Review Article Open Access
Adenosine��?s Autacoid Function in the Central Nervous System and the Behavioral State of Conservation-Withdrawal
| Brandy A. Briones1, Traci N. Plumb2 and Thomas R. Minor2,3,4* | ||
| 1Department of Psychology, Princeton University, | ||
| 2Department of Psychology, University of California, Los Angeles | ||
| 3Brain Research Institute, UCLA | ||
| 4Integrative Center for Learning and Memory, UCLA | ||
| Corresponding Author : | Thomas R. Minor, Ph. D Department of Psychology Campus Box: 156304, UCLA Los Angeles, CA 90095-1563 Tel: (310) 625-3611 Email: minor@psych.ucla.edu |
|
| Received August 04, 2014; Accepted October 16, 2014; Published October 18, 2014 | ||
| Citation: Briones BA, Plumb TN, Minor TR (2014) Adenosine’s Autacoid Function in the Central Nervous System and the Behavioral State of Conservation- Withdrawal. Autacoids 3:106. doi: 10.4172/2161-0479.1000106 | ||
| Copyright: © 2014 Briones BA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Journal of Autacoids and Hormones
Abstract
The purine nucleoside adenosine has the critical autacoid function of directly linking cellular excitability to energy availability. The mechanism is activated whenever the rate of adenosine triphosphate (ATP) utilization exceeds the rate of synthesis. In CNS neurons, adenosine is produced by the rapid intracellular hydrolysis of purine nucleotides during neural excitation and then is extruded into extracellular space. The nucleoside is also produced by the extracellular hydrolysis of ATP by ectonucleotidases. Extracellular adenosine interacts with G-protein linked stereospecific receptors to reestablish metabolic homeostasis by exerting extraordinarily potent inhibition of neural excitation via a number of mechanisms. This autacoid mechanism is directly linked to the production of a depression like behavioral state termed conservation-withdrawal during times of physical stress or severe emotional distress. We review evidence here that adenosine produces a transition to conservation-withdrawal by activation of A2A receptors in the ventral-medial striatum.
| Keywords | |
| Adenosine; A2A receptor; Autacoid; Behavioral depression; Conservation-withdrawal; Learned helplessness; Striatum | |
| Introduction | |
| Adenosine as an autacoid | |
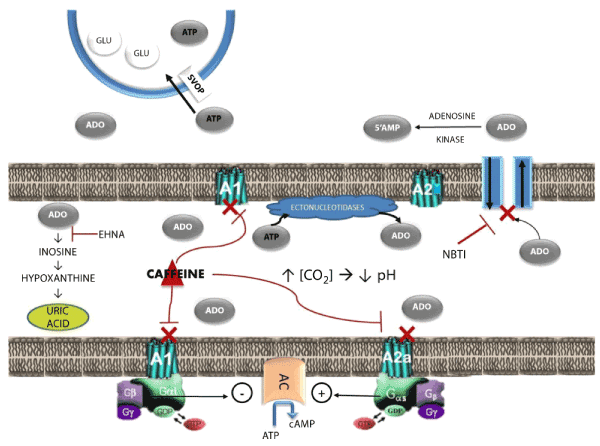
| The purine nucleoside adenosine functions as an autacoid that links cellular excitability to energy availability in most forms of excitable tissue [1-2]. Adenosine signaling meets all of the criteria of an autocrine mechanism: the nucleoside is produced at the synapse to regulate signal transduction; its production and release are highly regulated; and there are multiple mechanisms of reuptake, deactivation, and degradation to limit its action to a discrete region (Figure1). | |
| Adenosine plays a crucial homeostatic role in regulating neural excitability in the central nervous system. Figure 1 shows a generic synapse in the CNS to summarize adenosine pharmacology and function. Brain adenosine concentrations normally are about 10,000 fold lower than those of ATP (adenosine triphosphate) [3]. Extracellular adenosine is increased by one of two mechanisms. Adenosine’s autacoid function is engaged whenever the rate of ATP utilization exceeds the rate of synthesis. In brain neurons, this type of imbalance in the energy supply/demand ratio can result from excessive neural activation or from a shortage in brain glucose or oxygen. The nucleoside is produced in nanomolar concentrations as cellular work increases via S-adenosyl- L-homocysteine (SAH) metabolism and is extruded into extracellular space via bidirectional transporters [4-5]. Adenosine is also produced by the rapid hydrolysis of extracellular ATP [6]. Adenine nucleotides are actively transported into and co-localized with neurotransmitter in synaptic vesicles. ATP is co-released with neurotransmitter from the presynaptic terminal at the time of synaptic activation. Carbon dioxide [CO2] concentrations increase in the synaptic cleft during the course neural excitation and cellular respiration. Extracellular pH decreases as a consequence. Acidification of the cleft activates membranebound ectonucleotidases, which convert ATP into adenosine. | |
| Extracellular adenosine exerts its homeostatic and regulatory actions by interacting with four G-protein coupled stereospecific receptors: A1, A2A, A2B, and A3 [7,8]. A1 receptors are widely distributed in the brain and mediate adenosine’s inhibitory actions by coupling with a Gi protein that inhibits adenylyl cyclase [9]. A2 receptors mediate adenosine’s excitatory actions by coupling a Gs protein that excites adenylyl cyclase [10-11]. The A2B subtype is a lowaffinity receptor that is widely distributed in most brain regions. The high-affinity A2A subtype has a much more limited distribution, being localized primarily on enkephalinecontaining GABAergic neurons in the striatopallidal tract of the striatum [12-13]. Limited concentrations of A2A receptors also are found in the thalamus [14-16], nucleus tractus solitarius [17-18], and cholinergic neurons of the pontine reticular formation [19-20]. A3 receptors are found primarily in the periphery, with high concentrations in testes and mast cells, and are not heavily expressed in the brain. These receptors play an important role in regulating inflammatory reactions [21-22]. | |
| The primary mechanism by which adenosine reestablishes metabolic homeostasis is to produce profound and prolonged inhibition of neural excitation. A number of highly selective receptor agonists that mimic the effects of adenosine at each receptor subtype are now available. By contrast, caffeine and theophylline are widely used to elevate mood, combat fatigue, and reverse the effects of sleep. These methylxanthine stimulants derive their stimulant properties by acting as nonselective antagonists at brain adenosine receptors [1-2]. | |
| Extracellular adenosine is regulated by two mechanisms. The nucleoside is rapidly transported into the presynaptic terminal by a bidirectional transporter and converted into 5’AMP by adenosine kinase [4]. The reuptake transporter is blocked by nitrobenzyltheoinosine (NBTI). Blockade of the adenosine reuptake also is at least partially responsible for the anxiolytic and anticonvulsant actions of the benzodiazepines [23]. The second regulatory mechanism is a degradation pathway involving adenosine deaminase, which converts the nucleoside into inactive inosine that is then degraded into stable uric acid [24]. The degradation pathway is blocked by erythro-9-(2- hydroxy-3-nonyl)adenine (EHNA). | |
| Adenosine and the behavioral state of conservation withdrawal | |
| This molecular regulatory mechanism has the unusual effect of directly mediating the transition to a depression-like state under highly aversive circumstances. We borrowed a term from the psychiatric literature (conservation-withdrawal) to characterize this reaction [25]. | |
| Engel and Schmale [26] originally coined this term in describing an exaggerated withdrawal response of a psychiatric patient to emotional challenges. The reaction unconditionally follows periods of intense catabolic output. The sensory unresponsiveness, cognitive dullness, and behavioral depression that characterize this state serve as adaptive mechanisms for husbanding limited resources and facilitating the recovery of metabolic homeostasis. The term is used more broadly in modern parlance to refer to enervated states associated with physical or psychological stress. | |
| Conservation-withdrawal is an integral component of major depression and related mood disorders [26-28]. The state most closely corresponds to the affect-less, fatigue components of depression, rather than subsuming the entirety of the behavioral, cognitive, emotional, and motivational symptoms that comprise the disorder. It also represents the aspects of affective disorders that are most accurately modeled in animals. Conservation-withdrawal is also a key component of the after-reaction to physical and psychological stress. Symptoms of conservation-withdrawal are seen after a patient leaves the intensive care unit following a serious injury and are often confused with major depression [28-30]. These same symptoms also are the hallmark of the after-reaction to traumatic uncontrollable stress that has been variously termed learned helplessness [31], behavioral despair [32], behavioral depression [33], and the distress syndrome [34]. Finally, conservationwithdrawal is a critical component of sickness behavior—the lethargy, hypoactivity, decreased libido, anorexia, anhedonia, and increased sleep that accompanies infectious disease [35-37]. This dramatic shift in ongoing activity, along with the induction of fever, is a highly adaptive strategy for fighting infection [35]. The overlap among mood disorders, the after-reaction to traumatic stress, recuperation from injury, and sickness behavior [38-40] suggests a common biological mechanism underlying these enervated states. We have argued that the overlap is well accommodated by the concept of conservation-withdrawal [41-44]. Here we review recent data, from Plumb et al. indicating that adenosine mediates the behavioral depression component of a conservation-withdrawal reaction via purine receptors in the ventralmedial striatum. Previous research implicated adenosine signaling at A2A receptor in the production of conservation-withdrawal in a number of animal models of depression [45]. Most adenosine receptor subtypes have a wide distribution in the CNS. However, A2A receptors are primarily located in the striatum, with a dense population in the nucleus accumbens. These receptors interact with dopamine signaling to influence the motivational regulation of ongoing behavior. Thus, we hypothesized that adenosine signaling increases in the N. accumbens during times of extreme catabolic output and emotional distress to uncouple the dopamine signal from ongoing behavior. Normal commerce with the environment transitions to a state of conservationwithdrawal. If so, then we should be able to prevent the transition to a state of conservation-withdrawal via pharmacological blockade of N. accumbens A2A receptors. | |
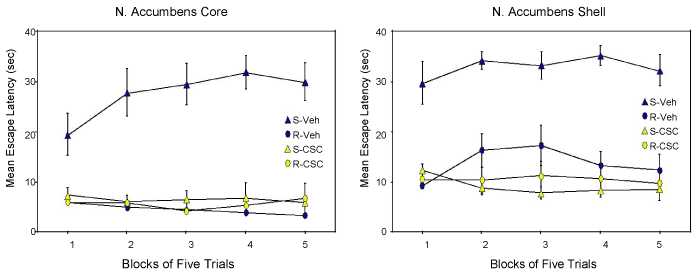
| Rats were surgically implanted with bilateral cannula in either the shell or core of nucleus accumbens. Following recovery from surgery, we exposed rats to traumatic shock stress (S: shock) or simple apparatus restraint (R: restraint) in the learned helplessness procedure. This is an animal model of post-traumatic stress disorder (PTSD) and comorbid depression. The behavioral syndrome induced by this procedure was considered to be a prototype for conservation-withdrawal by [26]. All rats were tested for shuttle-escape performance, the traditional measure of helplessness in rats [46], 24 hours later. Rats from each pretreatment condition received either bilateral microinfusion of the highly selective A2A receptor antagonist CSC (8-(3-chlorostyryl)caffeine) (S-CSC; R-CSC) or vehicle (S-Veh; R-Veh) 15 minutes before testing. Test data from experiments in the core (left panel) and shell (right panel) or N. Accumbens shown in Figure 2. A profound difference in escapable latencies occurred in vehicle-treated shocked (S-Veh) and restrained (R-Veh) groups in both experiments. The difference between these groups defines the learned helplessness effect and is our measure of the behavioral depression component of a conservation-withdrawal reaction. Treatment of the shocked group with the A2A receptor antagonist CSC shortly before testing (S-CSC) completly eliminated the performance deficits in both experiments (Figure 2). | |
| These data clearly implicate adenosine signaling the production of the learned helplessness effect and more generally a conservationwithdrawal reaction. Adenosine is acting at A2A receptors in the indirect pathway in the ventral-medial striatum to produce these effects. Adenosine regulates the motivational influence of mesolimbic dopamine signaling at these receptors. Activation of A2A receptors functionally uncouples dopamine from its receptor, undercutting the motivation for ongoing behavior. Conservation-withdrawal ensues. | |
| Conclusion | |
| Adenosine serves as an autacoid to regulated metabolic homeostasis in most forms of excitable tissue. The nucleoside is produced in CNS neurons during periods of excessive or unregulated excitation. The autacoid acts to reestablish metabolic homeostasis under these circumstances by exerting profound and prolonged inhibition of neural excitation [26] elaboration of a conservation-withdrawal reaction anticipates exactly this type of molecular mechanism. The sensory unresponsiveness, cognitive dullness, and behavioral depression that characterize this state were seen as unconditional reaction to severe emotional distress that husbanded limited energy resources and facilitated the recovery of metabolic homeostasis. Considerable evidence now suggests that activation of adenosine A2A receptors engenders exactly this type of reaction. | |
References
- Sattin A, Rall TW (1970) The effect of adenosine and adenine nucleotides on the cyclic adenosine 3'-5'-phosphate content of guinea pig cerebral cortex slices. Mol Pharmacol 6: 13-23
- Snyder SH, Katims JJ, Annau Z, Bruns RF, Daly JW (1981) Adenosine receptors and behavioral actions of methylxanthines. Proc Natl Acad Sci U S A 78: 3260-3264
- Chen X, Hui L, Geiger JD (2013) Adenosine and energy metabolism-relationship to brain bioengergetics. Adenosine 55-70
- Geiger JD, Fyda DM (1991) Adenosine transport in nervous tissues. In: Stone TW (ed) Adenosine in the central nervous system. Academic, London
- Parkinson FE, Damaraju VL, Graham K, Yao SY, Baldwin SA, et al. (2011) Molecular biology of nucleoside transporters and their distributions and functions in the brain. Curr Top Med Chem 11: 948-972
- Vorhoff T, Zimmermann H, Pelletier J, Sevigny J, Braun N (2005) Cloning and characterization of the ecto-nucleotidase NTPDase3 from rat brain: predicted and secondary structure and relation to other members of the E-NTPDase family and actin. Purinergic Signal 1: 259-270
- Haas HL, Selbach O (2000) Functions of neuronal adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol 362:375-381
- Phillis JW (2004) Adenosine and adenine nucleotides as regulators of cerebral blood flow: roles of acidosis, cell swelling, and KATP channels. Crit Rev Neurobiol 16: 237-270
- Linden J, Taylor HE, Robeva AS, Tucker AL, Stehle JH, et al. (1993) Molecular cloning and functional expression of a sheep A3 adenosine receptor with widespread tissue distribution. Mol Pharmacol 44: 524-532
- Bruns RF, Lu GH, Pugsley TA (1986) Characterization of the A2 adenosine receptor labeled by [3H] NECA in rat striatal membranes. Mol Pharmacol 29: 331-346
- Sebastião AM, Ribeiro JA (1996) Adenosine A2 receptor-mediated excitatory actions on the nervous system. Prog Neurobiol 48:167-189
- Calon F, Dridi M, Hornykiewicz O, Bédard PJ, Rajput AH, et al. (2004) Increased adenosine A2A receptors in the brain of Parkinson’s disease patients with dyskinesias. Brain 127:1075-1084
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB (1999) Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol 59: 355-396
- Ishiwata K, Mishina M, Kimura Y, Oda K, Sasaki T, et al. (2005) First visualization of adenosine A(2A) receptors in the human brain by positron emission tomography with [11C]TMSX. Synapse 55: 133-136
- Mishina M, Ishiwata K, Kimura Y, Naganawa M, Oda K, Kobayashi S, et al. (2007) Evaluation of distribution of adenosine A2A receptors in normal human brain measured with [11C]TMSX PET. Synapse 61: 778-784
- Weaver DR (1993) A2a adenosine receptor gene expression in developing rat brain. Brain Res Mol Brain Res 20: 313-327
- Castillo-Meléndez M, Krstew E, Lawrence AJ, Jarrott B (1994) Presynaptic adenosine A2a receptors on soma and central terminals of rat vagal afferent neurons. Brain Res 652: 137-144
- Scislo TJ, O’Leary DS (2006) Vasopressin V1 receptors contribute to hemodynamic and sympathoinhibitory responses evoked by stimulation of adenosine A2a receptors in NTS. Am J Physiol Heart Circ Physiol 290: H1889-H1898
- Coleman CG, Baghdoyan HA, Lydic R (2006) Dialysis delivery of an adenosine A2A agonist into the pontine reticular formation of C57BL/6J mouse increases pontine acetylcholine release and sleep. J Neurochem 96:1750-1759
- Ferré S, Diamond I, Goldberg SR, Yao L, Hourani SMO, et al. (2007) Adenosine A2A receptors in ventral striatum, hypothalamus and nociceptive circuitry: implications for drug addiction, sleep and pain. Prog Neurobiol 8: 332-347
- Gessi S, Merighi S, Varani K, Leung E, Mac Lennan S, et al. (2008) The A3 adenosine receptor: an enigmatic player in cell biology. Pharmacol Ther 117: 123-140
- Linden J, Taylor HE, Robeva AS, Tucker AL, Stehle JH, et al. (1993) Molecular cloning and functional expression of a sheep A3 adenosine receptor with widespread tissue distribution. Mol Pharmacol 44: 524–532
- Bender AS, Wu PH, Phillis JW (1981) The rapid uptake and release of [3H] Adenosine by rat cerebral cortical synaptosomes. J. Neurochem 6: 651
- Hask? G, Cronstein BN (2004) Adenosine: an endogenous regulator of innate immunity. Cell Press 25: 33-39
- Plumb TN, Sterlace SR, Cavanaugh KA, Minor TR (2013) Stress, brain adenosine signaling, and fatigue-related behavioral processes. Springer Science + Business Media, New York.
- Engel GL, Schmale AH (1972) Conservation-withdrawal: a primary regulatory process for organic homeostasis. In: Ciba Foundation Symposium on Physiology, Emotion and Psychosomatic Illness 8: 57-75
- Field T, Reite M (1984) Children’s responses to separation from mother during the birth of another child. Child Dev 55:1308-1316
- Weiner MF, Lovitt R (1979) Conservation-withdrawal versus depression. Gen Hosp Psychiatry 1: 347-349
- Mohta M, Sethi AK, Tyagi A (2003) Psychological care in trauma patients. Injury 34: 17- 25
- Weiner MF (1983) Conservation-withdrawal and mental retardation in medical and surgical patients. Psychosomatics 24: 41-43
- Overmier JB, Seligman ME (1967) Effects of inescapable shock upon subsequent escape and avoidance responding. J Comp Physiol Psychol 63: 28-33
- Porsolt RD, Bertin A, Jalfre M (1977) Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229: 327-336
- Weiss JM, Goodman PA, Losito BG, Corrigan S, Charry JM, et al. (1981) Behavioral depression produced by an uncontrollable stressor: relationship to norepinephrine, dopamine, and serotonin levels in various regions of rat brain. Brain Res Rev 3: 167-205
- Minor TR, Dess NK, Overmier JB (1991) Inverting the traditional view of “learned helplessness”. In: Denny MR (ed) Fear, avoidance, and phobias: a fundamental analysis. Lawrence-Erlbaum Associates, New Jersey.
- Dantzer R (2001) Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun 15: 7-24
- Hestad KA, Tønseth S, Støen CD, Ueland T, Aukrust P (2003) Raised plasma levels of tumor necrosis factor alpha in patients with depression: normalization during electroconvulsive therapy. J ECT 19:183-188
- Maes M (1995) Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry 19: 11-38
- Anisman H, Merali Z, Poulter MO, Hayley S (2005) Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des 11: 963-972
- Dantzer R, Wollman E, Vitkovic L, Yirmiya R (1999) Cytokines and depression: fortuitous or causative association? Mol Psychiatry 4: 328-332
- Yirmiya R (1996) Endotoxin produces a depressive-like episode in rats. Brain Res 711: 163-174
- Minor TR, Chang WC, Winslow JL (1994a) Stress and adenosine I: effects of methylxanthine and amphetamine stimulants on learned helplessness in rats. Behav Neurosci 108: 254-264
- Minor TR, Winslow JL, Chang WC (1994b) Stress and adenosine II: adenosine analogs mimic the effect of inescapable shock in shuttle-escape performance in rats. Behav Neurosci 108: 265-276
- Minor TR, Huang Q, Witt AE (2006) Cytokine-purine interactions in traumatic stress, behavioral depression, and sickness. CNS Neurol Disord Drug Targets 5: 547-560
- Minor TR, Plumb TN, Schell CJ, Pham AK (2010) Brain adenosine signaling in psychological trauma and comorbid depression. In: Sher L, Vilens A (eds) Neurobiology of posttraumatic stress disorder. Nova Science Publishers, Inc., New York.
- Plumb TN, Briones BA, Minor TR (2014) Striatal adenosine A2A receptors mediate stress-induced behavioral depression in rats. Biological Psychiatry, in press.
- Maier SF, Albin RW, Testa TJ (1973) Failure to learn to escape in rats previously exposed to inescapable shock depends on the nature of the escape response. J Comp Physiol Psychol 85: 581–592
Tables and Figures at a glance
 |
 |
|
| Figure 1 | Figure 2 |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14978
- [From(publication date):
November-2014 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 10394
- PDF downloads : 4584
