Research Article Open Access
Adhesion of Trypanosoma evansi to Red Blood Cells (RBCs): Implications in the Pathogenesis of Anaemia and Evasion of Immune System
M S Rossi S 1,2,3*, A A Boada-Sucre4, M T Simoes1, Y Boher4, P Rodriguez5, M Moreno6, M Ledezma de Ruiz7, M L Marquez8, H J Finol5, C Sanoja1 and G Payares11Laboratorio de Inmunología y Quimioterapia, Instituto de Biología Experimental, Facultad de Ciencias, Universidad Central de Venezuela, Caracas, Venezuela
2Sección de Investigaciones en Patología Ultraestructural y Biología Molecular, Instituto Anatomopatológico José A. O´Daly, Facultad de Medicina, Universidad Central de Venezuela, Caracas, Venezuela
3Laboratorio de Arbovirus y Enfermedades Virales Emergentes, Centro Nacional de Microbiología, Instituto de Salud Carlos III, Madrid, España
4Laboratorio de Microscopía Electrónica, Instituto de Estudios Científicos y Tecnológicos, Universidad Nacional Experimental Simón Rodríguez, Caracas, Venezuela
5Centro de Microscopía Electronica "Mitsuo Ogura", Facultad de Ciencias, Universidad Central de Venezuela, Caracas, Venezuela
6Instituto de Investigaciones en Biomedicina y Ciencias Aplicadas, Universidad de Oriente, Cumaná, Venezuela
7Cátedra de Bioquímica, Facultad de Farmacia, Universidad Central de Venezuela, Caracas, Venezuela
8Laboratorio Cultivo de Tejidos Animales, Instituto de Biología Experimental, Facultad de Ciencias, Universidad Central de Venezuela, Caracas, Venezuela
- *Corresponding Author:
- Marcello Salvatore Rossi Spadafora
Centro Nacional de Microbiología
Instituto de Salud Carlos III, Madrid, Spain
Tel: 0034-911265512
Email: rossimarcell@gmail.com
Received Date: January 23, 2017; Accepted Date: February 06, 2017; Published Date: February 11, 2017
Citation: Rossi MSS, Boada-Sucre AA, Simoes MT, Boher Y, Rodriguez P, et al. (2017) Adhesion of Trypanosoma Evansi to Red Blood Cells (RBCs): Implications in the Pathogenesis of Anaemia and Evasion of Immune System . Diagn Pathol Open 2:122.doi: 10.4172/2476-2024.1000122
Copyright: © 2017, Rossi MSS. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use,distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Diagnostic Pathology: Open Access
Abstract
Background: Trypanosoma evansi is the etiological agent of trypanosomosis, a disease of domestic (horses, cattle and goats) and wild (capybara, vampires) animals characterized by anaemia, degeneration, necrosis and inflammatory processes. This disease is of great concern because it produces growth retardation, loss of body weight, low production of animal proteins and diminished fertility and traction power. Because anaemia is considered the most characteristic symptom, the aim of this study was to assess the effects that T. evansi adhesion to erythrocytes has on their morphology, surface oligosaccharides profiles and trypanosome surface antigens.
Methods: Blood and tissues samples from mice experimentally infected, were studied using scanning (SEM) and transmission (TEM) electron microscopes and lectin's histochemistry (sConA, sWGA, PNA, LFA, etc.). Furthermore immunoprecipitation of T. evansi radio iodinated surface antigens with specific anti-hosts and anti-trypanosome sera was performed and antigens analysed by electrophoresis and autoradiography.
Results: T. evansi adhesion to erythrocytes as well as changes in the morphology of them, were frequent findings. SEM studies showed that adhesion of bloodstream trypomastigotes of T. evansi to mature erythrocytes and reticulocytes occurred through its flagellum, undulating membrane or cellular body and were mediated by filopodia. Minute pores and filamentous material were sometimes observed on erythrocytes membrane at the point of adhesion to the trypanosome. Oligosaccharides changes of RBCs glycocalix were characterized by a marked decrease in Man/Glc, terminal GalNAc and terminal Neu5Ac labelling accompanied by an increase in labelling of terminal GlcNAc. There were not labelling changes with the other lectins assayed. Preliminary immunoprecipitation studies of T. evansi radio iodinated surface antigens with anti-host sera, showed the presence of a of 45 kDa antigen from erythrocyte and host plasma on T. evansi plasma membranes. Furthermore, TEM studies demonstrated an increased abnormal erythrophagocytosis in adrenal gland, spleen and liver of experimentally infected mice.
Conclusion: Adhesion of T. evansi to erythrocytes together with morphological and biochemical changes of these cells must be responsible for abnormally incremented erythrophagocytosis. On the other hand, detection of antigens from host on T. evansi membranes, suggests the operability of the antigen masking as an alternate immune response evasion mechanism to antigenic variation described in African trypanosomes.
Keywords
Trypanosoma evansi; Cell adhesion; RBC; SEM; TEM; Erythrophagocytosis; Lectins; Surface Antigens; Antigen masking
Abbreviations
SEM: Scanning Electron Microscope; TEM: Transmission Electron Microscope; kDa: Kilodaltons; RBCs: Red Blood Cells; NMRI: Naval Medical Research Institute; IVIC: Instituto Venezolano de Investigaciones Científicas; Ip: Intraperitoneal; EDTA: Ethylene Diamine Tetra-Acetic Acid; DMSO: Dimethyl sulfoxide ; PSG: Phosphate Saline Glucose Buffer; ABC: Avidin-Biotin Complex; DAB: Diaminobenzidine; sConA: Succinyl Concanavalina A; sWGA: Succinyl Wheat Germen Agglutinin; SBA: Soybean Agglutinin; UEA-I: Ulex europaeus Agglutinin I; SNA: Sambucus nigra Agglutinin; PNA: Peanut Agglutinin; LFA: Limax flavus Agglutinin; MAA or MAL-II: Maackia Amurensis Agglutinin or Maackia Amurensis Lectin II; DMetil- man: D-Metil-mannose; D-Man: D-Mannose; D-Glc: DGlucose; GlcNAc: N-Acetyl Glucosamine; α- and β-GalNAc: α- and β- N-Acetyl Galactosamine; D-Gal: D-Galactose; D-Fuc: D-Fructose;Neu5Ac: N-Acetylneuraminic Acid; GalNAc: N-Acetyl Galactosamine; RαE: Rabbit Anti-Mouse Erythrocyte Membrane Serum; RαP: Rabbit Anti-Mouse Plasma Serum; RαT: Rabbit Anti-T. evansi serum; MαT: Mouse Anti- T. evansi serum; NR: Normal Rabbit Serum; NM: Normal Mouse Serum; DEAE-Cellulose: Diethyl-Amino-Ethyl Cellulose; KRT: Krebs Ringer Tris Buffer; PMSF: Phenyl Methyl Sulphonyl Fluoride; SDS-PAGE: Sodium Dodecyl Sulphate-Polyacrilamide Gel Electrophoresis; CPM: Counts Per Minute; 125I: 125Iodine Isotope; PCV: Packed Cell Volume; LD: Lectin Domain; TconTS: Trypanosoma Congolense Catalytic Domain of Transialidase; LacNAc: N-Acetyl Lactosamine; gp 68: 68 kDa Glycoprotein; gp 29: 29 kDa Glycoprotein; VSG: Variant Surface Glycoprotein.
Introduction
Of all the diseases caused by the different African trypanosomes species, infections produced by Venezuelan isolates of T. evansi and T. vivax have been the least studied. In Venezuela information concerning ultrastructural changes caused by these parasites in their host, as well as experimental animals is scarce. In this regard, Quinones-Mateu et al. [1] studied muscle changes in horses naturally infected with T. evansi in Hato El Frío (Apure State, Venezuela), and typical changes of autoimmune diseases, such as necrosis of skeletal muscle fibers and capillaries with mononuclear infiltration were found. Moreover, as a part of ultrastructural studies on the experimental infections of mice we described the development of intracellular forms of T. evansi [2,3] and varying degrees of ultrastructural abnormalities in cells of adrenal cortex [2], muscle fibers [4], hepatocytes [3], blood capillaries and endothelial cells [2,3,5].
Changes that occur in blood cells during infection by trypanosomes have been also studied. Anaemia is the most important finding in many trypanosomosis of veterinary importance [6] because it is a good indicator of infection severity. There is haemolytic in nature, occurring intravascularly in early stages of acute phase, and also extravascularly in sub-acute and chronic phase of disease [6].
Causes of anaemia in animal trypanosomosis are not entirely known, being haemolysis, hemodilution, bleeding and bone marrow dyserythropoiesis [7] the major events to which it relates. Haemolysis can be attributed to biologically active molecules produced by living or dead trypanosomes [8,9] as well as to mechanical damage that RBCs may experience due to the motility and adhesion of the flagellates to their surface [10].
Anaemia and systemic alterations caused by trypanosomes during infection are consequences of metabolic spoliation, mechanical cell injuries caused by trypanosomes motility and invasion of host's tissues [5], and secretion of biologically active macromolecules capable to produce biochemical alterations of cell surface. However effects of host's immune response against trypanosomes and the evasion mechanisms expressed by these flagellates in order to elude immune system act synergistically allowing colonization of host´s body.
Evasion of host´s immune system by African trypanosomes is achieved thanks to a well-studied and described genetic mechanism known as Antigenic Variation, however as will be discussed in this paper, the operability of other mechanisms described for haemoparasites such as antigen masking, extensively described for Schistosoma mansoni and other trypanosome species and protozoa, could constitute an alternate mechanism of immune system evasion to antigenic variation.
This work is a contribution to the knowledge and understanding of pathogenic mechanisms that affects RBCs during murine experimental infections. The aim of this study was to assess the effects that T. evansi adhesion to erythrocytes has on their morphology, surface oligosaccharides profiles and trypanosome surface antigens. In order to reveal morphological, ultrastructural and histochemical changes that occur in RBCs during infection, different electron microscopy, histochemical, biochemical and serological techniques were used. Results obtained allowed to propose adhesion of T. evansi to RBCs as a pathogenic mechanism responsible for morphological alterations which in turn render them more prone to erythrophagocytosis. In addition results obtained from study of T. evansi surface antigens during infection suggest occurrence of an alternate mechanism of immune response evasion different to antigenic variation.
Materials and Methods
Experimental infections with T. evansi
A total of 15 heterozygous albino mice (20 g body weight) of the NMRI strain were used. Animals were purchased at the Venezuelan Institute for Scientific Research (IVIC) and kept in an experimental animal facility in cages with shell rice as a bed. They were fed ad libitum with Ratarina® (Protinal) and filtered ozonized water.
The heterogeneous population of T. evansi used in this work was isolated in 1991 from a horse (Equus cabalus) infected at Hato El Frio (7°56' North latitude and 68°57' West longitude) in the Apure State of Venezuela. The infected horse showed signs of cachexia, prostration and paresis of the hindquarters knew as "Derrengadera" (equine trypanosomosis) in Venezuela.
The isolation was performed by intraperitoneal (Ip) inoculation of horse blood aliquots in two albino mice of NMRI strain. When trypanosomes were patent in tail vein blood, a subpasage in Sprague- Dawley rats was done by inoculation. Murine parasitaemia was evaluated daily from tail vein blood by microhaematocrit method [11] and when values reached 107-108 trypanosomes/mL, the whole animal blood was obtained by heart puncture under anaesthesia, using 15% mg/mL EDTA (ethylene diamine tetra-acetic acid) as an anticoagulant. Part of blood obtained was cryopreserved with dimethyl sulfoxide (DMSO) in phosphate saline glucose (PSG) buffer pH 8 (20 mm phosphate, 150 mm NaCl, 1% glucose and 15% DMSO) at -196°C (liquid nitrogen).
Trypanosomes present in blood of infected horse and inoculated mice and rats, were characterized as monomorphic trypomastigote forms, and were classified as T. evansi , according to clinical, morphological (T. brucei -like) and biological (susceptibility for experimentation animals) criteria. Furthermore trypanosomes were classified as T. evansi according to its ultrastructure feature (low number of coated vesicles in the flagellar pocket in relation to T. equiperdum a co-endemic specie of trypanosome) [12].
After thawing a stabilate vial in a water bath at 40°C, 0.50 ml of cryopreserved blood was inoculated into Sprague-Dawley rats in order to expand parasitic populations. After rats reached parasitemias of 108 trypanosomes/ml an inoculum was prepared by diluting aliquots of infected blood in PSG [13] in order to inoculate mice with 104 flagellated (500 trypanosomes/g body weight). Parasitemias of mice were evaluated daily from tail vein blood by Brenner method [14]. The deep circulation blood to be used in SEM studies was obtained by cardiac puncture using EDTA as anticoagulant. Samples of liver, spleen and adrenal glands to be studied by TEM were obtained from anesthetized animals. All the samples used were obtained at terminal stage of infection (parasitemia=109 trypanosomes/mL).
Electron microscopy
For scanning electron microscopy (SEM) studies, when parasitemia of mice reached values around 108-109 trypanosomes/mL, aliquots of blood were prefixed in Karnovsky's solution (2.5% glutaraldehyde and 4% p-formaldehyde in Millonig's phosphate buffer 0.10 M pH 7.4), leaving the blood dripping gently on the fixing solution. The biological material was allowed to settle and after 2 h of fixation at 4°C, excess of glutaraldehyde was removed by pipetting and washed 3 times with Millonig's buffer. The entire process was carried out by decantation to avoid the formation of cell aggregates. All next steps were performed according to Boada-Sucre et al. [15] methodology. Briefly 100 μL of prefixed blood were spotted onto coverslips pre-treated with poly-Llysine 0.1% v/v in order to promote adhesion of blood cells to glass. Excess of glutaraldehyde was removed by washing with Millonig's phosphate buffer and post-fixation was performed with 1% m/v osmium tetroxide (OsO4) for 1 hour at 4°C. Osmium excess was removed by washing with deionized water and samples were dehydrated by sequential incubation of glass coverslips in a battery of increasing ethanol concentration (50%, 70%, 80%, 90%, 95% and 100% at 4°C). Biological material adsorbed to coverslips was dried by evaporation on a critical point dryer (Hitachi HCP-2) using carbon dioxide (CO2) as liquid transition fluid. The dried samples (coverslips) were mounted on a metallic sample holder with double-sided tape and silver paste and then were covered with platinum in a thermionic coater (Electron Microscopy Science EMS-350). Observations and photographic records were performed on a field emission scanning electron microscope (FE Hitachi S-4500) with an accelerating voltage of 5 kV.
After taking samples of adrenal glands, liver and spleen, pieces of these organs were processed according to technique described in [2] and ultrafine sections of them were obtained for transmission electron microscopy (TEM) study. In this regard, sample blocks of tissuesamples were subjected to ultramicrotomy in order to obtain sections of 60 to 90 nm thick, which were collected on copper grids of 300 mesh and contrasted with an aqueous solution of 1% w/v uranyl acetate for 30 minutes and lead citrate for 10 minutes according to the methodology described by Reynolds [16]. Observations and photographic records were performed on a field emission scanning electron microscope (Hitachi H-7100) with an accelerating voltage of 75 kV.
For freeze-fracture studies performed by TEM, blood aliquots from mice infected with T. evansi were prefixed in Karnowsky's solution, washed with Millonig's buffer and cryoprotected for 30 minutes by incubation in 10 mm phosphate buffer pH 7.2 containing 25% v/v glycerol. Cell suspensions obtained were placed in a sample holder, quickly frozen in liquid nitrogen, fractured, freeze-etched and covered with platinum-carbon, to finally obtain replicas after removal all organic material with bleach (3% v/v sodium hypochlorite) [17]. Replicas obtained by freeze-fracture were observed and photographic recorded in transmission electron microscopy (Hitachi H-500) with an accelerating voltage of 75 kV.
Lectin's Histochemistry
Oligosaccharide composition of RBC surface from healthy and T. evansi experimentally infected mice was studied by lectin's histochemistry technique described by Gonzalez-Elorriaga and Canepa [18]. Briefly, samples of adrenal glands, liver and spleen of infected animals and control group were fixed in 10% buffered formalin (pH 7.2) for 72 h. Subsequently, fixed tissues were prepared according to conventional histological techniques. Histochemical reactions were performed on slides containing deparaffinized and hydrated 7 μm thick tissues sections. A total of seven biotinylated lectins with specificities for different configurations of specific carbohydrate residues in tissue (Table 1) were used at a final concentration of 10 μg/mL in 5 mM Tris-HCl pH 7.8 containing 150 mM NaCl, 0.10 mM CaCl2, 0.10 mm MgCl2, 0.7% w/v Carrageenan and 5% v/v Triton X100. Lectin-Oligosaccharides were revealed by Avidin-Biotin Complex (ABC) method using diaminobenzidine (DAB)-hydrogen peroxide as chromogenic substrate. Results were observed under an optical microscope (Nikon OptiPlus) and scored semi quantitatively using a scale from 0 to 5 plus (+).
| Source | Acronym | Inhibitors Saccharide Residues |
|---|---|---|
| Canavaliaensiformis | sConA | D-Metil-man >D-Man >D-Glc |
| Triticum vulgaris | sWGA | GlcNAcb(1→4)GlcNAcb(1→4)GlcNAc |
| Glycine max | SBA | α- and b-GalNAc terminal>>D-Gal |
| Ulex europaeus I | UEA-I | Fucα(1→2)Galb(1→4)GlcNAc>D-Fuc |
| Sambucus nigra | SNA | Neu5Ac-α(2→6)Gal y Neu5Ac-α(2→6)GalNAc |
| Arachishypogaea | PNA | D-Galb(1→3)GalNac |
| Limax flavus | LFA | Neu5Ac and Neu5-Glc terminal |
| Maackia amurensis | MAA or MAL-II | Neu5Ac-α(2→3)D-Galb |
Table 1: Set of lectins used for determination of changes in RBC surface oligosaccharides composition.
Specific antisera
Specific antisera to be used in immunoprecipitation assays were prepared according to Simoes [19]. Specificity of these antisera: rabbit anti-mouse erythrocyte membrane (RαE), anti-mouse plasma (RαP) and anti- T. evansi (RαT), mouse anti- T. evansi (MαT), normal rabbit sera (NR) and normal mouse (NM) were done by Outcherlony technique (agar double immunodiffusion).
Purification of T. evansi Bloodstream Trypomastigotes and Preparation of RBC Ghosts
In order to perform surface radio iodination of T. evansi , blood trypomastigotes from Sprague- Dawley rats experimentally infected were obtained by anion exchange chromatography on columns of diethyl-amino-ethyl cellulose (DEAE-cellulose) according to technique described by Lanham and Godfrey [13]. RBC ghosts free of cytoplasm and haemoglobin were obtained from heparinized blood of normal mouse as it has been described by Newbold et al. [20].
Preparation of Plasma Membrane Fractions from T. evansi and Studies of Surface Antigens Composition
Changes in composition of T. evansi surface antigens during experimental infections of mice were determined by preparation of a radio iodinated cell membrane fraction, and inmunoprecipitation of T. evansi surface antigens with specific antisera and Protein A-agarose. Radio-iodination of surface proteins with 125I was performed with 2 × 108 intact blood trypomastigotes obtained by DEAE-cellulose chromatography, using Iodogen method [21]. Plasma membrane fractions from bloodstream trypomastigotes (2 × 108) were obtained after 1 h lysis at 4ºC in Krebs Ringer Tris (KRT) pH 7.4 containing 1% Triton X-100, 1 mm phenyl-methyl-sulphonyl-fluoride (PMSF) and 1 mm EDTA (ethylene diamine tetraacetic acid) followed by centrifugation for 30 minutes at 38,000 x g. Immunoprecipitation with specific antisera was performed overnight at 4ºC by mixing 50 μl of radio iodinated fractions with 40 μl of specific antisera in 10 μl of 50 mM Tris-HCl pH 8.2 containing 150 mm NaCl, 10 mm EDTA and 1 mm PMSF. Overnight incubation was followed by an additional incubation of 1 hour with gentle mixing followed by 3 minutes boiling of fractions mixed with 50 μl of 2X sample buffer for sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) [22]. Surface antigens were then separated in 12.5% SDS-PAGE under dissociating conditions [22] and stained and dried in order to perform autoradiography.
Results
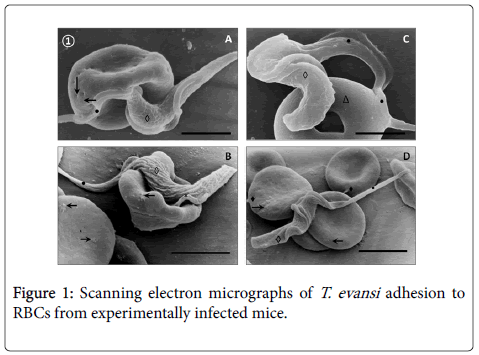
SEM of blood from T. evansi experimentally infected mice (Figure 1) revealed occurrence of abnormalities of RBCs characterized by presence of holes (Panel A), rough surfaces (Panel B and D) and donut-shaped RBCs (Panel C).
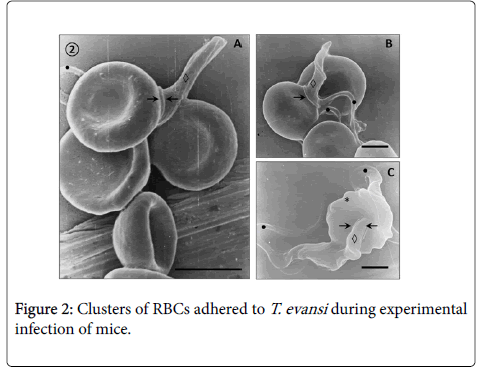
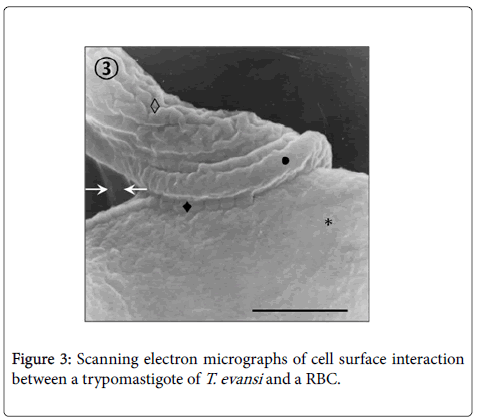
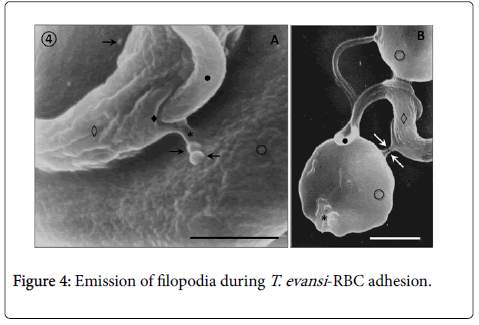
Adhesion of T. evansi to RBCs was through cell body and undulating membrane (Panels A, B and C) as well as flagellum (Panel D). In all preparations studied, adhesion of trypanosomes to RBCs was observed to one (Panel A and B) or more RBC (Figure 2) determining formation of large aggregates or trypanosomes-RBC microthrombi (Panels A and B). A rare finding was the presence of trypomastigotes crossing RBC body (Figure 2, Panel C). Adhesion appears to be mediated by secretion of a filamentous material (Figure 3) and the emission of filopodia from trypanosomes and erythrocytes (Figure 4; Panels A and B). Figure 3 shows that binding of T. evansi to the RBC surface was intimate, determining the formation of zip-type connection sealing structure. Figure 4 shows that formation of this ziptype connection (Figure 3) seems to be favoured by filopodia in the vicinity of the contact area, whose emission begins with formation of small bubbles on the RBC surface.
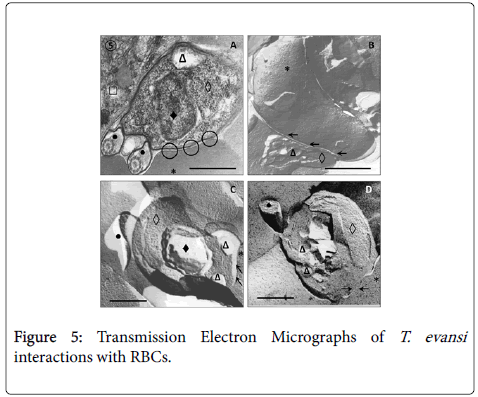
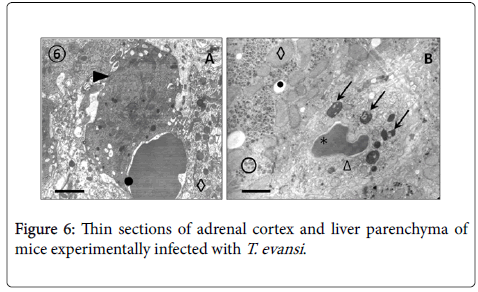
Results from TEM revealed close relationships between trypomastigotes and deep circulating RBCs of adrenal glands (Figure 5, Panel A) and blood (Panels B, C and D), as well as of spleen and liver (not showed). Panel A shows presence of an electron dense material in the RBC-trypanosome contact apparently coming from trypanosome surface coat. Freeze-fracture replicas from deep-circulating RBCs confirmed this superficial close interaction (Panel B and C). In some cases was a frequent finding the continuity between RBC and T. evansi plasma membranes and the presence of lysosome-like organelles in the proximity of trypanosome-RBC interaction (Panel C). Other frequent finding in ultrathin sections (Figure 6) was observation of abnormal and increased RBC phagocytosis by macrophages in adrenal gland (Panel A), Kupffer cells lining hepatic sinusoids (Panel B), and spleen macrophages (not showed).
(Figure 7) shows results of histochemical staining of RBC's surface with a panel of seven specific lectins. Results scored allowed to verify occurrence of changes in composition of RBCs surface oligosaccharides during infection with T. evansi .
Changes were characterized by a marked decrease in labelling of RBC's surface oligosaccharides containing Man/Glc by Canavalia ensiformis lectin (ConA), terminal GalNAc by peanut agglutinin (Arachis hypogaea) (PNA) and terminal Neu5Ac by Limax flavus agglutinin (LFA). These changes were accompanied by an increase in labelling of terminal GlcNAc residues with Triticum vulgaris agglutinin (sWGA). There were not changes in the intensity of labelling (+3) with the other three lectins assayed (SBA, UEA-I, SNA and MAA) when RBCs from infected mice were compared with those from control mice.
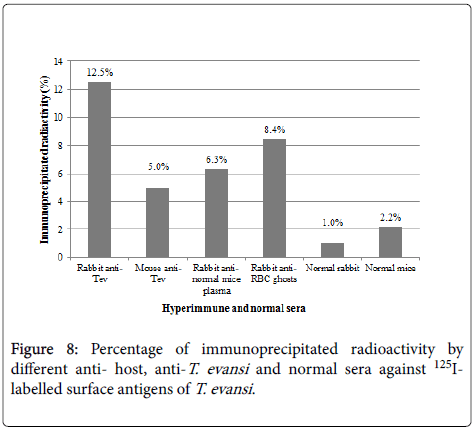
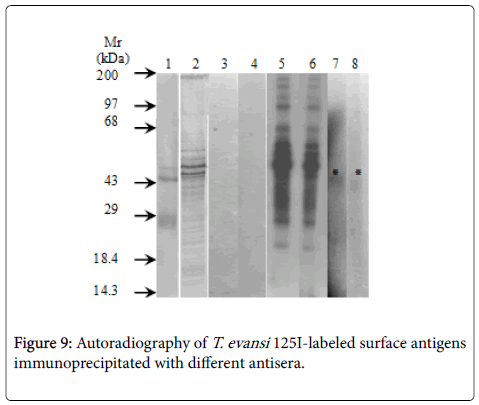
Specificity of antisera used in this work showed identity reactions with different fractions of T. evansi antigens by Outcherlony technique (results not shown). (Figure 8) shows percentages of radioactivity (cpm) immunoprecipitated from fractions of 125I- T. evansi surface antigens using these specific antisera. As it can be seen rabbit anti- T. evansi antiserum precipitated 12.5% of radioactivity, followed by rabbit antiserum against to RBC ghosts (8.4%) and rabbit antiserum against normal mice plasma (6.3%). Mice anti- T. evansi serum precipitated 5% of radioactivity, while normal rabbit and mice sera precipitated non-specifically between 1 and 2.2% of radioactivity (Figure 9).
Discussion
Haematological abnormalities in infections caused by African trypanosomes have been extensively studied and the existing literature about pathogenic mechanisms involved was reviewed by several authors [23]. Among the most striking changes reported, decreased RBC count, packed cell volume (PCV) and morphological abnormalities of RBC seem to be best indicators that have been associated to anaemia that develops during the disease.
In the early 70's it was reported that morphological abnormalities of RBCs during trypanosomosis are a consequence of changes that occur in small blood vessels which become abnormally thick and adopt tortuous shapes, affecting RBCs morphology and integrity [24]. Adhesion of T. evansi to mice RBCs and morphological changes of erythrocytes described in this paper such as perforations, warty surface, donuts-shape cells and emission of filopodia, are quite similar to those described in infections of deer mice (Peromyscus maniculatus) with T. brucei in absence of blood vessels damage [25].
Perforations on the surface of RBC's could be explained by the mechanical damage that bloodstream trypomastigotes cause to their membranes, especially when parasitemias are too high as 108 – 109 trypanosomes/ml. In this regard, mechanical damage "pinching out" caused by bloodstream forms of T. brucei has been demonstrated by cinematographic techniques with Normansky light interference, and may be a factor that contributes to characteristic haemolysis of African trypanosomosis [10] and less frequently to occurrence of bloodstream trypomastigotes of T. evansi going through RBC as it has been described in this study. In addition activation of effector mechanisms of the immune system as a consequence of formation and adsorption of antigen-antibody complexes and complement on RBC's surface, could explain these injuries as well as the erythrolysis, their antigenic recognition and removal of RBCs by mononuclear phagocytic system [26].
Morphological changes of RBCs have been also described in lectinstreated erythrocytes. RBC membranes have internal protein skeletons that govern cells shape and morphology changes (discocyteechinocyte) usually seen in conventional microscopy. Glycophorin (the RBCs transmembrane glycoprotein) presents all of its oligosaccharides outside cell and is intracellularly linked to the RBC cytoskeleton, enabling lectins binding to the external saccharides to gain profound control over internal cytoskeleton behavior expressed by governance of the visibly seen cell shape [27].
Decrease in lectin's labelling of RBCs from mice experimentally infected with T. evansi , such as sialic acids (desialation), not only implies removal of this saccharide residues from erythrocyte glycocalix but also the exposition of endosaccharides on RBC's glycophorin enabling lectins to gain a tight control over RBCs morphology in simple stoichiometric ratios of bound lectin molecules/glycophorin receptor [27].
Lectin-like molecules have been described in African trypanosomes, in this regard, transialidase from T. congolense is an enzyme composed by two domains, a Lectin domain (LD) and a catalytic domain (TconTS) whose enzymatic activity over several (foreign and own) Man-containing oligosaccharides, such as mannobiose, mannotriose and higher mannosylated glycans, as well as Gal, GalNAc and LacNAc containing oligosaccharides is modulated by the LD domain [28]. Although transialidases have not been described in T. evansi and other trypanosomes of brucei-group, the presence of lectin-like molecules on its surface could also contribute together with other factors to morphological changes described for RBCs during experimental infection.
Adhesion of T. evansi to peripheral and deep circulation RBCs of adrenal glands, liver and spleen, seems to be a shared character with T. brucei [25] and other African trypanosomes. During adhesion of T. evansi to RBC's it was observed involvement of undulating membrane and flagellum as it has been described in flagellum-mediated adhesion of T. congolense to bovine aortic endothelium [29] and T. vivax to ovine RBCs [26]. This process was accompanied by emission of erythrocyte's and trypanosome's filopodia, cytoplasmic extensions that were described for the very first time in bloodstream trypomastigotes of T. brucei brucei, T. brucei rhodesiense [30] and T. evansi [31], and act as elements that promote adhesion of bloodstream trypomastigotes to RBCs [32].
Adhesion of T. evansi trypomastigotes to RBCs occurs with one or more erythrocytes, to give rise to formation of aggregates or microthrombi with an important role in trypanosomosis systemic pathology. In this regard, complications triggered by anaemia together with microthrombi of parasitic origin not only compromise the normal gas and metabolites exchange between blood and host tissues by way of the occlusion of the vascular lumen of capillary, but also contribute to the cell necrosis aggravated by endothelial alterations that occur in visceral organs during disease [2-3].
Another peculiar feature of T. evansi adhesion to mice RBCs were electron microscopic evidences of secretion of a filamentous material in the contact zones between trypomastigotes and RBCs. In this regard, electron microscopic evidences during adhesion of T. congolense to bovine aortic endothelial (BAE) cell monolayers [29] suggested that this filamentous material is constituted by proteins from trypanosome surface coat that are shed to cell culture. Furthermore, lectin-gold labelling assays demonstrated that contains Sialic acid residues involved in adhesion.
In T. evansi adhesion to RBCs this filamentous material could be constituted by two glycoproteins: a cysteine-proteinase of 29 kDa (gp29) with gelatinolytic activity which has been identified in secretion supernatants and homogenates of T. brucei [33] and Venezuelan isolates of T. evansi [34] and another one of 68 kDa (gp68) whose biochemical characterization on the basis of its molecular weight and recognition by lectins corresponds to the Variant Surface Glycoprotein (VSG) [34].
Although it is unclear how adhesion determines different kind of abnormalities described by SEM and TEM, we suggest that adhesion of T. evansi to RBCs via Sialic acid receptors causes injuries to RBCs membrane [26] and should promote haemolysis and erythrophagocytosis in a direct association to parasitemia, since high trypanosomes densities in bloodstream at any given time increases the probability of contacts between parasites and RBCs [25,26]. This association is supported by the fact that increasing trend in parasitemia during the infection overlaps with the sharpest decline of the haematocrit during the experimental infections of cattle and goats with T. evansi [35], mice with T. brucei [23] and sheep with T. vivax [36].
Adhesion mechanisms through T. evansi can damage RBCs included also changes in the composition of surface oligosaccharides described by lectin's histochemistry. In this regard it has been found that during infections, live or dead trypanosomes produces a variety of biologically active molecules capable of producing modifications of host cells glicocalyx and cell damage [9]. Extracellularly-secreted cysteine proteinase (gp29) of T. evansi [34] shared with T. brucei and T. equiperdum [33,37] could act together with gp68 inducing not only the biochemical changes of RBC's surface but also cytoskeletal changes responsible for the morphological alterations described in this work. In this regard, it has been demonstrated that brucipain-like proteases induce changes in actin cytoskeleton of endothelial cells from central nervous system that allow T. brucei gambiense to enter into and trespass haemato-encephalic barrier in a process dependent on level of brucipain expressed by trypanosomes [38]. If to this process is added the fact that increased trypanosome motility during parasitemia peaks constitutes a mechanical stimulus that can trigger the release of intracellular Ca2+ followed by changes in actin cytoskeleton [38], operability of similar processes triggered by trypanosome swimming and secretion of an homologous cysteine proteinase (evansin), could explain morphology changes of RBCs such as donuts-shape cells, emission of filopodia from RBCs and the presence of trypanosomes crossing RBCs.
These processes could act synergistically with other biologically active molecules such as proteases and phospholipases from live or dead trypanosomes which could act favouring degradation of glycoproteins and other glycoconjugates from the RBC membranes, altering their oligosaccharides profiles and making them more prone to lysis and its removal by phagocytosis [9]. In this regard, this work provides evidences about changes in oligosaccharides composition of RBC surface, related to a decreasing of terminal D-Man/D-Glc, GalNAc and sialic acid, and an increase in labelling of terminal GlcNAc residues with Triticum vulgaris Agglutinin (sWGA). These changes could be a consequence of glycosidases secreted by trypanosomes [39-42] such as sialidases [41] and β-galactosidases [42], enzymes which have been described and kinetically characterized in bloodstream trypomastigotes of T. evansi .
β-galactosidase catalyzes the hydrolysis of β-galactosides from lectins, glycoproteins and gangliosides. So far, among all the species of trypanosomes, β-galactosidase has only been described in T. evansi [42], T. congolense [43] and T. cruzi [44]. The enzyme has also been purified and characterized in Tritrichomonas foetus [45] where it is required for mucin breakdown during invasion process [46].
Its mechanism of action is in part due to action of sialidases whose levels are marked increased in plasma of bucks experimentally infected with African isolates of T. evansi [41]. This enzyme independently they come from living or dead trypanosomes, produces cleavage of surface sialic acids from erythrocytes, exposing β-galactosyl residues which in turn could be breakdown by β-galactosidase or been recognized by β- D-galactose specific lectins on the surface of adrenal cortex macrophages and Kupffer cells from hepatic sinusoids, leading to the abnormal extravascular erythrophagocytosis observed in this work and responsible for the subsequent anemia developed [47,48].
Detection of a 45 kDa polypeptide from RBC and host plasma in plasma membrane fraction of T. evansi provides antigenic evidences about the occurrence of an unusual mechanism of immune evasion in Africa trypanosomes. The mechanisms that trypanosomes of bruceigroup have developed to escape immune surveillance are currently under investigation in many laboratories, because their elucidation may provide new approaches to the prevention of the diseases that parasites cause [49] . One possible way of evasion supported by results herein presented is the uptake of host antigen by trypanosomes. This phenomenon of masking of T. evansi surface antigens by host proteins has been extensively studied and documented in schistosomiasis. In this regard, the acquisition of mouse antigens, human A, B, and H blood group antigens, glycolipids from erythrocytes, serum factors and mouse histocompatibility antigens have been demonstrated in Schistosoma mansoni and offer an effective mechanism for antigenic mimicry of host and subsequent escape from the immune response [50,51].
This mechanism would allow T. evansi to evade the immune system by a different mechanism than the antigenic variation previously described by Perrone et al. [52] for Venezuelan isolates as well as for T. evansi and other African trypanosomes [49,53]. Masking of host antigens, such as immunoglobulins has been described for bloodstream forms of many trypanosomes such as T. congolense , T. brucei gambiense, T. brucei rhodesiense, T. cruzi , T. musculi and T. lewisi . Other host proteins, such as albumin, have also been detected on surface of protozoa and some trypanosomes species. In this respect it has been suggested that surface immunoglobulins are responsible for the persistence of T. lewisi in the bloodstream of rats.
The acquisition of host antigens by T. evansi could be achieved by absorption from host plasma but also during T. evansi -RBC surface interactions and adhesion. In this regard, freeze-fracture replicas results showed areas in which trypanosome-RBC contacts appear as continuity regions between the plasma membrane of T. evansi and RBC. A similar feature of the adhesion phenomenon has also been described during cytoadherence of Trichomonas vaginalis to epithelial cells of the human vagina [54], T. brucei to RBCs [21] and T. congolense to endothelial [22] and RBCs [25]. These intimate contacts trypanosome-RBCs together with presence of filamentous material shed from the surface coat of trypanosomes to RBCs membrane and interaction of RBCs filopodia with those produced by T. evansi could account for the antigenic modifications of RBCs (not studied in this work), and those described for trypanosomes.
Conclusion
This work not only reports for the very first time, the adhesion of T. evansi to RBCs in a process mediated by emission of a filamentous material derived from surface coat and filopodia from trypanosomes and erythrocytes, but also fusion of plasma membranes between trypanosomes and RBC, changes in the oligosaccharide composition of RBC plasma membrane and antigenic modification of plasma membrane of T. evansi . While changes in antigenic composition of T. evansi plasma membrane could allow it to evade immune system by masking their own antigens with antigens taken from host, changes in RBC morphology and membrane oligosaccharides, surely contribute to changes in RBC antigenicity (not studied in this work) that determine cell damage and lysis, as well as abnormal phagocytosis in adrenal glands, skeletal muscle, liver and spleen of experimentally infected mice. However, further research are needed to: (1) identify T. evansi antigens that could be responsible for the modification of RBC surface, (2) define the role that biologically active molecules secreted by T. evansi have on morphological and biochemical changes of host cells surface, (3) know the potential role of antigen masking on immune system evasion and (4) know precise nature of the adsorption of host antigens to plasma membrane of T. evansi .
References
- Quinones Mateu ME, Finol HJ, Sucre LE, Torres SH (1994) Muscular Changes in Venezuelan Wild Horses Naturally Infected with Trypanosoma evansi. J Comp Pathol 110: 79-89.
- Rossi M, Boada-Sucre A, Finol HJ, Tejero F, Aso P, et al. (1999) Ultrastructural alterations in the adrenal gland cortex of mice experimentally infected with a Venezuelan isolate of Trypanosoma evansi. J Submicrosc Cytol Pathol 31: 509-513.
- Finol HJ, Boada-Sucre A, Rossi M, Tejero F (2001) Skeletal muscle ultrastructural pathology in mice infected with Trypanosoma evansi. J Submicrosc Cytol Pathol 33: 65-71.
- Rossi S MS, Boada-Sucre AA, Hernández G, Bello B, Finol HJ, et al. (2009) Análisis Ultraestructural del Hígado en Ratones Infectados Experimentalmente con un Aislado Venezolano de Trypanosoma evansi (KINETOPLASTIDA: TRYPANOSOMATIDAE). Acta Microscópica 17: 5-12.
- Rossi S MS, Boada-Sucre AA, Márquez ML, Hernández G, Payares G, et al. (2017) Description of an intracellular stage in the experimental infections of albino mice with a Venezuelan isolated of Trypanosoma evansi using scanning and transmission electron microscopy techniques. Submitted to Vet Ital (In press).
- Van Den Ingh TS, Zwart D, Schootman AJ, Van Miert AS, Veenendaal GH, et al. (1976) The pathology and pathogenesis of Trypanosoma vivax infection in goat. Res Vet Sci. 1: 264-270.
- Igbokwe IO (1989) Dyserythropoiesis in animal trypanosomiasis. Rev Elev Med VetPaysTrop 42: 423-429.
- Esievo KAN, Saror DI (1991) Immunochemistry and Immunopathology of Animal Trypanosomiasis. Vet Bull 61: 755-777.
- Igbokwe IO (1994) Mechanisms of cellular injury in African trypanosomiasis. Vet Bull 64: 611- 620.
- Jenkins GC (1980) Effects of trypanosomes on the haemopoietic system. In: Tweentieth Seminar on Trypanosomiasis. Trans R Soc Trop Med Hyg 74: 268-270.
- Woo PTK, Soltys MA (1970) Animals as reservoir hosts of human trypanosomes. J Wildl Dis 6: 313-322.
- Brun R, Hecker H, Lun ZR (1998) Trypanosoma evansi and Trypanosoma equiperdum: distribution, biology, treatment and phylogenetic relationship. Vet Parasitol 79: 95-107.
- Lanham SM, Godfrey DG (1970) Isolation of salivarian Trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol 28: 521-534.
- Brener Z (1962) Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev Inst Med TropSao Paulo 4: 389-396.
- Boada-Sucre AA, De Stefano H, González B, Soto H, Godoy S, et al. (1999) Microscopía Electrónica de Barrido de las alteraciones presentes en los espermatozoides de toros mestizos Siboney. Rev Cient FCV-LUZ 9: 235-242.
- Reynolds ES (1963) The Use of Lead Citrate at High pH as an Electron-Opaque Stain in Electron Microscopy. J. Cell Biol 17: 208-212.
- Rodríguez P.S (Personal communication).
- González-Elorriaga M, Cánepa G (2002) Los mastocitos en la lengua del sapo Bufo marinus L. Caracterización histoquímica de glicoconjugados con técnicas convencionales y con lectinas. Acta Cien Venez 53: 183-194.
- NewboldCI,Boyle DB,Smith CC,Brown KN (1982 Identification of a schizont- and species-specific surface glycoprotein on erythrocytes infected with rodent malarias. Mol Biochem Parasitol 5: 45-54.
- Simoes MT (1990) Trypanosoma evansi: proteins, parasite and host antigens on the plasma membrane of trypomastigotes isolated from mice blood. Special Degree Thesis presented to the Central University of Venezuela to qualify for a bachelor's degree in Biology. Science Faculty. Central University of Venezuela. Venez 59 pages.
- Fraker PJ, Speck JC (1978) Protein and cell membrana iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphenyl-glycoluril. Biochem Biophys Res Commun 80: 849-857.
- Laemmli UK (1979) Cleavage of Structural Proteins During the Assembly of the Head of the Bacteriophage T4. Nature 227: 680-685.
- Poltera AA (1985) Pathology of human African trypanosomiasis with reference to experimental African trypanosomiasis and infections of the central nervous system. Br Med Bull 41: 169-174.
- Goodwin LG (1970) The pathology of African trypanosomiasis. Trans R Soc Trop Med Hyg 64: 797-812.
- Anosa VO, Kaneko JJ (1983) Pathogenesis of Trypanosoma brucei infection in deer mice (Peromyscus maniculatus): Light and electron microscopic studies on erythrocyte pathologic changes and phagocytosis. Am J Vet Res 44: 645-651.
- Boada-Sucre AA, Rossi S MS, Tavares-Marques LM, Finol HJ, Reyna-Bello A, et al. (2016) Trypanosoma vivax: Adhesion to Red Blood Cells in Experimentally Infected Sheep. Pathol Res Int, Article ID 4503214.
- Anderson RA, Paquette S, Lovrien R (2002) Lectin-erythrocyte interaction with external transmembrane glycophorin saccharides controlling membrane internal cytoskeleta. J Agric Food Chem 50: 6599-6604.
- Waespy M,Gbem TT,Elenschneider L,Jeck A-P,Day CJ, et al. (2015) Carbohydrate Recognition Specificity of Trans-sialidase Lectin Domain fromTrypanosoma congolense. PLoS Negl Trop Dis 9: e0004120.
- Hemphill A, Ross CA (1995) Flagellum-mediated adhesion of Trypanosoma congolense to bovine aorta endothelial cells. Parasitol Res 81: 412-420.
- Macadam RF, Herbert WJ (1970) Fine Structural Demostration of Cytoplasmic Protrusions (Filopodia) in Trypanosomes. Exp Parasitol 27: 1-8.
- Hernández-Páez R (1980) Consideraciones Ultraestructurales sobre el Trypanosoma venezuelense, Mesnil 1910; Aspecto Morfológico Normal del Trypanosoma venezuelense. Rev Fac Cs Vets UCV 28: 83-106.
- Banks KL (1979) In Vitro Binding of Trypanosoma congolense to Erythrocytes. J Protozool 26: 103-108.
- Knowles G, Black SJ, Whitelaw DD (1987) Peptidase in the plasma of mice infected with Trypanosoma brucei brucei. Parasitology 95: 291-300.
- Rossi M, Bremo A, Gonzatti MI, Giardina S (1995) Trypanosoma evansi: secretes proteins into extracellularly. Acta Cien Venez 46 Suppl 1: 88.
- Rossi S MS. Data not published.
- Boada-Sucre AA, Rossi S MS, Gómez E, González R, Rodríguez JP, et al. (2013) Experimental Infection of Sheeps with Trypanosoma vivax (Strain LIEM-176): changes in the body temperature, haematocrit, haemoglobin and plasma proteins. Rev Iberolatinoam Parasitol 72: 132-141.
- Giardina S, Paganico G, Urbani G, Rossi M (2003) A Biochemical and Immunological Comparative Study on Trypanosoma equiperdum and Trypanosoma evansi. Vet Res Comm. 27: 289-300.
- Nikolskaia OV,De A Lima AP,Kim YV,Lonsdale-Eccles JD,Fukuma T,et al. (2006) Blood-brain barrier traversal by African trypanosomes requires calcium signaling induced by parasite cysteine protease. J Clin Invest 116: 2739-2747.
- Engstler M, Schauer R (1993) Sialidases from african trypanosomes. Parasitol Today 9: 222-225.
- Nok AJ, Humprey C, Zelibe N, Yako SK (2003) Sialidase: surface localization, properties and hydrolysis of ghost red blood cells and brain cells-identification in tripanosomiasis. Z Naturforsch C 58: 504-601.
- Shehu SA, Ibrahim NDG, Esievo KAN, Mohammed G (2006) Neuraminidase (Sialidase) Activity and its Role in Development of Anaemia In Trypanosoma evansi Infections. JAppl Sci 6: 2779-2783.
- Sallau AB, Ibrahim MA, Salihu A, Yusuf IA (2008) Bloodstream Form of Trypanosoma evansi Contains β-Galactosidase. Middle East J Sci Res 3: 49-52.
- Nok AJ, Balogun EO (2003) A Bloodstream Trypanosoma congolense Sialidase could be involved in anemia during experimental trypanosomiasis. J Biochem 133: 725-730.
- Buckner FS, Wilson JA, Vanvoohirs WC (1999) Detection of live Trypanosoma cruzi in tissues of infected mice by using histochemical stain for β-Galactosidase. Infect Immun 67: 403-409.
- Vella M, Greenwell P (1997) Purification and partial characterization of β-Galactosidase from Tritrichomonas foetus. Glycoconjugate J 14: 883-887.
- Connaris S, Greenwell P (1997) Glycosidases in mucin-dwelling protozoans. Glycoconj J 14: 879-882.
- Muller R, Franco NW, Schauer R (1981) Involvement of membrane galactose in the in vitro sequestration of desialylated erythrocytes. Hoppe SeylersZ Physiol Chem 364: 1615-1620.
- Nok AJ, Nzelibe HC, Yako SK (2003) Trypanosoma evansi sialidase: surface localization, properties and Hydrolysis of Ghost Red Blood Cells and brains cells-implication in Trypanosomiasis. Z Naturforsch C 58: 594-601.
- Stijlemans B, Caljon G, Van Den Abbeele J, Van Ginderachter JA, et al. (2016) Immune evasion Strategies of Trypanosoma brucei within the Mammalian Host: Progression to Pathogenicity. Front Immunol 7: 233.
- Ferreira De Miranda-Santos IK, Campos-Neto A (1981) Receptor For Immunoglobulin Fc On Pathogenic But Not On Nonpathogenic Protozoa Of The Trypanosomatidae. J Exp Med 154: 1732–1742.
- Ramalho-Pinto FJ, Carvalho EM, Horta MF (1992) Mechanisms of evasion of Schistosoma mansoni schistosomula to the lethal activity of complement. Mem Inst Oswaldo Cruz 87 Suppl 4: 111-116.
- Turner MJ, Donelson JE (1990) Cell Biology of African Trypanosomes. In: D.J. Wyler editor. Modern Parasite Biology, Cellular, Immunological and Molecular Aspects, W.H. Freeman and Company. New York, USA P- 51-63.
- Touratier L (1993) Isolation of Variant Antigenic Types from Venezuelan Stock of Trypanosoma evansi. In: First International Seminar on Non TseTse-Transmitted Animal Trypanosomes: conclusions and recommendations. Rev Sci Tech 12: 273-281.
- Furtado MB, Benchimol M (1998) Observation of membrane fusion on the interaction of Trichomonas vaginalis with human vaginal epithelial cells. Parasitol Res 84: 213-220.
Relevant Topics
- Classic Diagnostic Pathology
- Diagnostic Molecular Pathology
- Diagnostic Pathology
- Diagnostic Pathology of Infectious Diseases
- Diagnostic Pathology Reports
- Diagnostic Surgical Pathology
- Diagnostic Techniques in Pathology
- Forensic Pathologist Communications
- Immunohistochemistry (IHC):
- Immunopathology
- Molecular Pathology
- Pathobiology
- Pathology Diagnostics Market Analysis
- Prognosis-related diagnosis
- Stereology
- Tissue based Diagnosis
- Virtual Microscopy
- Virtual Pathology
Recommended Journals
Article Tools
Article Usage
- Total views: 5125
- [From(publication date):
March-2017 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 4163
- PDF downloads : 962