Addressing the Ineffectiveness of Antimicrobial Drugs: A Global Challenge
Received: 20-Oct-2023 / Manuscript No. JIDT-23-117579 / Editor assigned: 23-Oct-2023 / PreQC No. JIDT-23-117579(PQ) / Reviewed: 08-Nov-2023 / QC No. JIDT-23-117579 / Revised: 15-Nov-2023 / Manuscript No. JIDT-23-117579(R) / Published Date: 22-Nov-2023
Abstract
The ineffectiveness of antimicrobial drugs, especially antibiotics, represents one of the most pressing challenges for global health. In 2019, 1.27 million people died from common bacterial infections that had become antibioticresistant, underscoring the need for a global response. This article examines the dimensions of the problem, identifies challenges in pharmaceutical innovation, emphasizes the importance of financial incentives, and highlights the crucial role of global health authorities in combating antimicrobial drug ineffectiveness.
Keywords: Antimicrobial drug ineffectiveness; Antibiotic resistance; Global health; Pharmaceutical innovation; Financial incentives
Introduction
The ineffectiveness of antimicrobial drugs, particularly antibiotics, stands as one of the most pressing challenges for global public health [1]. According to the Global Research on Antimicrobial Resistance published in The Lancet 2, 1.27 million people succumbed to common bacterial infections that had become antibiotic-resistant in 20193. This escalating antimicrobial resistance poses a global threat to human health, necessitating a coordinated and worldwide response. The European Union (EU) and the European Economic Area (EEA) are particularly affected by this crisis, losing over 1 million life-years annually due to the ineffectiveness of antimicrobial drugs [4]. This devastating impact translates into costs amounting to approximately 1.1 billion euros per year for healthcare systems [4,5]. These alarming figures underscore the urgent need for targeted interventions to address this growing crisis (Table 1) [4].
| Bacteria | Antibiotic | Avergae resistance Percentage |
|---|---|---|
| Escherichia coli | Third generation cephalosporins | 22% |
| Kleibsiella pneumoniae | Third generation cephalosporins | 32.20% |
| Staphylococcus aureus | Methicillin | 29.20% |
| Streptococcus pneumoniae | Penicillin | 15.10% |
| Pseudomonas aerugenosa | Carbapenems | 25.20% |
| Acinetobacter baumanii | Carbapenems | 35.70% |
Table 1: Percentage of resistance to major antibiotics worldwide (2021). Source: ECDC (European Centre for Disease Prevention and Control). The State of Antimicrobial Resistance in Europe 2022. Stockholm: ECDC; 2022.
The situation is expected to worsen over time if not addressed urgently. Therefore, it is crucial to focus on innovation in the field of antimicrobial drugs. However, there are significant obstacles hindering the development of new drugs with innovative features [6]. These obstacles encompass scientific, economic, structural, and regulatory challenges [6]. For example, most major pharmaceutical companies have abandoned antimicrobial drug research due to the high risk of failure and lower profitability [7]. This has led small and mediumsized enterprises, which now lead the research, to struggle to secure funding for studies and face significant economic risks [7].
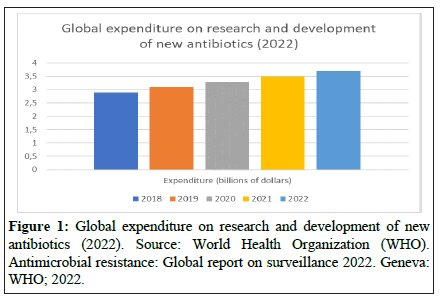
To revitalize the pipeline of antimicrobial drugs, a comprehensive approach with adequate incentives is necessary [8]. Financial incentives, such as direct funding, can help reduce research and development costs [8]. However, it is essential for these incentives to be sufficiently substantial to be effective [8]. For instance, financial rewards tied to research outcomes and reimbursement reforms could increase revenue and create vital markets for antimicrobial drugs [8]. Alignment of incentives is crucial, taking into account public health factors, market dynamics, and operational feasibility (Figure 1) [8].
As it can be seen from the table, expenditures for research and development of new antibiotics have been increasing in recent years. However, expenditures are still relatively low compared to other pharmaceutical sectors, such as oncology drugs or drugs for chronic diseases.
Furthermore, ensuring timely access to both new and existing antimicrobial drugs is essential. Limited access poses a risk to patients and contributes to treatment ineffectiveness [9]. Access to new antimicrobial drugs is often restricted to larger markets, while access to existing ones is compromised by supply chain issues [9]. To improve access to effective antimicrobial drugs, short-term and longterm measures are required [9]. These include streamlining administrative procedures, adopting good procurement practices, and enhancing production capacity [9].
Health authorities in more developed countries play a crucial role in addressing antimicrobial drug ineffectiveness (AMR) [10]. They can and must support antimicrobial drug research and development, participate in international initiatives, and use their global influence [10]. Only through international cooperation and strong leadership is it possible to successfully address the threat of antimicrobial drug ineffectiveness and promote global solutions [10-15].
In conclusion, antimicrobial drug ineffectiveness is a global challenge that requires immediate and coordinated actions worldwide. Research and innovation are essential to address this growing threat. The World Health Organization (WHO) and national health authorities have a crucial role to play in promoting effective global solutions, including the use of precision medicine.
References
- O'Neill J (2016) Tackling drug-resistant infections globally: Final report and recommendations. Rev Antimicrobial Resist.
- The Lancet (2019) Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis.
- World Health Organization (2019) Surveillance of antimicrobial resistance in the WHO African Region: 2018/2019 report.
- European Centre for Disease Prevention and Control (2020) Antimicrobial Resistance in the EU/EEA (EARS-Net) - Annual Epidemiological Report 2019.
- Gandra S (2014) The economics of antibiotic resistance: The need for action. The Lancet Infect Dis 15: 581-592.
- Roca I, Akova M, Baquero F, Carlet J, Cavaleri M, et al. (2015) The global threat of antimicrobial resistance: Science for intervention. New Microbes New Infect 6:22-29.
[Crossref] [Google Scholar] [PubMed]
- Centers for Disease Control and Prevention (2019) Antibiotic resistance threats in the United States, 2019.
- Outterson K (2016) New business models for sustainable antibiotics. Duke Law J 66: 1275-1361.
- World Health Organization (2015) Global action plan on antimicrobial resistance.
- Laxminarayan R, Matsoso P, Pant S, Brower C, Rottingen J, et al. (2020) Access to effective antimicrobials: A worldwide challenge. The Lancet 395: 1689-1699.
- World Health Organization (2019) Ten threats to global health in 2019.
- Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, et al. (2018) Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. The Lancet Infect Dis 18: 318-327.
[Crossref] [Google Scholar] [PubMed]
- European Medicines Agency (2020) Regulatory support for the development of new antibiotics.
- Bush K, Courvalin P, Dantas G, Davies J, Eisenstein B, et al. (2011) Tackling antibiotic resistance. Nat Rev Microbiol 9: 894-896.
[Crossref] [Google Scholar] [PubMed]
- Ventola CL (2015) The antibiotic resistance crisis: part 1: causes and threats. Pharmacy Ther 40: 277-283.
[Google Scholar] [PubMed]
Citation: Mirijello M (2023) Addressing the Ineffectiveness of Antimicrobial Drugs: A Global Challenge. J Infect Dis Ther 11:571.
Copyright: © 2023 Mirijello M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 651
- [From(publication date): 0-0 - Mar 14, 2025]
- Breakdown by view type
- HTML page views: 527
- PDF downloads: 124