Research Article Open Access
Adequate Timing of Diagnostic CT-Scan in Colorectal Patients Suffering From Anastomotic Leakage Can Improve Survival
| JRD Reuvers*, Milan Richir, Ingrid Kappers and Hermien Schreurs | |
| Department of Surgery, Medical Centre Alkmaar, Postbus, Netherlands | |
| Corresponding Author : | JRD Reuvers Department of Surgery Medical Centre Alkmaar Postbus 50, 1800 AM, Alkmaar, The Netherlands Tel: 0031725484444 Fax: 0031725482168 E-mail: reinderreuvers@icloud.com |
| Received: June 29, 2015 Accepted: August 03, 2015 Published: August 07, 2015 | |
| Citation: Reuvers JRD, Richir M, Kappers I, Schreurs H (2015) Adequate Timing of Diagnostic CT-Scan in Colorectal Patients Suffering From Anastomotic Leakage Can Improve Survival. OMICS J Radiol 4:199. doi:10.4172/2167-7964.1000199 | |
| Copyright: © 2015 Reuvers JRD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Radiology
Abstract
Introduction: Anastomotic leakage (AL) is a feared complication of gastrointestinal surgery and has a high morbidity and mortality. Although several studies have investigated risk factors for AL and its diagnosis, little is known about treatment strategies for AL and the relationship between mortality and the time interval between the diagnosis of the AL and its treatment. The aims of this study were to gain insight into the influence of the time between diagnosis and treatment of AL and to investigate what interventions are used.
Methods: Retrospective study of patients surgically treated for AL between January 2008 and December 2012 in our hospital in the Netherlands.
Results: In total 2095 abdominal gastrointestinal surgeries were performed, 120 patients were included in our study (5.7%). Non-survivors were significantly older, had a higher CRP level on the day of reoperation, and had to wait longer for surgery after the diagnostic CT scan. A probit model described mortality risk as a function of age and time to corrective surgery.
Conclusion: Older age and longer delay between diagnostic CT and surgery for AL were associated with an increased mortality. This emphasizes the fact that urgent corrective surgery is necessary to decrease AL mortality, especially in the older patient. We advise to standardize the treatment of AL; this prevents delay and increases chances of survival.
|
Abstract
Introduction: Anastomotic leakage (AL) is a feared complication of gastrointestinal surgery and has a high morbidity and mortality. Although several studies have investigated risk factors for AL and its diagnosis, little is known about treatment strategies for AL and the relationship between mortality and the time interval between the diagnosis of the AL and its treatment. The aims of this study were to gain insight into the influence of the time between diagnosis and treatment of AL and to investigate what interventions are used.
Methods: Retrospective study of patients surgically treated for AL between January 2008 and December 2012 in our hospital in the Netherlands. Results: In total 2095 abdominal gastrointestinal surgeries were performed, 120 patients were included in our study (5.7%). Non-survivors were significantly older, had a higher CRP level on the day of reoperation, and had to wait longer for surgery after the diagnostic CT scan. A probit model described mortality risk as a function of age and time to corrective surgery. Conclusion: Older age and longer delay between diagnostic CT and surgery for AL were associated with an increased mortality. This emphasizes the fact that urgent corrective surgery is necessary to decrease AL mortality, especially in the older patient. We advise to standardize the treatment of AL; this prevents delay and increases chances of survival. Introduction
Anastomotic leakage (AL) is a feared complication of colorectal surgery and is associated with increased morbidity and mortality. Despite advances in surgical technology, the reported risk of 1–22% is still high [1,2]. AL results in prolonged hospitalization, re-operation, and permanent enterostomy. It also leads to an increased mortality (10–16%) in the immediate postoperative period and a decreased 5-year survival [3,4]. Despite numerous studies investigating the relationship between patient risk factors and AL and different surgical techniques, the pathogenesis of AL remains unclear. Early diagnosis of AL, preferably before it becomes symptomatic, is an important determinant of outcome [2,5]. The level of C-reactive protein (CRP) has been investigated as presymptomatic marker but there is as yet no consensus about its predictive value [6,7] . Clinical signs of a systemic inflammatory response, such as fever, ileus and pain, are common in patients with AL but have a low specificity [8,9].
A delay in managing peritonitis will increase the risk of exacerbation of the inflammatory response [10], but relatively little is known about the most appropriate treatment of AL [5,11]. In a study involving 137 surgeons the most important determinants of treatment for managing colorectal AL, whether to create an enterostomy or a new anastomosis, were the localization and presentation of the leak, and the patient’s overall physical condition [12]. The aims of this study were to gain insight into which factors in the diagnosis and management of AL are of influence on mortality in our patient population. This can improve treatment strategies. Methods
Patients
The medical records were reviewed of all patients who had undergone abdominal gastrointestinal surgery with construction of an anastomosis in the small or large bowel, and who were diagnosed with AL, within 30 days of primary surgery at our institute between January 2008 and December 2012. Only patients who were diagnosed with AL during surgery and who underwent surgical treatment of AL were included in the study. After inclusion, patient characteristics such as age, sex, C-reactive protein (CRP), leucocytes, time between request of CT-scan and the moment the scan was actually made, and the time between CT diagnosis of AL and corrective surgery (CT-time), duration of primary surgery, treatment of AL, American Society of Anesthesiologists (ASA)-score and whether initial surgery was acute or elective, were recorded. Exclusion criteria were; no anastomotic leakage during surgery, and time between CT-scan and corrective surgery >24 hours. We consider duration longer than 24 hours indicates not enough clinical and radiological evidence of AL to perform laparotomy.
Statistical analysis
Data are expressed as means ± standard deviation (SD). Differences between survivors and non-survivors were analyzed by using Students T-test for nominal values and logistics regression when data were binary. A two-tailed p<0.05 was considered statistically significant. Statistical analyses were performed using SPSS 21.0 (SPSS Inc., Chicago, IL), the probit analysis was performed using MATLAB R2013b (Mathworks Inc., Illinois, MA).
Probit analysis was used to determine the relationship between mortality and the time between the diagnostic CT scan (CT time) and surgical treatment of AL. Then the effect of age and time to corrective surgery in combination was tested: Pr (death=1 | X) = φ(X’β), where mortality is a dependent variable, X is a matrix containing time from diagnostic CT to corrective surgery (CT time), and/or age, β is a vector containing the coefficients of the variables of interest, and finally φ is the cumulative distribution function. The full model containing CT time and age is: Y*=α+β1*cttime+β2*age+ε. Results
Patients
In our study period a total of 2095 gastrointestinal surgeries were performed at the Department of Surgery in our hospital in the Netherlands. Of this number 120 patients (5.7%) suffered from AL and were surgically treated. Patient characteristics are listed in Table 1.
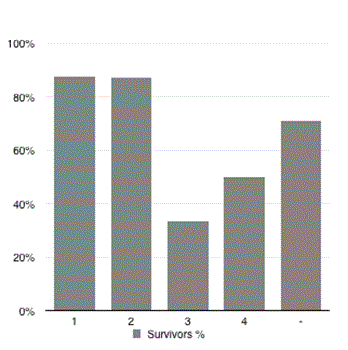
Thirty patients (25.0%) died within 30 days of primary surgery. The non-survivors were significantly older (p<0.001). There was a trend towards higher levels of CRP (p=0.06) on the day of reoperation for AL compared to the survivors. Data on ASA-score was missing in 55% of the patients. More ASA 3 and 4 patients were seen in the deceased group. The percentage of unknown ASA-scores was equal in each subgroup. There was no correlation between ASA-score and mortality. Graph. 1 shows the survival in each ASA-classification subgroup. Diagnostic CT scan was performed in 72 of 120 patients. The time between diagnostic CT and corrective surgery was significantly longer among the non-survivors compared to the survivors (465 ± 387 minutes vs. 311 ± 216 minutes, respectively; p<0.05). Time between diagnosis and re-operation could not be determined when AL was diagnosed without CT scan. The preoperative characteristics of patients developing AL are shown in Table 2. Treatment of AL
Table 3 shows the surgical managements that were used for the treatment of AL. Enterostomy was used most often (80.8% of patients); construction of a new anastomosis was performed in 12.5%. There was no significant difference in the treatment of AL between surviving and non-surviving patients, and treatment was not correlated with mortality.
In Table 4 the types of initial surgery and indications for surgery are shown. Most patients were treated for colon carcinoma. There were no significant differences in initial surgery and initial disease between survivors and non-survivors. Mortality
Univariate and multivariate logistic regression revealed age and time between CT and surgery (CT time) to be significantly associated with mortality (p<0.05) (Table 5).
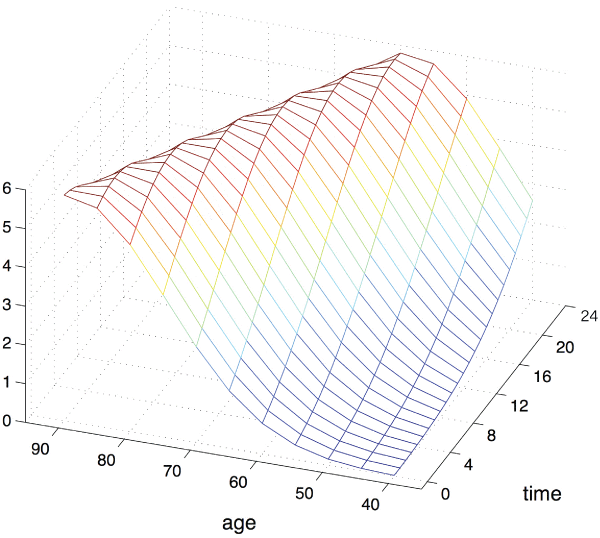
A probit analysis was performed to investigate the relationship between age and the time between diagnostic CT and surgical treatment of AL on mortality (Figure 1). The relative mortality risk for a 60-year-old patient doubled when surgery was performed 6 hours instead of 2 hours after AL diagnosis. If surgery was performed immediately after AL diagnosis, the mortality risk was twice as high in a 70-year-old patient compared to a 60-year-old patient. Discussion
This study shows that an older age and longer delay between diagnostic CT and surgery for AL are associated with an increased mortality. Therefore, it is of vital importance that surgery to manage AL should be performed as soon as possible after AL diagnosis, especially in older patients. Type of treatment for AL was not associated with mortality, and the mortality rate was similar to other studies [13,14].
This is the first study that endeavors to provide insight into mortality risk and age after AL. In our study, the non-survivors were significantly older than the survivors (p=<0.001). On probit analysis, we found that the mortality risk increased with age, i.e. the mortality risk in an 80-year-old patient was five times higher than in a 60-year-old patient. The association between time to corrective surgery and mortality risk was still present after correction for age. The association between time to corrective surgery and mortality is probably due to the impact of prolonged exposure to bacterial peritonitis. Barnett et al. showed the effect of inflammation on mortality in mice with bacterial peritonitis. Interestingly, the inflammatory response was disturbed in the mice that died compared with the mice that survived, despite the bacterial count being the same [10]. We found that the non-survivors had a higher CRP level on the day of corrective surgery (p=0.06), which is consistent with the literature and may reflect the above-mentioned disturbed inflammatory response [15-17]. Unlike other studies, our study failed to show a correlation between comorbidity (ASA score >3) and mortality [18,19]. This was probably due to the fact that the ASA score was lacking in a large amount of patients. The second goal of this study was to evaluate the various procedures to correct AL. Most surgeons (79%) chose to create an enterostomy. We did not find the choice of treatment to correct AL to be associated to mortality. Even so, initial disease and type of initial surgery were also not associated with mortality. With its retrospective nature, this study was not designed to demonstrate any differences in mortality for different management of AL. Few studies have reported about the different treatment approaches that were used for the management of AL, but the preference for enterostomy is consistent with another study [18]. Fraccalviere et al. found that the creation of a loop stoma and salvage of the anastomosis were accompanied by a lower mortality and morbidity and higher stoma reversal compared with breaking down the initial anastomosis and creating a stoma [20]. Although the decision of treatment for each patient was made by skilled surgeons, the lack of protocol how to treat a patient with (suspected) AL is one of the problems we found. We assume the lack of standardization causes the delay we observed, and thereby the increased risk of mortality. The only protocol in the treatment of the patients in our hospital was post-operative admission to the ICU and intravenously Cefuroxime® and Metronidazole® for five days. The decisions towards surgical treatment are not standardized. Therefore it is hard to assume what steps in the post-operative time are of major influence. For example, as shown by den Dulk et al. standardizing the surveillance after surgery leads to less delay in diagnosis and better outcome after AL [21]. Since we show the effect of delay in the CT-surgery time, we suggest that a system should be developed to indicate the urgency of surgery for specific patients. For example, patients aged 70 years and older should undergo surgery within 2 hours of AL diagnosis, whereas younger patients should undergo surgery within 5 hours, of course taking into account the clinical condition of the individual patient. One could also consider skipping the CT-scan, especially in patients with high clinical suspicion of AL. This will decrease delay in treatment and improve the chances of survival. The major problem with this approach is the increased chances of negative laparotomy and its negative impact on the patient's already poor condition. More research needs to be done to explore the benefits and risks of this approach. Conclusion
In conclusion, this study shows no differences in mortality between different treatments of AL. It does show that older age and longer interval between diagnostic CT and corrective surgery for AL are associated with an increased mortality. These results stress the importance of early intervention when patients are diagnosed with or clinically suspected of AL, especially for the elderly patient. We were able to illustrate an exponential increase in mortality when surgery is delayed in older patients. This may help in developing a treatment logarithm for standardizing the management of AL, from the time of diagnosis up to type of surgery, including a maximum waiting time for different patient categories, depending on age, CRP level and comorbidity.
We stress the importance, for the severe complication AL is, to start standardizing treatment from suspicion until the actual intervention. In the future, prospective studies should be done to gain insight into the effects of standardization. |
References
- Rullier E, Laurent C, Garrelon JL, Michel P, Saric J, et al. (1998) Risk factors for anastomotic leakage after resection of rectal cancer. Br J Surg 85: 355-358.
- Sabbagh C, Maggiori L, Panis Y (2013) Management of failed low colorectal and coloanal anastomosis. J Visc Surg 150: 181-187.
- Buchs NC, Gervaz P, Secic M, Bucher P, Mugnier-Konrad B, et al. (2008) Incidence, consequences, and risk factors for anastomotic dehiscence after colorectal surgery: a prospective monocentric study. Int J Colorectal Dis 23: 265-270.
- McArdle CS, McMillan DC, Hole DJ (2005) Impact of anastomotic leakage on long-term survival of patients undergoing curative resection for colorectal cancer. Br J Surg 92: 1150-1154.
- Lindgren R, Hallböök O, Rutegård J, Sjödahl R, Matthiessen P (2011) What is the risk for a permanent stoma after low anterior resection of the rectum for cancer? A six-year follow-up of a multicenter trial. Dis Colon Rectum 54: 41-47.
- Warschkow R, Beutner U, Steffen T, Müller SA, Schmied BM, et al. (2012) Safe and early discharge after colorectal surgery due to C-reactive protein: a diagnostic meta-analysis of 1832 patients. Ann Surg 256: 245-250.
- Garcia-Granero A, Frasson M, Flor-Lorente B, Blanco F, Puga R, et al. (2013) Procalcitonin and C-reactive protein as early predictors of anastomotic leak in colorectal surgery: a prospective observational study. Dis Colon Rectum 56: 475-483.
- Doeksen A, Tanis PJ, Vrouenraets BC, Lanschot van JJ, Tets van WF (2007) Factors determining delay in relaparotomy for anastomotic leakage after colorectal resection. World J Gastroenterol 13: 3721-3725.
- den Dulk M, Marijnen CA, Collette L, Putter H, Påhlman L, et al. (2009) Multicentre analysis of oncological and survival outcomes following anastomotic leakage after rectal cancer surgery. Br J Surg 96: 1066-1075.
- Barnett RE, Keskey RC, Rao JM, Billeter AT, Kanaan Z, et al. (2013) Poor outcome in bacterial peritonitis is associated with dysregulated microRNAs and an increased inflammatory response. Surgery 154: 521-527.
- Merad F, Hay JM, Fingerhut A, Yahchouchi E, Laborde Y, et al. (1999) Is prophylactic pelvic drainage useful after elective rectal or anal anastomosis? A multicenter controlled randomized trial. French Association for Surgical Research. Surgery 125: 529-535.
- Daams F, Slieker JC, Tedja A, Karsten TM, Lange JF (2012) Treatment of colorectal anastomotic leakage: results of a questionnaire amongst members of the Dutch Society of Gastrointestinal Surgery. Dig Surg 29: 516-521.
- Daams F, Luyer M, Lange JF (2013) Colorectal anastomotic leakage: aspects of prevention, detection and treatment. World J Gastroenterol 19: 2293-2297.
- Branagan G, Finnis D; Wessex Colorectal Cancer Audit Working Group (2005) Prognosis after anastomotic leakage in colorectal surgery. Dis Colon Rectum 48: 1021-1026.
- Ramanathan ML, Mackay G, Platt J, Horgan PG, McMillan DC (2013) Impact of day 2 C-reactive protein on day 3 and 4 thresholds associated with infective complications following curative surgery for colorectal cancer. World J Surg 37: 2705-2710.
- Platt JJ, Ramanathan ML, Crosbie RA, Anderson JH, McKee RF, et al. (2012) C-reactive protein as a predictor of postoperative infective complications after curative resection in patients with colorectal cancer. Ann Surg Oncol 19: 4168-4177.
- Lagoutte N, Facy O, Ravoire A, Chalumeau C, Jonval L, et al. (2012) C-reactive protein and procalcitonin for the early detection of anastomotic leakage after elective colorectal surgery: pilot study in 100 patients. J Visc Surg 149: e345-349.
- Rickert A, Willeke F, Kienle P, Post S (2010) Management and outcome of anastomotic leakage after colonic surgery. Colorectal Dis 12: e216-223.
- Alves A, Panis Y, Pocard M, Regimbeau JM, Valleur P (1999) Management of anastomotic leakage after nondiverted large bowel resection. J Am Coll Surg 189: 554-559.
- Fraccalvieri D, Biondo S, Saez J, Millan M, Kreisler E, et al. (2012) Management of colorectal anastomotic leakage: differences between salvage and anastomotic takedown. Am J Surg 204: 671-676.
- den Dulk M, Noter SL, Hendriks ER, Brouwers MA, van der Vlies CH, et al. (2009) Improved diagnosis and treatment of anastomotic leakage after colorectal surgery. Eur J Surg Oncol 35: 420-426.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 | Table 5 |
Figures at a glance
 |
 |
| Graph 1 | Figure 2 |
Relevant Topics
- Abdominal Radiology
- AI in Radiology
- Breast Imaging
- Cardiovascular Radiology
- Chest Radiology
- Clinical Radiology
- CT Imaging
- Diagnostic Radiology
- Emergency Radiology
- Fluoroscopy Radiology
- General Radiology
- Genitourinary Radiology
- Interventional Radiology Techniques
- Mammography
- Minimal Invasive surgery
- Musculoskeletal Radiology
- Neuroradiology
- Neuroradiology Advances
- Oral and Maxillofacial Radiology
- Radiography
- Radiology Imaging
- Surgical Radiology
- Tele Radiology
- Therapeutic Radiology
Recommended Journals
Article Tools
Article Usage
- Total views: 14233
- [From(publication date):
August-2015 - Apr 26, 2025] - Breakdown by view type
- HTML page views : 9635
- PDF downloads : 4598
