Adaptations to Treatment for Opioid Use Disorder during the COVID-19 Pandemic: A Survey of Office-Based Addiction Treatment Programs in California
DOI: 10.4172/2155-6105.1000429
Abstract
Objective: To describe the adaptations made in office-based opioid treatment settings in response to the COVID-19 pandemic.
Methods: A survey was distributed to 202 staff from 95 primary care clinics across the state of California. The response rate was 67 individuals (33.2%) from 41 clinics (43.2%). The survey inquired about changes in service delivery for patients with opioid use disorder (OUD) between April 21 and May 8, 2020. Data on active OUD patient panel size, patients with a new medication for OUD (MOUD) prescription or refill within the past 90 days, were collected through staff submitted monthly reports between January and August 2020.
Results: 39 of 41 clinics (95.1%) reported making adaptations to OUD patient practices during the pandemic. In general, clinics transitioned from in-person to virtual care for initial (39.0%) and follow-up (46.3%) medication visits. Similarly, behavioral health visits shifted from in-person to virtual care for initiation (29.2%) and follow-up (43.9%) appointments. Most of the clinics reduced the frequency of administering toxicology monitoring (70.7%). Remarkably, the number of active OUD patients increased by 9.3% between January and August of 2020.
Conclusion: This study demonstrated that many primary care clinics implemented adaptations in response to COVID-19
regulatory changes, including virtual visits, reduced toxicology screens while increasing the active MOUD patient panel size. Although these adaptations indicate continued or improved access to care, further evaluation is needed to determine the impact of these adaptations, as well as suggestions for their sustainment on quality of care and patient outcomes.
Keywords: COVID-19; Opioid use disorder; Telehealth; Primary care; Medications for opioid use disorder; Behavioral health; Urine toxicology screening
Introduction
COVID-19 has caused significant disruptions, placed unprecedented burdens on the health care system, and posed substantial barriers to treatment. The opioid overdose epidemic has been particularly exacerbated amidst a global pandemic [1]. A record high rate of 92,000 drug overdose deaths occurred in the past year in the United States (US), which continue to escalate [2]. The state of California observed a 45.9% increase in overdose related deaths between December 2019 and 2020 [2]. Concerns are raised for people with an opioid use disorder (OUD) who have faced healthcare delivery and treatment barriers due to the pandemic [3].
Many barriers have prevented the initiation and retention on medications to treat OUD (MOUD), including buprenorphine, naltrexone, and methadone. Only 18% of individuals with OUD initiate treatment [4], of which only a small proportion is retained in care [5]. Yet, these medications effectively reduce overdoses, severe opioid related morbidity, and mortality, but patient access has been limited [6]. Limitations to patient access have included waiver requirements to prescribe MOUD [7], stigma among patients and providers [5], workforce shortages [8], outdated payment structures, and system wide emphasis on acute care models over long term treatment [9]. The availability of treatment for MOUD has increased since 2012. Still, only 10.3 physicians are waivered to prescribe buprenorphine per 100,000 residents nationally, and more than half of rural counties in the US still lack a buprenorphine waivered provider [10]. Treatment for OUD also faced substantial regulations imposed by federal and state laws before the pandemic intended to monitor adherence, reduce diversion, and address psychosocial determinants [11]. A long standing call has been made by experts to ease restrictions and remove barriers to MOUD due to their safety and effectiveness [12].
In response to COVID-19 regulations minimizing in person visits during the pandemic, temporary federal policy changes were made to increase and maintain access to MOUD [13-15]. These changes included allowing buprenorphine waivered prescribers to provide MOUD out of state; to prescribe buprenorphine without an in-person medical evaluation [16]; to ease restrictions for methadone dispensing using 14 or 28 day take home privileges; to allow advanced practice providers to prescribe MOUD [14]. Penalties were waived for prescribers, who did not comply with the Health Insurance Portability and Accountability Act (HIPAA) for technology [17], and funding was increased for telephone and video telehealth encounters [13].
Prescribers across treatment settings were impacted by federal regulations, including those in methadone dispensing opioid treatment programs (OTP), office based opioid treatment (OBOT) settings, and non OTP specialty treatment clinics [11]. Clinics have adjusted their protocols and practices to allow for the initiation of MOUD through telemedicine to expand treatment and reduce overdose deaths [16,18]. Adaptations include decreased onsite medication visits, offering virtual appointments, modifying outreach methods, and lengthening prescription durations [18-20]. The number of individuals filling prescriptions for buprenorphine increased by 26.01% between May 2019 and March 2020 but then plateaued during the national emergency declaration [21,22]. Clinics also reported decreases in weekly drug tests performed on urine specimens since March 2020 [23]. There is growing evidence that these adaptations can increase engagement of individuals with OUD in treatment, especially for patients unable to attend frequent in person visits [11].
These federal and clinic level policy changes were designed to maintain continuity of care and decrease structural barriers for MOUD [24]. These sudden shifts offer a natural experiment to document the impact of the relaxation of regulations for MOUD during the pandemic [25]. To date, most studies have examined adaptations made in OTP settings [26-28]. Few studies have investigated the changes in OBOTs and whether the pandemic has impacted service delivery of MOUD in these settings [18,19,29].
In this report, we describe the treatment adaptations made in OBOT settings to the treatment of patients with OUD during the COVID-19 pandemic, examine the number of patients actively in treatment for an OUD diagnosis before and since the first detection of COVID-19 in the US, and investigate the staff experience in OBOT programs during the pandemic. The results may inform which temporary adaptations might be considered post pandemic [25].
Methods
Design
This cross sectional study surveyed a sample of clinics in primary care, and OBOT settings enrolled in a statewide expansion project to increase MOUD. The survey was concurrently administered in another subsample of primary care clinics [19]. Sites included federally qualified health centers (FQHC), behavioral health centers, hospitals, and private practitioners waivered to prescribe buprenorphine [30]. The clinics varied in patient panel size and number of buprenorphine waivered prescribers.
Measures measures
Primary care practice adaptations for patients with opioid use disorder during COVID-19: A 14 item survey was constructed to identify adaptations to service delivery at office based treatment settings for patients with OUD during the COVID-19 pandemic. A team of subject matter experts developed the survey items. The survey and its development have been described [19].
The survey comprises 14 items across 7 domains: (1) urine drug screens, (2) medication visits, (3) medication management, (4) behavioral health and counseling visits, (5) protocol or workflow adaptations, (6) patient demand, (7) staff well-being. The survey included an open ended question for respondents to elaborate on their experience adapting to their clinic's practices during the COVID-19 pandemic. Participants selected statements ranging from no adaptation (all in person visits) to complete adaptation (entirely virtual telehealth) for MOUD services. Respondents could choose multiple response options, selecting all that apply (Table 1).
| Please indicate changes that have been made to medication visit type (in person=In clinic; virtual=Phone or video). [Check all that apply] |
| We have not made any changes to our in person initial MOUD visits or follow-up MOUD |
| We do not offer any virtual visits for patients starting MOUD |
| Some visits for patients starting MOUD are in person and some are virtual |
| All visits for patients starting MOUD are virtual |
| We do not do any virtual options for follow-up MOUD visits |
| Some follow-up MOUD visits are in person and some are virtual |
| All follow-up MOUD visits are virtual |
| In person follow-up MOUD visits are less frequent but no longer than one month apart |
| Follow-up MOUD visits are as frequent but a combination of in person and virtual |
| Follow-up MOUD visits are as frequent and all virtual |
| Follow-up MOUD visits are less frequent and all virtual |
Table 1: Sample Item from the Survey on Primary Care Practice Adaptations for Patients with Opioid Use Disorder during COVID-19.
Active MOUD Patient Panel Size
This analysis used monthly clinic reports submitted by staff between January and August 2020 collected through a statewide expansion project [31]. The measurement of active MOUD patient panel size included a report of the number of patients with OUD who had either a new MOUD prescription or a refill of MOUD within the past 90 days. Patients were included in these reports if they were prescribed buprenorphine or naltrexone. Clinics also reported demographic information, such as urban city, clinic type, and the number of buprenorphine waivered providers.
Procedures
The surveys were distributed to 202 clinic members across 95 participating clinics through a direct Qualtrics link and administered between April 21 and May 8, 2020. Program leaders sent one reminder email per week over 3 weeks to ensure a timely response from staff members. Staff was surveyed across several role categories, including prescribers (medical director, physician, and nurse practitioner), administration (clinical administrator and nurse manager), behavioral health personnel (behavioral health manager and clinician, and substance use counselor), and other (nurse, medical assistant, or other).
Ethics
Before distributing the survey a waiver of consent was obtained from the Institutional Review Board at Stanford University and the University of California, Los Angeles. A de-identified survey link was used to maintain the anonymity and confidentiality of participant data. No identifiable information was collected from participants, patients, or health records.
Statistical analysis
All analyses were performed using STATA Version 17. Descriptive statistics were used to summarize the characteristics at the respondent and clinic levels. For descriptions of the survey data at the clinic level, cases with discordant responses were resolved by prioritizing the prescriber or clinic leadership's response (medical director, clinical administrator, or nurse manager). Individuals in these roles likely have more familiarity with the clinic level adaptations made for patients with OUD during the pandemic. In cases with multiple respondents within the same position (n=4 clinics), of 236 responses possible, 25.8% were discordant. Discordant observations were excluded from the analysis.
Results
Out of 95 clinics, at least one team member from 41 clinics (43.2%) responded to the survey (Figure 1). On the respondent level, the response rate on the survey was 33.2% (n=67). Data on the active MOUD patient panel size were reported from 40 clinics. The descriptive statistics of the staff and clinics are presented in Table 2. The majority of clinics were FQHC (n=20; 48.8%), in predominantly urban areas (n=32; 78.1%). Most of the respondents were prescribers (n=33; 50.8%). A majority were clinic leaders (n=51; 78.5%), including prescribers and clinical administration roles. On average, each clinic employed around 4 buprenorphine-waivered providers.
| Characteristics of clinics (N=41) | n (%) |
|---|---|
| Urbanicity | |
| Rural | 9 (22.0) |
| Urban | 32 (78.1) |
| Clinic type | |
| Federally Qualified Health Center (FQHC) | 20 (48.8) |
| FQHC look-alikes | 7 (17.1) |
| Hospital | 5 (12.2) |
| Indian Health Center | 4 (9.8) |
| Behavioral Health Practice | 2 (4.9) |
| Private Practice | 3 (7.3) |
| Number of buprenorphine-waivered providers | |
| Mean (SD) | 3.6 (4.1) |
| Any adaptations made to MOUD practice | |
| No | 2 (4.9) |
| Yes | 39 (95.1) |
| Characteristics of respondents | n (%) |
| Respondent role | |
| Prescribers | 33 (50.8) |
| Administration | 8 (12.3) |
| Behavioral Health Staff | 19 (29.2) |
| Other | 5 (7.7) |
| Leadership role | |
| Clinic Leadership | 51 (78.5) |
| Other Staff | 14 (21.5) |
Table 2: Demographic Characteristics of Clinics (n=41) and Survey Respondents (n=67).
Medication and behavioral health visits
"We hope that after COVID-19, a hybrid of in person and virtual visits will be possible and reimbursed. This is in the best interest of our patients”.
Nearly all clinics reported making adaptations to OUD treatment practices during the COVID-19 pandemic (n=39; 95.1%) (Table 2). Clinics started offering MOUD in person and virtually for initial (n=16; 39.0%) and follow up (n=19; 46.3%) medication visits (Table 3). Similarly, behavioral health visits shifted from in person to virtual care for initiation (n=12; 29.2%) and follow up (n=18; 43.9%) appointments. Only a minority of clinics (n=8; 19.5%) offered solely virtual visits for medication induction, as most clinics still offered in person medication visits. Most of the ongoing behavioral health counseling and medication visits transitioned to a virtual format, with an increase estimated from 9.3% to 75.7% before and since COVID-19 control measures were instated. The ongoing medication visits (n=12; 29.3%) and behavioral health counseling (n=12; 29.3%) remained as frequent and virtual during and before the pandemic.
| Reported adaptations for medication visits | n (%) |
|---|---|
| Initial medication visits for patients with OUD | |
| No changes | 1 (2.4) |
| Do not offer virtual | 6 (14.6) |
| Both in-person and virtual | 16 (39.0) |
| All virtual | 8 (19.5) |
| Follow-up medication visits for patients with OUD | |
| Do not offer virtual | 0 (0.0) |
| Both in-person and virtual | 19 (46.3) |
| All virtual | 9 (22.0) |
| Follow-up visit frequency | |
| In-person less frequent, but not >1 month apart | 3 (7.3) |
| As frequent in-person and virtual | 12 (29.3) |
| As frequent, all virtual | 12 (29.3) |
| Less frequent, all virtual | 1 (2.4) |
| Reported adaptations for behavioral health and/or counseling visits | n (%) |
| Initial evaluations for patients with OUD | |
| No changes | 2 (4.9) |
| Do not offer virtual | 1 (2.4) |
| Both in-person and virtual | 9 (22.0) |
| All virtual | 12 (29.3) |
| Ongoing counseling for patients with OUD | |
| Do not offer virtual | 0 (0.0) |
| Both in-person and virtual | 12 (29.3) |
| All virtual | 18 (43.9) |
| Ongoing counseling frequency | |
| In-person less frequent, but not >1 month apart | 2 (4.9) |
| As frequent in-person and virtual | 8 (19.5) |
| As frequent, all virtual | 12 (29.3) |
| Less frequent, all virtual | 3 (7.3) |
| Adaptations for patient retention, preference, and demand | |
| Engaging patients and keeping them in care | |
| Unchanged | 18 (43.9) |
| Harder | 4 (9.8) |
| Easier | 6 (14.6) |
| Perceived patient preference for behavioral health and/or counseling visits | |
| In-person | 9 (22.0) |
| Virtual | 8 (19.5) |
| Perceived patient preference for medication visits | |
| In-person | 7 (17.1) |
| Virtual | 13 (31.7) |
| Demand for behavioral health and/or counseling visits | |
| Unchanged | 10 (24.4) |
| Decreased | 1 (2.4) |
| Increased | 17 (41.5) |
| Demand for medication visits | |
| Unchanged | 21 (51.2) |
| Decreased | 5 (12.2) |
| Increased | 13 (31.7) |
| Clinics could select multiple response options, such that percentages may not add up to 100%. | |
Table 3: Adaptations for Initial and Ongoing Visits by Medication and Behavioral and Counseling services (n=41 Clinics)
Medication management and urine drug Screenings
"We are a little concerned that some of our clients are taking advantage of not drug testing (because of virtual visits) to expand their use of other drugs, usually meth and possibly opioids. Our long term clients, however, appear to be stable in their recovery”.
Nearly all clinics made changes to their urine drug screenings during the pandemic. Specifically, 70.7% (n=29) of clinics reduced their frequency of urine drug screenings for established patients. Clinics also reported writing prescriptions for longer durations in comparison to pre COVID-19 (n=18; 43.9%). The injectable buprenorphine and naltrexone rates did not change pre and post COVID-19, with only slight decreases in buprenorphine (0.1%) and naltrexone (1.4%).
Workflow adaptations
"We have a spike in counseling/encounter measures for our SUD BH staff, but that is because we are being intentional about reaching out for many different projects that remain despite COVID-19. If it were not for that, I am not sure if we would see a spike in counseling/therapy.”
Clinics reported adaptations to workflows, protocols, and outreach. The most significant changes included: changes to CPT codes to bill for virtual visits (n=20; 48.8%); lowered barriers for patients to start and continue on medications (n=19; 46.3%); more assertive outreach to patients by phone and email (n=16; 39.0%).
Patient demand
"We are doing virtual visits using OTTO and NextGen our patients love virtual and telephonic visits. We hope we can continue to provide devices like this and bill for these services. Follow up and retention in care has increased."
The responses to the quantitative survey questions about patient retention and engagement were equally distributed across response options (Table 2). The qualitative responses indicated that follow up and demand for care have increased. More clinic respondents reported an increase in patient demand for behavioral health visits (n=17; 41.1%) than for medication visits (n=13; 31.7%). Around half of the respondents indicated demand for medication visits has remained largely unchanged (n=21; 51.2%).
Staffing changes
"Our substance abuse counselor works from home. She has had more 1:1 counseling sessions but feels very connected to patients still."
Changes to staff wellness, including layoffs, remote working, and anxiety about COVID-19 affecting work, were reported individually. Only 22.4% (n=15) of staff members reported layoffs, and 25.4% (n=17) indicated reduced work hours. A majority of staff stated that members worked partly onsite and partly at home (n=40; 59.7%), while only 6% (n=4) reported working onsite as usual, and 22.4% (n=15) worked entirely at home. Anxiety level about COVID-19 impacted functioning at home and work for about a quarter of staff members (25.4%; n=17). In general, most staff members felt supported by their organization during the pandemic (80.6%; n=54).
Number of patients receiving MOUD
"It has been easier to reach some patients now that everything is virtual. Also, my schedule has become more flexible as a result of telehealth."
"My new patient inquiries have dropped dramatically. I am concerned that many illicit opioid users are too overwhelmed to seek treatment during this period."
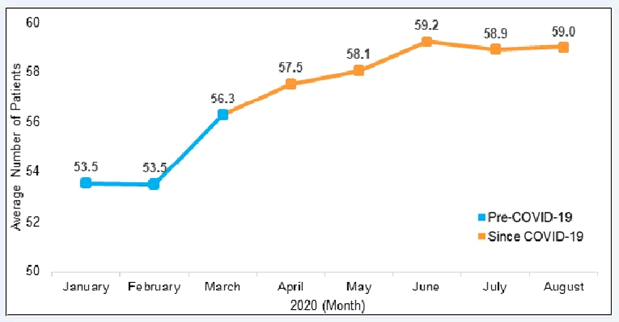
Although clinics offered more virtual options for OUD care, clinic members still reported some concerns over engaging and initiating medication treatment for patients with OUD. The monthly reports show that the average number of active OUD patients increased from 54 to 59 (9.3%) between January and August of 2020 (Figure 2). The most significant change occurred between February and March of 2020, increasing the average number of patients by approximately 3.
Discussion
Summary of findings
This study documents adaptation made in a subset of primary care clinics in California adjusting to changes in federal guidelines during the COVID-19 pandemic to reduce care access barriers for patients with OUD. Nearly all clinics reported changes to their practices. Almost half of all clinics started offering both in person and virtual medication visits for initial and follow up visits. Only a small subset of clinics provided only virtual visits for medication induction. Nearly half of the clinics offered behavioral counseling follow up visits completely virtual. A majority of clinics reduced their frequency of urine drug screenings for established patients. Several clinics reported prescriptions and refills of buprenorphine over a longer duration. Survey results indicated that the demand for behavioral health increased while medication visits remained essentially unchanged. The active MOUD patient size across clinics increased steadily between February and March until plateauing from June to August 2020.
These results complement other recent qualitative and quantitative studies in primary care and specialty opioid treatment programs during the pandemic [18,19,27]. Previous studies have described rapid transitions from in person care to telehealth, reductions in the frequency of urine drug screenings and behavioral health visits, and modifications to prescription durations [11]. Our findings underscore the shift in provider preference for hybrid models that incorporate in person and telehealth visits [11]. There is evidence that clinics in states with shelter in place policies were more likely to socially distance for in person visits than states without such a policy [26], which may have contributed to the swift changes observed in these primary care settings across California. This study replicated the methodology used by Caton, et al. [19], but with a different sample of primary care clinics in California. Across studies, modifications to practices have generally been received positively, and providers have expressed support for making these temporary regulations permanent [11]. Continued exploration of the benefits and consequences to patient outcomes and quality of care over a more extended period is needed to understand whether these changes should be maintained beyond the acute period in the pandemic.
The findings from our monthly reports indicated increases in MOUD prescriptions in the early months of the pandemic, which contrasts with studies showing that buprenorphine prescriptions remained stable and new treatment initiation decreased [21,32]. One study found increases in the number of new patients receiving outpatient prescriptions for buprenorphine despite a decline in established patients during the pandemic [33]. Our findings on active MOUD patient panel size were collected using data from the past 90 days. Thus, the plateau in prescriptions from the early months of the pandemic might be reflected in the data between June and August 2020, as providers started to transition to virtual formats (Figure 2). These inconsistent findings emphasize a need for further research on buprenorphine prescription rates and the impact on patients as COVID-19 restrictions lift.
In terms of staffing changes, a majority of participants reported that staff worked partly onsite and remote. Many staff members felt supported by their organization during the pandemic, and few stated anxieties around COVID-19 and their ability to work. These findings were in line with a previous study using the same survey [19]. Prevention of pandemic burnout among staff is crucial to provide patients with OUD continuity of care, which is essential to preventing overdose and adverse health outcomes [34]. Lower barrier service models might better accommodate staff work schedules and allow for increased MOUD retention [35]. Continued refinement and improvement of workflows and reimbursement structures will be necessary to sustain services for patients with OUD during the pandemic and beyond [27].
Limitations
The survey results should be interpreted in the context of their limitations. First, these results are descriptive and non-causal. The survey design included select all that apply answers, which rendered the assessment of missing data challenging and resulted in conflicting responses. Data was primarily collected at a small sample of largely FQHCs, predominantly in urban settings, limiting the generalizability to primary care settings in areas with higher accessibility to telehealth and MOUD [8,10]. These clinics and prescribers were already prescribing medications and may have been more willing to prescribe MOUD than other primary care settings. Multiple responses were resolved by prioritizing the response of the prescriber or clinic leadership, but these nuanced perspectives within the clinic require further exploration [15]. However, the respondents included predominantly prescribers, who offer invaluable insights into MOUD service delivery changes during the pandemic [36-39]. Patient preferences were also reported by providers, which may not accurately reflect the patient experience of care [18,40]. Future research should explore patient preferences for virtual MOUD treatment initiation and behavioral health services and whether these preferences relate to increases in active MOUD patients [39].
Conclusion
Overall, our findings demonstrate the widespread adaptations to MOUD treatment in primary care settings. The Biden Harris Administration has appropriated nearly $ 4 billion to expand access to behavioral health services for people with OUD and recommends the continuation of relaxed regulations for prescriptions of MOUD. A report by the Office of National Drug Control Policy advises making the emergency provisions implemented during the pandemic concerning MOUD authorizations permanent.
Many experts have recommended the continuation of adapted practices in the future to provide low barrier MOUD care. Targeted urine drug screenings based on clinical reasoning have been advised over routine tests to reduce healthcare costs. Hybrid models that incorporate in person and telehealth to provide patient centered care may benefit patients and ensure the effective delivery of care for patients with OUD in the future. However, investment in training, infrastructure, and telehealth equipment is needed to increase adoption among patients and providers to reduce barriers to telehealth and sustain treatment for OUD.
Despite the many benefits from these temporary adaptations made during the pandemic, some patients may still have experienced decreased access to care. The engagement of individuals with OUD residing in rural areas, living with disabilities, lacking access to technology, or facing housing insecurity has posed a significant challenge during the pandemic. Further research is needed to determine if telehealth visits are equally effective as in person appointments and whether longer prescriptions, less frequent toxicology monitoring, and virtual behavioral counseling benefit patient outcomes. Data are needed to evaluate these temporary adaptations' benefits and untoward effects to inform future practice guidelines and innovate addiction treatment.
In conclusion, this survey revealed a rapid and widespread transition to virtual care for MOUD visits, a reduction in urine drug screening, growth in the number of active MOUD patients, and increased demand for behavioral health counseling. Temporary regulatory changes during the pandemic have allowed clinics to expand access to care for MOUD and engage more patients. Whether these regulatory and practice changes should be maintained and become the new status quo remains an open research question.
Funding
California Department of Health Care Services (DHCS) "MAT Expansion Project" (DHCS Contract #17-94653) by the SAMHSA State Targeted Response to the Opioid Crisis Grants (TI-17-014). The contents do not represent the official views of the State of California.
Conflict of Interest
None
Acknowledgement
The authors are particularly grateful to the staff, clinicians, and patients of the clinics involved. The study was made possible through funding from the California Department of Health Care Services by the SAMHSA State Targeted Response to the Opioid Crisis Grants. We also thank Eva Vazquez for her data collection efforts.
References
- Striley CW, Hoeflich CC (2021) Converging public health crises: Substance use during the coronavirus disease 2019 Pandemic. Curr Opin Psychiatry. 34(4): 325–331.
- Ahmad FB, Rossen LM, Sutton P (2021) Provisional drug overdose death counts. National Center for Health Statistics
- Malik M, Francis A (2021) COVID-19 and The opioid use disorder: a syndemic perspective and their neuropsychiatric manifestations. Biol Psychiatry. 89(9): S142–S143.
- Nunes EV, Levin FR, Reilly MP, El-Bassel N (2021) Medication treatment for opioid use disorder in the age of covid-19: can new regulations modify the opioid cascade? J Subst Abuse Treat. 122: 108196.
- Wakeman SE, Larochelle MR, Ameli O, Chaisson CE, McPheeters JT, et al. (2020) Comparative effectiveness of different treatment pathways for opioid use disorder. J American Med Asso Netw Open. 3(2): e1920622.
- Doernberg M, Krawczyk N, Agus D, Fingerhood M (2019) Demystifying buprenorphine misuse: has fear of diversion gotten in the way of addressing the opioid crisis? Subst Abuse. 40(2): 148–153.
- Snell-Rood C, Pollini RA, Willging C (2021) Barriers to integrated medication-assisted treatment for rural patients with co-occurring disorders: The Gap in Managing Addiction. Psychiatr Serv. 72(8): 935-942.
- Weimer MB, Wakeman SE, Saitz R (2021) Removing one barrier to opioid use disorder treatment: is it enough? J American Med Asso. 325(12): 1147-1148.
- Andrilla CHA, Moore TE, Patterson DG, Larson EH (2019) Geographic distribution of providers with a dea waiver to prescribe buprenorphine for the treatment of opioid use disorder: A 5 year update. J Rural Health. 35(1): 108–112.
- Treitler PC, Bowden CF, Lloyd J, Enich M, Nyaku AN, et al. (2022) Perspectives of opioid use disorder treatment providers during covid-19: adapting to flexibilities and sustaining reforms. J Subst Abuse Treat. 132: 108514.
- Fiscella K, Wakeman SE, Beletsky L (2019) Buprenorphine deregulation and mainstreaming treatment for opioid use disorder: X the X Waiver. J American Med Asso Psychiatry. 76(3): 229–230.
- US DHHS (2020) FAQs on Telehealth and HIPAA during the COVID-19 Nationwide Public Health Emergency.
- SAMHSA (2020) FAQs: Provision of Methadone and Buprenorphine for the Treatment of Opioid Use Disorder in the COVID-19 Emergency.
- Executive office of the president office of national drug control policy (2021) The Biden-Harris Administration’s Statement of Drug Policy Priorities for Year One.
- US Department of Justice Drug Enforcement Administration, COVID-19 information page (2020).
- U.S. Department of Health & Human Services, Office for Civil Rights Notification of Enforcement Discretion for Telehealth (2020).
- Cales RH, Cales SC, Shreffler J, Huecker MR (2021) The COVID-19 pandemic and opioid use disorder: expanding treatment with buprenorphine, and combining safety precautions with telehealth. J Subst Abuse Treat. 108543.
- Caton L, Cheng H, Garneau HC, Fisher T, Harris-Mills B, et al. (2021) COVID-19 adaptations in the care of patients with opioid use disorder: a survey of california primary care clinics. J Gen Intern Med. 36(4): 998-1005.
- Joseph G, Torres-Lockhart K, Stein MR, Mund PA, Nahvi S (2021) Reimagining patient-centered care in opioid treatment programs: Lessons from the Bronx during COVID-19. J Subst Abuse Treat. 122:108219.
- Nguyen TD, Gupta S, Ziedan E, Simon KI, Alexander GC, et al. (2021) Assessment of filled buprenorphine prescriptions for opioid use disorder during the coronavirus disease 2019 pandemic. J American Med Asso Intern Med. 181(4): 562.
- Huskamp HA, Busch AB, Uscher-Pines L, Barnett ML, Riedel L, et al. (2020) Treatment of opioid use disorder among commercially insured patients in the context of the COVID-19 pandemic. J American Med Asso, 324(23): 2440.
- Niles JK, Gudin J, Radcliff J, Kaufman HW (2021) The opioid epidemic within the COVID-19 pandemic: Drug testing in 2020. Popul Health Manag. 24(S1): S43-S51.
- Sofuoglu M, DeVito EE, Carroll KM (2019) Pharmacological and behavioral treatment of opioid use disorder. Psychiatr Res Clin Pract. 1: 4–15.
- Blanco C, Compton WM, Volkow ND (2021) Opportunities for research on the treatment of substance use disorders in the context of COVID-19. J American Med Asso Psychiatry. 78:357.
- Cantor J, Laurito A (2021) The new services that opioid treatment programs have adopted in response to COVID-19. J Subst Abuse Treat. 130: 108393.
- Hunter SB, Dopp AR, Ober AJ, Uscher-Pines L (2021) Clinician perspectives on methadone service delivery and the use of telemedicine during the COVID-19 pandemic: A qualitative study. J Subst Abuse Treat. 124: 108288.
- Tracy K, Wachtel L, Friedman T (2021) The impact of COVID-19 on opioid treatment program (OTP) Services: Where do we go from here? J Subst Abuse Treat. 131: 108394.
- O’Gurek DT (2021) Designing and evaluating COVID-19 protocols for an office-based opioid treatment program in an urban underserved setting. J Am Board Fam Med. 34: S136–S140.
- Miele GM, Caton, L, Freese TE, McGovern M, Darfler K, et al. (2020) Implementation of the hub and spoke model for opioid use disorders in California: Rationale, design and anticipated impact. J Subst Abuse Treat. 108: 20–25.
- Caton L, Shen H, Miele GM, Darfler K, Sandoval JR, et al. (2021) Opening the “Black Boxâ€: four common implementation strategies for expanding the use of medications for opioid use disorder in primary care. Implement Res Pract. 2: 1-11.
- Thornton JD, Varisco TJ, Bapat SS, Downs CG, Shen C (2020) Impact of COVID-19 related policy changes on buprenorphine dispensing in Texas. J Addict Med. 14(6): e372–e374.
- Cance JD, Doyle E (2020) Changes in outpatient buprenorphine dispensing during the COVID-19 pandemic. J American Med Asso. 324(23): 2442–2444.
- Williams AR, Samples H, Crystal S, Olfson M (2020) Acute care, prescription opioid use, and overdose following discontinuation of long-term buprenorphine treatment for opioid use disorder. Am J Psychiatry. 177(2): 117–124.
- Henry BF, Campbell A, Hunt T, Johnson JK, Mandavia AD, et al. (2021) COVID-19 related substance use services policy changes: Policymaker perspectives on policy development & implementation. J Subst Abuse Treat. 108550.
- Carter J, Zevin B, Lum PJ (2019) Low barrier buprenorphine treatment for persons experiencing homelessness and injecting heroin in San Francisco. Addict Sci Clin Pract. 14(1): 20.
- Priest KC, McCarty D, Lovejoy TI (2020) Expanding access to medications for opioid use disorder: program and policy approaches from outside the veterans health administration. J Gen Intern Med. 35(3): 886–890.
- Pytell JD, Rastegar DA (2021) Down the drain: Reconsidering routine urine drug testing during the COVID-19 pandemic. J Subst Abuse Treat. 120: 108155.
- Hughto JMW, Peterson L, Perry NS, Donoyan A, Mimiaga MJ, et al. (2021) The provision of counseling to patients receiving medications for opioid use disorder: Telehealth innovations and challenges in the age of COVID-19. J Subst Abuse Treat. 120: 108163
- Andrilla CHA, Coulthard C, Larson EH (2017) Barriers rural physicians face prescribing buprenorphine for opioid use disorder. Ann Fam Med. 15(4):359–362.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2047
- [From(publication date): 0-2021 - Dec 22, 2024]
- Breakdown by view type
- HTML page views: 1567
- PDF downloads: 480