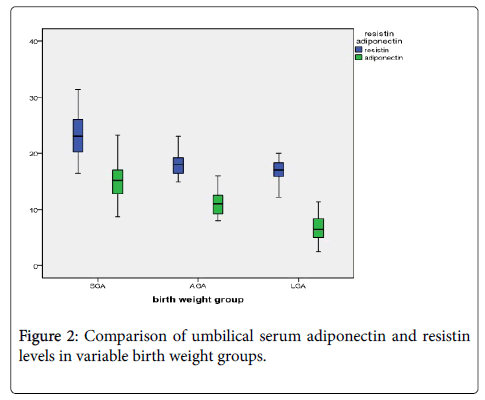
Umbilical serum adiponectin levels were significantly lower among LGA newborns than AGA newborns (6.74 ± 2.23 versus 11.04 ± 1.91 แถ?g⁄mL; P<0.001), but elevated in SGA newborns compared with that in control newborns (14.92 ± 3.19 versus 11.04 ± 1.91 แถ?g⁄mL; P<0.001). Similarly, umbilical resistin levels were diminished in macrosomic newborns than that in control newborns (16.9 ± 1.92 versus 17.98 ± 1.89 ng⁄ml; P<0.001), but significantly higher in FGR newborns than that in control newborns (23.03 ± 3.97 versus 17.98 ± 1.89 ng ⁄ml; P<0.001) as shown in Table 2 and Figure 1.
| Variable | SGA (n=40) | AGA (n=40) | LGA (n=40) | P values | ||
|---|---|---|---|---|---|---|
| All groups | LGA vs. AGA | SGA vs. AGA | ||||
| Resistin (ng/ml) | 23.03 ± 3.97 | 17.98 ± 1.89 | 16.9 ± 1.92 | <0.001 | 0.013 | <0.001 |
| Adiponectin (แถ?g/ml) | 14.92 ± 3.19 | 11.04 ± 1.91 | 6.74 ± 2.23 | <0.001 | <0.001 | <0.001 |
Table 2: Resistin and Adiponectin among studied groups.
Correlation of cord resistin and adiponectin with various parameters
Umbilical adiponectin and resistin were negatively correlated with birth weight (Figure 2), length and head circumference (P<0.001). No significant correlation found with maternal age or gestational age. Adiponectin had significant negative correlation with maternal BMI (P: 0.029) (Table 3).
| Variables | Cord resistin | Cord adiponectin | ||
|---|---|---|---|---|
| r | p | R | P | |
| Maternal age | 0.003 | 0.97 | 0.015 | 0.87 |
| Maternal BMI | -0.083 | 0.37 | -0.2 | 0.029 |
| Gestational age | -0.17 | 0.07 | -0.17 | 0.07 |
| Birth weight | -0.74 | <0.001 | -0.82 | <0.001 |
| Length | -0.57 | <0.001 | -0.67 | <0.001 |
| Head circumference | -0.66 | <0.001 | -0.71 | <0.001 |
Table 3: Correlation of cord resistin and adiponectin with various parameters.
Discussion
Adiponectin is a member of the growing group of adipose secreted proteins, sometimes described as adipocytokines [24]. Resistin is an adipocyte-derived peptide, which is involved in glucose metabolism, by increasing insulin resistance [22]. Changes in the maternal hormones can affect fetal growth, which may result in increased susceptibility to diseases in postnatal life [9].
The present study demonstrated that adiponectin and resistin levels of umbilical serum were lower in the LGA group than in the control group, but higher in SGA group than in the control group.
Maternal hormones can affect fetal growth by altering placental function [10]. Maternal IGF-I promotes placental nutrient uptake and transport [25]. In human placenta, IGF-I increases GLUT1 expression [26] and stimulates glucose and system A-mediated AA uptake [27,28]. Also, IGF-I receptor protein levels were reduced in IUGR [29] and elevated in pregnancies complicated by macrosomia [30].
Adiponectin might inhibit insulin-stimulated AA transport [31-33]; while leptin stimulate placental system A activity [34,35].
In pregnancy, the role of resistin has yet to be determined. Several studies have reported that resistin is generated in the placenta, and its levels are increased during pregnancy [36]. Di Simone, et al. found that resistin can induce the production of vascular endothelial cell growth factor and assumed that it may modulate the invasive behavior of the trophoblast and the proliferation of the feto-placental vascular system [37].
Few studies examined the relation between resistin and fetal growth. In early reports from Korea, Cho et al. [38] demonstrated that umbilical serum resistin levels were positively correlated with maternal serum resistin levels and negatively with neonatal birth weight. In addition, no significant differences in resistin levels were discovered between the female and male neonates. Furthermore, there were no correlations between the umbilical resistin levels and maternal body mass indices, umbilical leptin levels, or insulin levels.
Similar to our results, Wang, et al. [39] found that serum adiponectin and resistin levels in control babies were significantly higher than that in macrosomic babies, whereas significantly lower than that in FGR babies. Umbilical serum adiponectin levels and placental adiponectin expression were inversely correlated with birth weight. Farid et al. [40] reported that the umbilical blood resistin level was not correlated with maternal age or BMI. Resistin level of blood cord in AGA group was much elevated than in SGA group (P <0.001). More recently, Giapros, et al. [41] reported significant higher level of resistin in the SGAs than the AGAs newborns (P=0.03) at 12 months of life and there was significant correlation with birth weight. In addition, matching with our results, Cortelazzi, et al. [22] demonstrated that resistin levels showed a negative correlation with gestational age (P<0.0001, r=-0.7).
In contrast, a recent study concluded that cord blood levels of adiponectin and resistin were not associated either with birth weight or newborn weight change [12].
Thus, it has been suggested that high levels of resistin, besides its effect on energy homeostasis in fetus, may have an important role in weight control by its effects regulating adipogenesis with negative feedback [38,40].
In contrast to resistin, adiponectin is well described as associated to birth weight. Several studies have demonstrated a positive correlation between cord blood adiponectin and birth weight [42,43] while others have found a negative association, when examining macrosomic newborns [44,45].
In contrast to our results, a recent study reported non-significant difference between 30 LGA and 30 AGA newborns as regard umbilical serum adiponectin (P=0.057). However, they included both term and late preterm newborns, which might have affected the results.
Because many reports showed that the adiponectin level was significantly lower in large-for-gestation newborns, a negative feedback mechanism between adipose tissue and adiponectin level might be exerted, even in the fetus. This is compatible with the suggestion that adiponectin may be involved in fetal development during pregnancy and may be related to the occurrence of fetal macrosomia and FGR [39].
In summary, adiponectin and resistin have direct correlation with neonatal birth weight indicating the important role in controlling fetal growth which may result in the occurrence of fetal macrosomia and intrauterine growth restriction.
Conclusion
Our findings pointed to the potential role of adiponectin and resistin in regulation of fetal growth, probably through their effects on supply of nutrients to the placenta and mediated by insulin receptors. These results might help understanding the pathophysiology of fetal growth aberrations; thus, better insights to the control of various complications that associated with these disorders, either early-onset complications as hypoglycemia and other metabolic disturbances or late-onset complications including cardiovascular, metabolic and endocrine abnormalities.
Conflict of Interest
None declared
References
- Buck Louis GM, Grewal J, Albert PS, Sciscione A, Wing DA, et al. (2015) Acial/ethnic standards for fetal growth: the NICHD Fetal Growth Studies. Am J ObstetGynecol 213: 449e1-449e41.
- Rossen LM, Schoendorf KC (2014) Trends in racial and ethnic disparities in infant mortality rates in the United States, 1989-2006. Am J Public Health 104:1549-1556.
- Vasak B, Koenen SV, Koster MPH, Hukkelhoven CW, Franx A, et al. (2015) Human fetal growth is constrained below optimal for perinatal survival. Ultrasound ObstetGynecol 45:162-167.
- Carlo WA (2015) Prematurity and Intrauterine Growth Restriction. Nelson Textbook of Pediatrics. Elsevier 20: 821-830.
- American College of Obstetricians and Gynecologists (2013) ACOG Practice bulletin no. 134: fetal growth restriction. ObstetGynecol 121:1122-1133.
- Campbell S (2014) Fetal macrosomia: a problem in need of a policy. Ultrasound ObstetGynecol 43:3-10.
- Walsh JM, McAuliffe FM (2012) Prediction and prevention of the macrosomic fetus. Eur J ObstetGynecolReprodBiol 162:125-130.
- Sferruzzi-Perri AN, Vaughan OR, Forhead AJ, Fowden AL (2013) Hormonal and nutritional drivers of intrauterine growth. CurrOpin Clin NutrMetab Care 16: 298-309.
- Dimasuay KG, Boeuf P, Powell TL, Jansson T (2016) Placental Responses to Changes in the Maternal Environment Determine Fetal Growth. Front Physiol 7:12.
- Fowden AL, Forhead AJ, Sferruzzi-Perri AN, Burton GJ, Vaughan OR (2015) Review: endocrine regulation of placental phenotype. Placenta 36: S50-S59.
- Jamurtas AZ, Stavropoulos-Kalinoglou A, Koutsias S, Koutedakis Y, Fatouros I (2015) Adiponectin, Resistin, and Visfatin in Childhood Obesity and Exercise. PediatrExercSci 27:454-462.
- Fonseca MJ, Santos AC (2015) Umbilical cord blood adipokines and newborn weight change. Arch GynecolObstet 291:1037-1040.
- Caselli C (2014) Role of adiponectin system in insulin resistance. Mol Genet Metab 113:155-160.
- Jansson N, Nilsfelt A, Gellerstedt M, Wennergren M, Rossander-Hulthen L, et al. (2008) Maternal hormones linking maternal body mass index and dietary intake to birth weight. Am J Clin Nutr 87:1743-1749.
- Lowe LP, Metzger BE, Lowe WL, Jr Dyer AR, McDade TW (2010) Inflammatory mediators and glucose in pregnancy: results from a subset of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. J Clin EndocrinolMetab 95:5427-5434.
- Ategbo JM, Grissa O, Yessoufou A, Hichami A, Dramane KL, et al. (2006) Modulation of adipokines and cytokines in gestational diabetes and macrosomia. J Clin EndocrinolMetab 91:4137-4143.
- Aye IL, Powell TL, Jansson T (2013) Review: Adiponectin--the missing link between maternal adiposity, placental transport and fetal growth? Placenta 34: S40-S45.
- Bouchard L, Hivert MF, Guay SP, St-Pierre J, Perron P, et al. (2012) Placental adiponectin gene DNA methylation levels are associated with mothers’ blood glucose concentration. Diabetes 61:1272-1280.
- Hanson MA, Gluckman PD (2014) Early developmental conditioning of later health and disease: physiology of pathophysiology. Physiol Rev 94:1027-1076.
- Codoñer-Franch P, Alonso-Iglesias E (2015) Resistin: insulin resistance to malignancy. Clin ChimActa 438: 46-54.
- Sartori C, Lazzeroni P, Merli S, Patianna VD, Viaroli F, et al. (2016) From Placenta to Polycystic Ovarian Syndrome: The Role of Adipokines. Mediators Inflamm 2016:4981916.
- Cortelazzi D, Corbetta S, Ronzoni S, Pelle F, Marconi A, et al.(2007) Maternal and foetal resistin and adiponectin concentrations in normal and complicated pregnancies. Clin Endocrinol (Oxf) 66:447-453.
- Sferruzzi-Perri AN, Camm EJ (2016) The Programming Power of the Placenta. Front Physiol 7:33.
- Winer JC, Zern TL, Taksali SE, Dziura J, Cali AM, et al.(2006) Adiponectin in childhood and adolescent obesity and its association with inflammatory markers and components of the metabolic syndrome. J Clin EndocrinolMetab 91: 4415-4423.
- Sferruzzi-Perri AN, Owens JA, Pringle KG, Roberts CT (2011) The neglected role of insulin-like growth factors in the maternal circulation regulating fetal growth. J Physiol 589:7-20.
- Baumann MU, Schneider H, Malek A, Palta V, Surbek DV, et al. (2014) Regulation of human trophoblast GLUT1 glucose transporter by insulin-like growth factor I (IGF-I). PLoS One 9: e106037.
- Karl PI (1995) Insulin-like growth factor-1 stimulates amino acid uptake by the cultured human placental trophoblast. J Cell Physiol 165:83-88.
- Roos S, Jansson N, Palmberg I, Säljö K, Powell TL, et al. (2007) Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J Physiol 582:449-459.
- Laviola L, Perrini S, Belsanti G, Natalicchio A, Montrone C, et al. (2005) Intrauterine growth restriction in humans is associated with abnormalities in placental insulin-like growth factor signaling. Endocrinology 146: 1498-1505.
- Jiang H, Xun P, Luo G, Wang Q, Cai Y, et al. (2009) Levels of insulin-like growth factors and their receptors in placenta in relation to macrosomia. Asia Pac J Clin Nutr 18:171-8.
- Rosario FJ, Schumacher MA, Jiang J, Kanai Y, Powell TL, et al. (2012) Chronic maternal infusion of full-length adiponectin in pregnant mice down-regulates placental amino acid transporter activity and expression and decreases fetal growth. J Physiol 590:1495-1509.
- Aye IL, Gao X, Weintraub ST, Jansson T, Powell TL (2014) Adiponectin inhibits insulin function in primary trophoblasts by PPARα-mediated ceramide synthesis. MolEndocrinol 28:512-524.
- Aye IL, Rosario FJ, Powell TL, Jansson T (2015) Adiponectin supplementation in pregnant mice prevents the adverse effects of maternal obesity on placental function and fetal growth. ProcNatlAcadSci USA.112:12858-12863.
- Jansson N, Greenwood SL, Johansson BR, Powell TL, Jansson T (2003) Leptin stimulates the activity of the system A amino acid transporter in human placental villous fragments. J Clin EndocrinolMetab 88:1205-1211.
- Von Versen-Höynck F, Rajakumar A, Parrott MS, Powers RW (2009) Leptin affects system A amino acid transport activity in the human placenta: evidence for STAT3 dependent mechanisms. Placenta 30: 361-367.
- Yura S, Sagawa N, Itoh H, Kakui K, Nuamah MA, et al. (2003) Resistin is expressed in the human placenta. J Clin EndocrinolMetab 88:1394-1397.
- Di Simone N, Di Nicuolo F, Sanguinetti M, Castellani R, D'Asta M, et al. (2006) Resistin regulates human choriocarcinoma cell invasive behaviour and endothelial cell angiogenic processes. J Endocrinol 189:691-699.
- Cho GJ, Yoo SW, Hong SC, Oh MJ, Kim T, et al. (2006) Correlations between umbilical and maternal serum resistin levels and neonatal birth weight. ActaObstetGynecolScand 85:1051-1056.
- Wang J, Shang LX, Dong X, Wang X, Wu N, et al. (2010) Relationship of adiponectin and resistin levels in umbilical serum, maternal serum and placenta with neonatal birth weight. Aust N Z J ObstetGynaecol 50:432-438.
- Farid SD, Najati N, Gharebaghi MM, Haghjo AG, Ghojazadeh M (2013) Resistin in cord blood of small for gestation age and appropriate for gestation age term neonates. Iran J Pediatr 23:659-63.
- Giapros V, Vavva E, Siomou E, Kolios G, Tsabouri S, et al. (2016) Low-birth-weight, but not catch-up growth, correlates with insulin resistance and resistin level in SGA infants at 12 months. J Matern Fetal Neonatal Med 1-6.
- Tsai PJ, Yu CH, Hsu SP, Lee YH, Chiou CH, et al. (2004) Cord plasma concentrations of adiponectin and leptin in healthy term neonates: positive correlation with birth weight and neonatal adiposity. Clin Endocrinol 61: 88-93.
- Kamoda T, Saitoh H, Saito M, Sugiura M, Matsui A (2004) Serum adiponectin concentrations in newborn infants in early postnatal life. Pediatr Res 56: 690-693.
- Cekmez F, Canpolat FE, Pirgon O, Çetinkaya M, Aydinoz S, et al. (2011) Apelin, vaspin, visfatin and adiponectin in large for gestational age infants with insulin resistance. Cytokine 56: 387-391.
- Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Schiff E, et al. (2005) Cord blood adiponectin in large-for-gestational age newborns. Am J ObstetGynecol 93: 1238-1242.
Relevant Topics
- About the Journal
- Birth Complications
- Breastfeeding
- Bronchopulmonary Dysplasia
- Feeding Disorders
- Gestational diabetes
- Neonatal Anemia
- Neonatal Breastfeeding
- Neonatal Care
- Neonatal Disease
- Neonatal Drugs
- Neonatal Health
- Neonatal Infections
- Neonatal Intensive Care
- Neonatal Seizure
- Neonatal Sepsis
- Neonatal Stroke
- Newborn Jaundice
- Newborns Screening
- Premature Infants
- Sepsis in Neonatal
- Vaccines and Immunity for Newborns
Recommended Journals
Article Tools
Article Usage
- Total views: 3770
- [From(publication date):
June-2017 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 2918
- PDF downloads : 852