Acute Toxicity of Aqueous Leaf extract of Newbouldia laevis in Swiss Albino Mice
Received: 01-Apr-2023 / Manuscript No. jabt-23-93846 / Editor assigned: 03-Apr-2023 / PreQC No. jabt-23-93846 / Reviewed: 17-Apr-2023 / QC No. jabt-23-93846 / Revised: 17-Apr-2023 / Manuscript No. jabt-23-93846 / Accepted Date: 27-Apr-2023 / Published Date: 28-Apr-2023 QI No. / jabt-23-93846
Abstract
The acute toxicity of aqueous leaf extract of Newbouldia laevis was evaluated in Swiss albino mice. For the first phase, the mice were divided into four groups of three animals each at random. The aqueous leaf extract of Newbouldia laevis was administered both orally and intraperitoneally to the groups in doses of 0, 10, 100, and 1000 mg/kg body weight, respectively. The animals were separated into four groups of three animals each for the second phase of administration, and single doses of the extract at 0, 1600, 2900, and 5000 mg/kg body weight were administered. Animals in both phases were closely monitored for 24 hours and 14 days, respectively. For the animals that got the extract through the oral route, no death was recorded in any of the groups in both phase. The LD50 of the aqueous leaf extract of Newbouldia laevis when administered orally was calculated to be up to 5000 mg/kg. The results indicate that the extract may be safe to be used at high doses through the oral route. For the intraperitoneal administration, no death was recorded in any of the groups in phase I, however, all the animals that got the extract in phase II died within 24 hours after intraperitoneal administration. The LD50 of the aqueous leaf extract of Newbouldia laevis when administered intraperitoneally was calculated to be 1264.9 mg/kg. The results indicate that the extract may be toxic at a high dose in short-term exposure and non-toxic when taken at low doses through the intraperitoneal route.
Keywords
Newbouldia laevis; Acute toxicity; Aqueous extract; Albino mice
Introduction
Toxicity could be described as the relative ability of a substance or a chemical to cause adverse effects on living organisms, it also explains the extent to which biologically active substances elicit harm or cause mild to severe damage to an organism (Ene 2021 [1]). Toxicology could be simply described as the science of poison and the source from which the poison was gotten, the chemical composition of the poison, diagnosis/tests for the poison, and its antidotes (Bentur, 2008 [2]). Toxicology, the scientific evaluation or study of the adverse effects of substances or chemicals on a cell, tissue, organ, or the whole organism, has served human society in many diverse ways, which is not limited to only protecting human lives and the environment from the hazardous effects of biological, physical or chemical toxicants but has helped in the development of bioactive substances that are relatively harmless (Liu 2017 [3]). In Africa, medicinal plants have been among the common sources of medicines, either utilized when prepared traditionally or used to extract pure active principles. Due to the enormous chemical diversity among natural products, many researchers have geared their research interests toward the screening of plant extracts to obtain new bioactive therapeutic agents for infectious diseases. Local people and herbalists in Africa utilize medicinal plants in the treatment of illnesses (Ene, 2014 [4]). Herbal medicines are considered safe because they are obtained from natural origin. However, studies have demonstrated that not all are safe for direct use, especially in pediatric patients. Although, medicinal plants may have relatively fewer side effects when compared to synthetic drugs, studies have shown that they may cause acute toxicity in children even with fatal outcomes (Farzaei , 2020 [5]). Moreover, a large number of medicinal plants to an extent possess a degree of toxicity. For instance, studies have shown that about one-third of medicinal plants used as anti-diabetic agents are toxic (Bnouham , 2006 [6]). Also, toxicity studies of medicinal plants using animal models have shown some of these medicinal plants used in the treatment of chronic eczema and other skin diseases may induce serious life-threatening symptoms such as bradycardia, depression of respiratory and central nervous system (CNS), hepatotoxicity and nephrotoxicity (Kane, 1995 [7]). Patients consuming Thevetia peruviana (yellow oleander) may develop arrhythmia and become hypertensive (Farzaei, 2020 [5]). Herbal toxicities are concerning the presence of active secondary metabolites such as ephedrine-type alkaloids regarded as intrinsic effects. Toxicities may occur due to extrinsic factors such as contamination, adulteration, and misidentification of plant medicinal products because of the presence of toxic foreign substances which are not constituents of the plant (Wo., 2012 [8]). The enormous reports of toxicity of medicinal plants as well as indiscriminate use of herbal medicines have necessitated the need to evaluate herbal products and establish their safety and make global standard toxicity testing methods for effective toxicological characterization of herbal products (Verma, 2012 [9]). Furthermore, in evaluating the safety of any plant medicinal product, two important factors may be considered; the nature or significance of the toxicity and the extent of exposure to the toxicity observed (Obidike and Oluwakayinsola, 2013 [10]). Newbouldia laevis (N. laevis) is commonly known in English as the Tree of life, Fertility tree, and African nut tree, among the Igbos (Eastern Nigeria), it is known as Oke-ogirishi or Ogirisi, Akoko in Yoruba (Western Nigeria), Adurukuu in Hausa (Northern Nigeria) is a widely distributed plant in many of Africa countries (Okagu, 2021 [11]). Some of the medicinal benefits of this plant in folk treatment include anti-malaria agent, used in the treatment of fever, cough, breast cancer, pain (pelvic pain in females, chest pain, and ear ache), sickle cell anemia, sexually transmitted infections (STIs), toothache, stomach ache, ulcer, epilepsy, convulsion, infertility, snake bite, diabetes, etc. Some scientific investigations have validated some of these folkloric uses of this plant (Osigwe, 2017 [12]).
Acute toxicity is defined as the adverse effect which is caused by a single exposure to drugs or chemical substances by any route which could change the normal state and functioning of the whole organism or respective organs for a short period (usually 24 hours). Any drug intended for pharmaceutical use in humans is considered necessary to undergo acute toxicity studies using animal models. The results from acute toxicity studies serve as a guide in dosage selection for long-term toxicity studies as well as other studies involving animals (Ene, 2021 [4]) However, the safety of Newbouldia laevis is critical concerning its medicinal benefits, hence this study.
Materials and Methods
Plant material and extract preparation
The leaves of the Newbouldia laevis plant were collected from the Federal University of Technology Owerri (FUTO) forest reserve behind the Biochemistry Department at the Federal University of Technology Owerri (FUTO) in Imo State, Nigeria. Dr. Faruwa Francis of the Department of Forestry and Wildlife Technology, School of Agriculture and Agricultural Technology (SAAT), the Federal University of Technology Owerri, identified the plant, prepared and kept it in the University herbarium with voucher number FUTO/ FWT/ERB/2021/61. Newbouldia laevis leaves were air-dried in the laboratory for four weeks before being processed into a powder. To protect the powdered sample from sunlight, it was stored in airtight polythene bags. The aqueous extract of the leaf powder was made using quantitative extraction, in which 100g of the powder was steeped in 700ml of distilled water in a conical flask packed airtight with cotton wool and foil for 72 hours and then filtered using Whatman no 1 filter paper. The decoction liquid was concentrated at 45°C in a water bath to provide a semi-solid residue. The extract was kept at 4°C in the refrigerator until it was used for evaluation.
Experimental animals
The albino mice used in this study were purchased from the Department of Pharmacology and Physiology, Faculty of Veterinary Medicine, the Michael Okpara University of Agriculture Umudike, Abia state. They were kept in normal cages in the Department of Biochemistry’s animal house at the Federal University of Technology Owerri, Imo state. The mice used for this study were aged 8-9 weeks and weighed between 20-30 g. They were acclimatized for two weeks before the commencement of the study. They were fed finisher pellet feeds with tap water ad libitum throughout the study period. The animals fasted for several hours before the commencement of the experiment.
Acute toxicity evaluation/ determination of median lethal dose
Lorke’s method was used to test the acute toxicity of aqueous leaf extract of Newbouldia laevis (Lorke, 1983 [13]). The procedure involved two phases in which the extract was delivered through oral intubation and intraperitoneal route of administration. For the first phase of the treatment, 12 albino mice of both sexes with similar body weights were divided into four groups of three mice each. Each group was respectively administered both orally and intraperitoneally single dose of Newbouldia laevis’ extract at 10, 100, and 1000 mg/kg body weight, while the fourth group (control) received only the normal saline. The animals were thoroughly monitored for the first hour and then every 30 minutes for the next 24 hours for any early signs of toxicity, as well as daily for the next 14 days to record any delayed acute effects. Their weights were recorded every day throughout this time, and clinical signs of toxicity such as loss of appetite, immobility, convulsions, and so on were recorded. There was no death in any of the groups. In the second phase of the treatment, higher doses of the extract were used. 12 albino mice of both sexes with similar body weights were divided into four groups of three mice each. Each group was respectively administered both orally and intraperitoneally single dose of Newbouldia laevis’ extract at 1600, 2900, and 5000 mg/kg body weight, while the fourth group (control) received only the normal saline. The doses for the second phase were set based on the first phase’s results. In the second phase of treatment, the animals were watched as frequently as previously mentioned, and mortality was recorded. Writhing, gasping, palpitation, and increased or decreased breathing rate were all observed in the treated animals over the course of two weeks. The result obtained from the first and second phases of the experiment was used to calculate the LD50 of the plant extract.
The LD50 was calculated according to the method outlined by Lorke (1983 [13]):
√The geometric mean of the maximal dose without mortality × geometric mean of the minimal dose with mortality = Xmg/kg.
Statistical analysis
All data generated during the cause of the research were expressed as mean ± standard deviation (SD) and analyzed statistically by analysis of variance (ANOVA). Means were compared using LSD post hoc test and differences between treatment and control groups were accepted as significant at p ≤ 0.05.
Results
Result of quantitative extraction
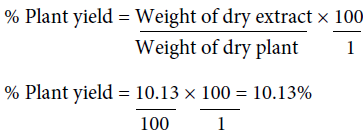
The percentage yield of the aqueous leaf extract of N. laevis is 10.13%. The quantity extracted may be dependent on the solvent used for extraction.
Acute toxicity evaluation of the aqueous leaf extract of Newbouldia laevis
For the animals administered the extract via the oral route
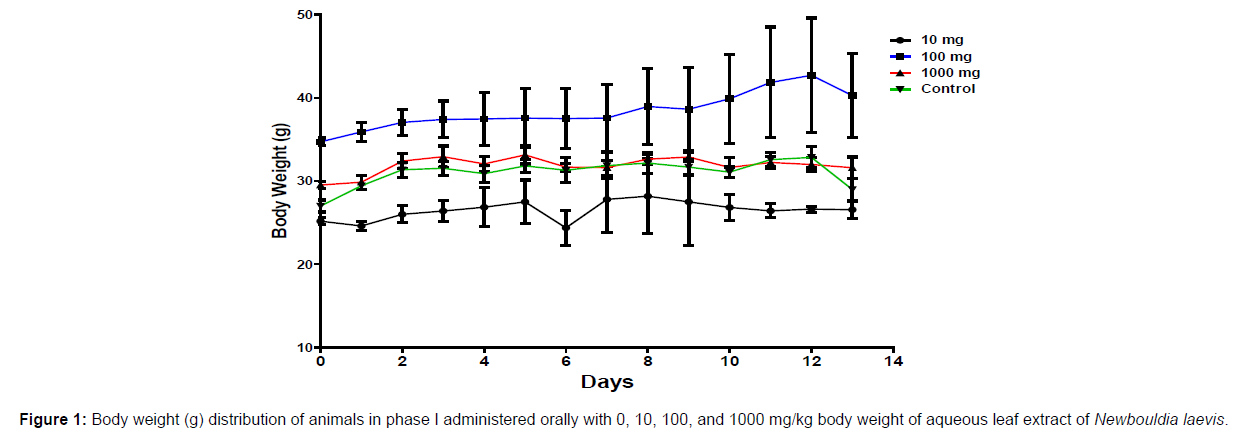
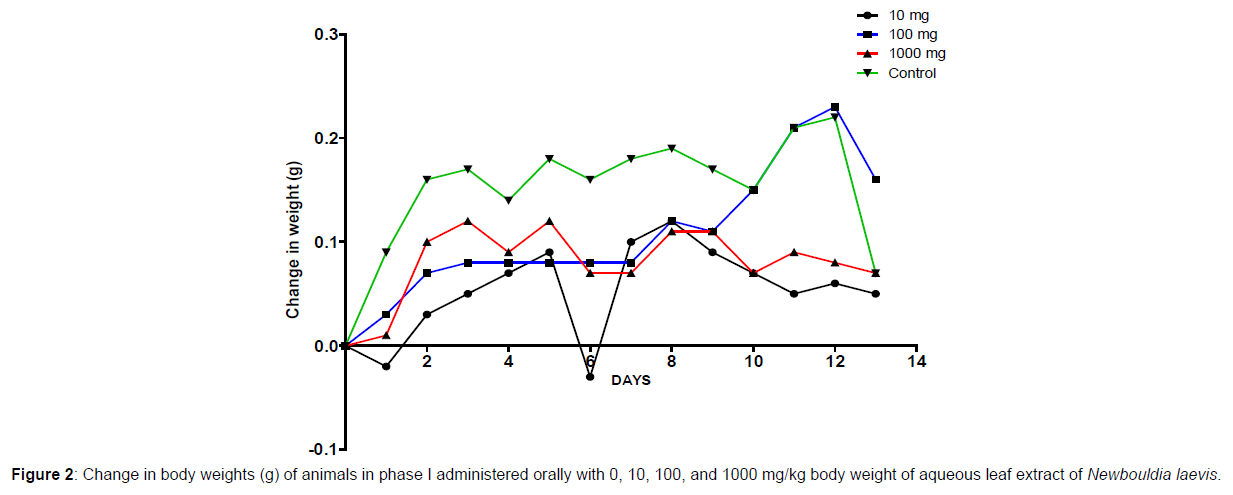
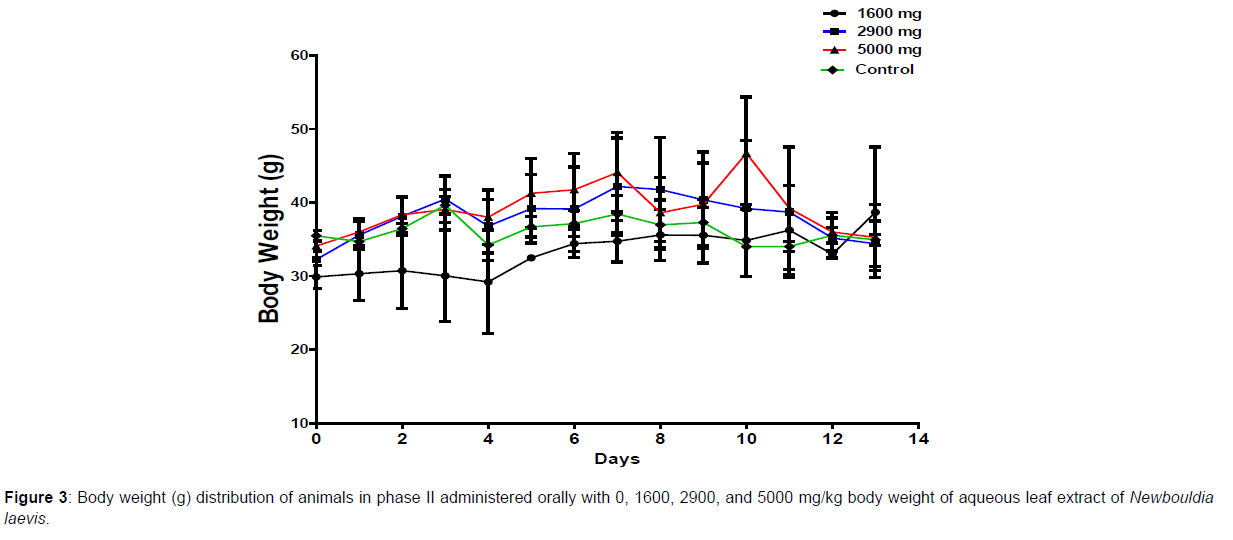
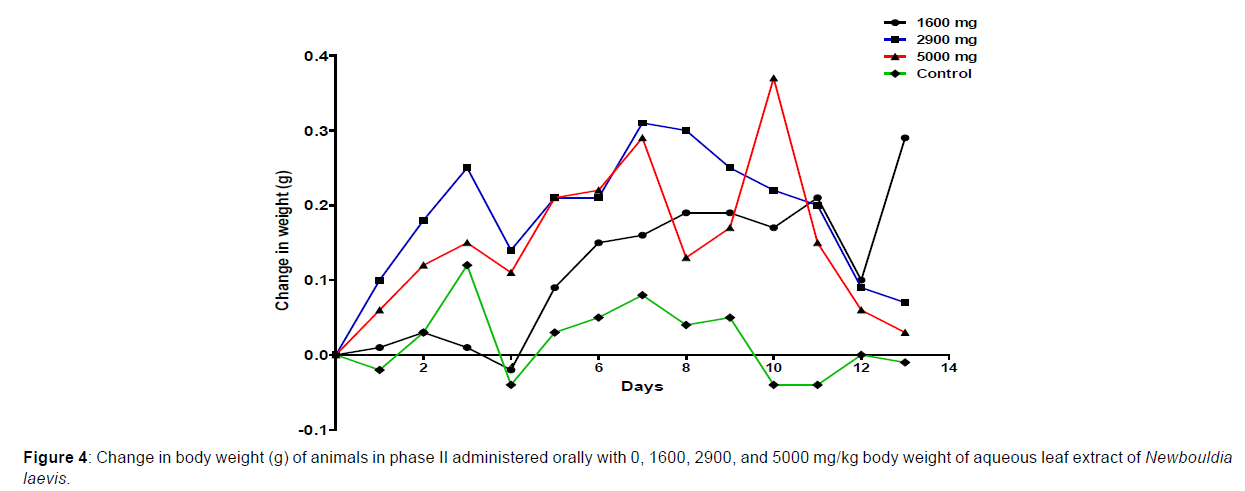
In phase 1 treated with the aqueous leaf extract of N. laevis, mice were administered the extract orally. Mild clinical signs of toxicity (loss of appetite, loss of stimuli sensitivity, loss of agility, and immobility) were developed within the group that was administered 1000 mg/kg within 30 minutes of administration. There were no signs of toxicity observed within the groups that got 10 mg/kg, 100 mg/kg, and the control either mediately or during the post-treatment period. No mortality was recorded either within 24 hours or during the 14- day observation period. In the second phase of the treatment, mice administered with the extract developed clinical signs of toxicity (loss of appetite, loss of stimuli sensitivity, loss of agility, and immobility) after 30 minutes of the post-treatment period with 1600 mg/kg, 2900 mg/kg and 5000 mg/kg of the extract. No mortality was recorded after 24 hours of treatment or during the 14-day observation period. No mortality occurred in the control group during the 14-day observation period. From the result, the LD50 of the aqueous extract of Newbouldia laevis was calculated to be up to 5000 mg/kg for the oral route of administration (Table 1). The body weight distribution and changes of the animals treated with aqueous leaf extract of Newbouldia laevis and the control groups treated with normal saline only are shown in (Figures 1-4).
| PLANT | EXPERIMENT | DOSE (mg/kg) | Proportion of Death | |
|---|---|---|---|---|
| After 24 hours | After 2 weeks | |||
| Newbouldia laevis (aqueous leaf extract) | Phase I | 10 100 1000 Control |
0/3 0/3 0/3 0/3 |
0/3 0/3 0/3 0/3 |
| Newbouldia laevis (aqueous leaf extract) | Phase II | 1600 2900 5000 Control |
0/3 0/3 0/3 0/3 |
0/3 0/3 0/3 0/3 |
Table 1: Oral acute toxicity studies (LD50) of aqueous leaf extract of Newbouldia laevis.
For the animals administered the extract via the intraperitoneal route
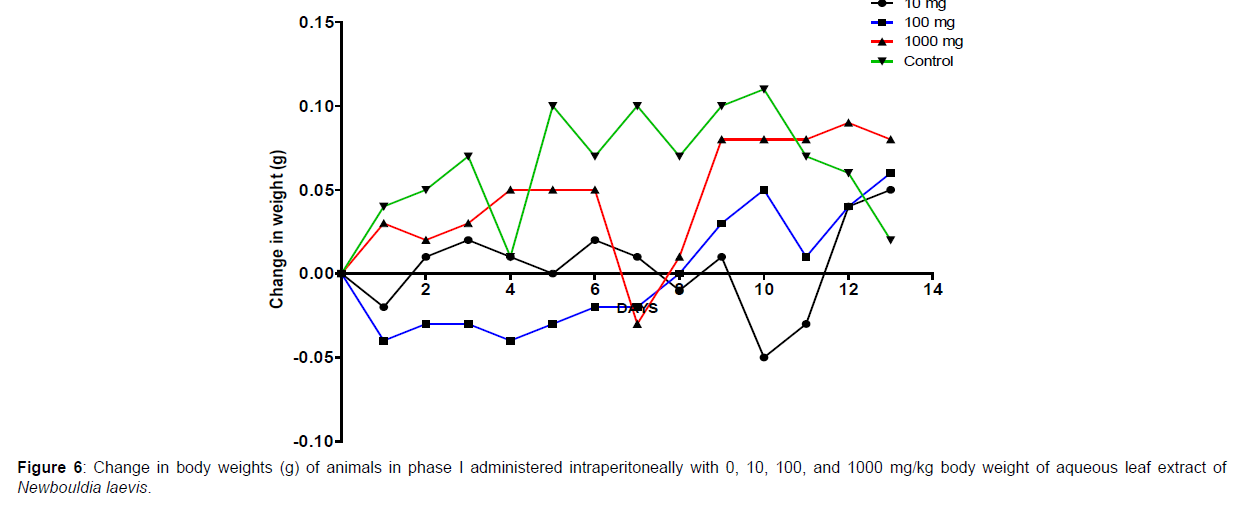
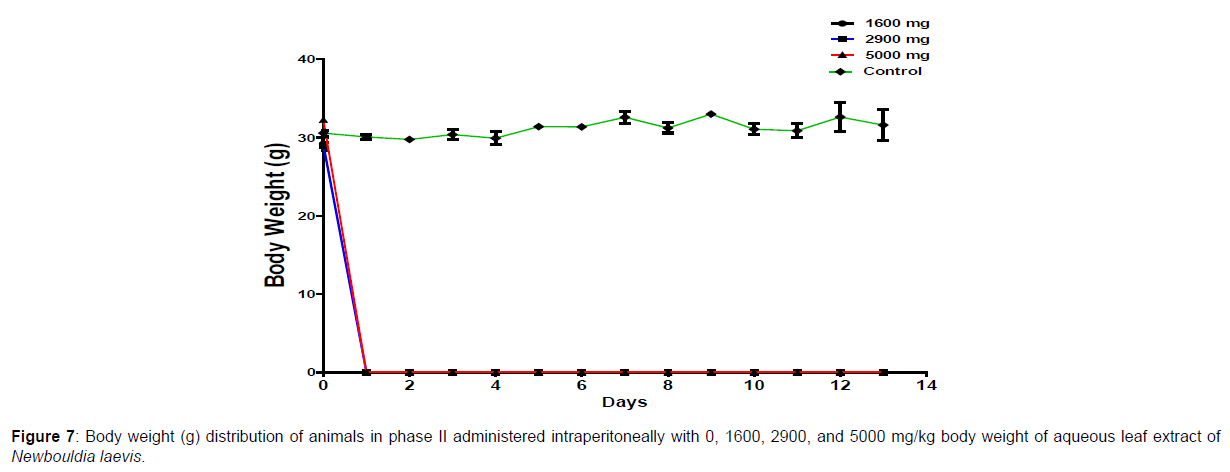
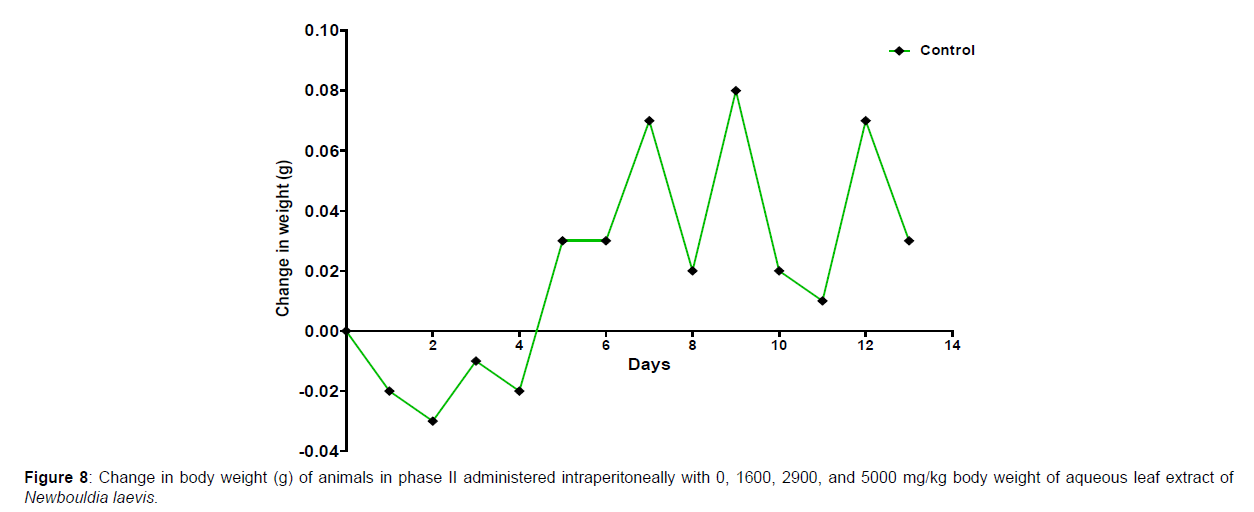
The results of the oral LD50 determination of methanol leaf extract of Newbouldia laevis are presented in Table 2 below. In phase 1 treated with the aqueous leaf extract of N. laevis, mice were administered the extract through the intraperitoneal route. Clinical signs of toxicity (loss of appetite, loss of stimuli sensitivity, loss of agility, and immobility) were observed within the groups that were administered 1000 mg/kg and 100 mg/kg within 30 minutes of administration. There were no signs of toxicity observed within the groups that got 10mg/kg and the control either immediately or during the post-treatment period. In all the groups, no mortality was recorded either within 24 hours or during the 14 days observation period. In the second phase of the treatment, mice administered with the extract developed clinical signs of toxicity (loss of appetite, loss of stimuli sensitivity, loss of agility, and immobility) immediately after administration with 1600 mg/ kg, 2900 mg/kg, and 5000 mg/kg of the extract. All the experimental animals treated with 1600 mg/kg, 2900 mg/kg, and 5000 mg/kg of the extract died within 24 hours after administration. No death was recorded within the control either within 24 hours or during the 14 days observation period. The body weight distribution and changes of the animals treated with aqueous leaf extract of Newbouldia laevis and the control groups treated with normal saline only are shown in (Figures 5-8).
| PLANT | EXPERIMENT | DOSE (mg/kg) | Proportion of Death | |
|---|---|---|---|---|
| After 24hours | After 2weeks | |||
| Newbouldia laevis (aqueous leaf extract) | Phase 1 | 10 100 1000 Control |
0/3 0/3 0/3 0/3 |
0/3 0/3 0/3 0/3 |
| Newbouldia laevis (aqueous leaf extract) | Phase 2 | 1600 2900 5000 Control |
3/3 3/3 3/3 0/3 |
3/3 3/3 3/3 0/3 |
Table 2: Acute toxicity studies (LD50) of aqueous leaf extract of Newbouldia laevis.
Discussion
Africa has a long history of using medicinal plants to create herbal medications or to isolate active compounds (Ene, 2022). Due to the enormous chemical diversity among natural products, many researchers have concentrated their research interests on screening plant extracts to discover new bioactive therapeutic agents for infectious diseases. Indigenous Africans and herbalists use medicinal herbs to treat illnesses ((Ene, 2014 [4]). Although traditional herbal treatment has many advantages, the handling, preparation, and use of medicinal herbs can be hazardous to human health (Langlois-Klassen, 2007 [14]).
Toxicology research should be done to expand understanding of the plant or plant preparation supplied to populations, and medicinal plants should be utilized with prudence (Yuan, 2011 [15]). Being toxic is a way of saying that something is harmful and denotes a condition of unfavorable consequence brought on by interactions between toxicants and cells. To assure the safety of use, a plant extract’s toxicological evaluation looks for any potential side effects. Some things can affect how poisonous a therapeutic plant extract is. This toxicity may be brought on by the manufacturing of the extract or it may be a property of the vegetable medicine itself. The toxicity of the drug is related to its compound(s) and could be ascribed to the active principle or not (Ene, 2021 [1]). Toxicity studies examine whether a drug or chemical has a short-term or long-term detrimental effect on animals. As stated by Paracelsus over 500 years ago, toxicity is dose-dependent (Saganuwan, 2017 [16]). Newbouldia laevis has been used in the folk treatment of various diseases such as an anti-malaria agent, used in the treatment of fever, cough, breast cancer, pain (pelvic pain in females, chest pain, and earache), sickle cell anemia, sexually transmitted infections (STIs), toothache, stomach ache, ulcer, epilepsy, convulsion, infertility, snake bite, diabetes, etc. (Osigwe, 2017 [12]). Because of its numerous medicinal benefits, it is paramount to investigate the intraperitoneal acute toxicity of the aqueous leaf extract of Newbouldia laevis in Swiss albino mice. The result of the quantitative extraction of the aqueous leaf extract of Newbouldia laevis was given to be 10.13%. Also, Anaduaka (2013 [17]), investigated the phytochemicals, nutritional and toxicological potentials of the ethanol extracts of the leaf and stem of Newbouldia laevis. The percentage yields of N. laevis ethanol leaf and stem extracts were found to be 7.44 and 3.30% (w/w), respectively. In the first phase of the treatment with the leaf extract of Newbouldia laevis administered orally, there was a significant (p <0.05) increase in the body weight of experimental animals treated with 10 mg/kg and 100 mg/kg of the extract when compared with the untreated animals (control). Also, there was no significant (p < 0.05) difference in the body weight of the experimental animals treated with 1000 mg/kg when compared with the control. This implies that the aqueous leaf extract of Newbouldia laevis has the potential to support weight gain at 10 mg/kg and 100 mg/kg. In a study by Ene [14], it was found that the tannins in Chrysophyllum albidum extract significantly (p< 0.05) increased the body weight of treated rats in comparison to control rats. A tiny amount of tannins were discovered during a phytochemical analysis of the ethanolic leaf and stem extract of Newbouldia laevis (Anadarko, 2013[17]). The report has shown that tannins from therapeutic plants are successful at increasing body mass (Marcus, 2003[18]). In the second phase of the oral treatment, there was no significant difference in the body weight of the experimental animals treated with 1600 mg/kg, 2900 mg/kg, and 5000 mg/kg. This shows that the extract was not toxic at the given doses (Ene, 2022[19]). No Clinical signs of toxicity (loss of appetite, loss of agility, and loss of immobility) were observed within the groups administered 10 mg/kg, and 100 mg/ kg of the extract. The animals treated with 1000 mg/kg of the aqueous leaf extract showed mild clinical signs of toxicity within 30 minutes after administration. No death was recorded either within 24hrs or during the 14-day observation period. This shows that the extract was not toxic at the given doses (Ene, 2021 [1]). Also, in the second phase of the treatment, all the experimental animals administered 1600 mg/kg, 2900 mg/kg, and 5000 mg/kg of the extract developed clinical signs of toxicity (loss of appetite, loss of agility, loss of stimuli sensitivity and loss of immobility) within 30 minutes after the administration. No death was recorded either within 24 hours or during the 14-day observation period. It could be seen that the aqueous leaf extract of Newbouldia laevis was not toxic at the given doses (Ene, 2022). The LD50 of aqueous leaf extract of Newbouldia laevis when administered orally was estimated to be above 5000 mg/kg. The result of this experiment shows that the aqueous leaf extract of Newbouldia laevis is not toxic when administered orally. This is in line with the acute toxicity investigation of ethanol leaf and stem extract of Newbouldia laevis using rat models reported by Anaduaka (2013[17]). The report showed that the ethanol leaf and stem bark extracts showed no toxicity up to 5000 mg/kg of body weight. Also, a recent study by Ene (2022 [19]) on the acute toxicity of aqueous leaf extract of Chrysophyllum albidum in Swiss Albino rats shows that the LD50 of aqueous leaf extract of Chrysophyllum albidum was calculated to be 1265 mg/kg when administered through the intraperitoneal route and 5000 mg/kg when administered through the oral route. This justifies the choice of methodology. For the extract administration through the intraperitoneal route, in the first phase of the treatment, there was a significant (p<0.05) decrease in body weight across the groups treated with 10mg/kg and 100mg/kg of the extract after the first day of treatment when compared with the control. The experimental animals treated with 1000 mg/kg of the extract showed a significant (p<0.05) increase in body weight when compared with the control after the first day of treatment. Subsequently, there was a significant (p<0.05) increase in the body weight of the experimental animals treated with 10mg/kg, 100 mg/kg, and 1000 mg/ kg of the extract. Aqueous leaf extract of Newbouldia laevis could be seen as relatively toxic and has the potential to support gain. The potency to support weight gain could be the result of the presence of tannins in the extract. Clinical signs of toxicity (loss of appetite, loss of agility, and loss of immobility) were observed within the groups Administered 100 mg/kg and 1000 mg/kg of the extract. The group that got 10mg/kg of the extract did not show any sign of toxicity. No death was recorded. Also, in the second phase of the treatment, all the experimental animals administered 1600 mg/kg, 2900 mg/kg, and 5000 mg/kg of the extract developed clinical signs of toxicity (loss of appetite, loss of agility, loss of stimuli sensitivity and loss of immobility) immediately after administration and all the animals that got the extract died within 24 hours of the post-treatment period. It could be seen that the aqueous leaf extract of Newbouldia laevis was very toxic at the given doses intraperitoneally (Ene, 2021 [1]). The LD50 of the aqueous leaf extract of Newbouldia laevis when administered intraperitoneally was calculated to be 1264.9 mg/kg. The observed toxicity is in line with the study reported by Ndarubu (2020 [20]). In their study, the experimental animals were divided into a group of four animals and were administered 100 mg/kg, 200mg/kg, 300 mg/kg, and 400 mg/kg of methanol extract of Newbouldia laevis respectively. No death was recorded from the group administered 100 mg/kg, 200 mg/ kg, and 300 mg/kg of the extract. One-fourth of the animals that got 400 mg/kg died while a third-fourth of the animals that got 500 mg/kg died. The LD50 was calculated to be 471.9 mg/kg (Ndarubu, 2020[20]).
Conclusion
From the result obtained from this study, it could be concluded that a single oral dose of 10 mg/kg and 100 mg/kg of aqueous leaf extract of Newbouldia laevis was not able to induce mortality or toxic effects in mice, while a single oral dose of 1000 mg/kg, 1600 mg/kg, 2900 mg/ kg and 5000 mg/kg was able to induce clinical signs of toxicity (loss of appetite, loss of stimuli sensitivity, loss of agility and immobility) with no mortality. The LD50 was estimated to be above 5000 mg/kg and the plant extract was said to be safe and non-toxic when taken through the oral route. Furthermore, it was gathered that a single intraperitoneal administration of 10 mg/kg of aqueous leaf extract of Newbouldia laevis was not able to induce mortality or toxic effects in mice, while a single oral dose of 100 mg/kg and 1000 mg/kg, were able to induce clinical symptoms of toxicity (loss of appetite, loss of stimuli sensitivity, loss of agility and immobility) with no mortality and a single oral dose of 1600 mg/kg, 2900 mg/kg and 5000 mg/kg of aqueous leaf extract of Newbouldia laevis were able to induce mortality or toxic effects in mice. The LD50 was calculated to be 1264.9 mg/kg. This shows that this extract is toxic at higher doses but safe at very low doses when administered through the intraperitoneal route.
Acknowledgment
We are grateful to Rev. Chinekeokwu of the Biochemistry department, FUTO for his assistance during the laboratory work.
References
- Ene AC, Obianuju NR, Blessing ON, Chibueze OV, Amah GC, et al. (2021) Acute Toxicity Studies of Methanol Leaf Extract of Chrysophyllum albidum in Swiss Albino Rats. J Anal Bioanal Tech 12: 001.
- Bentur Y (2008) Medical Toxicology: A Distinct Medical Subspecialty Sprouting from Ancient Roots. IMAJ 10: 747–748.
- Liu S, Yin N, Faiola F (2017) Prospects and Frontiers of Stem Cell Toxicology.Stem cells and development26: 1528–1539.
- Ene AC, Atawodi SE, Fatihu MY (2014) Acute Toxicity of Chloroform Extract of Artemisia macivera Linn in Swiss Albino Mice. Journal of Pharmaceutical Research International 4: 1900-1908.
- Farzaei MH, Bayrami Z, Farzaei F, Aneva I, Das SK, et al. (2020) Poisoning by Medical Plants. Arch Iran Med 23:117-127.
- Bnouham M, Merhfour FZ, Elachoui M, Legssyer A, Mekhfi H, et al. (2006) Toxic effects of some medicinal plants used in Moroccan traditional medicine. Moroccan J Biol 2: 21-30.
- Kane JA, Kane SP, Jain S (1995) Hepatitis induced by traditional Chinese herbs; possible toxic components. Gut 36:146-7.
- Woo CSJ, Lau JSH El-Nezami (2012) Herbal Medicine: Toxicity and Recent Trends in Assessing Their Potential Toxic Effects. Advances in Botanical Research 62: 365-384.
- Verma A, Gupta AK, Kumar A, Khan PK (2012) Cytogenetic toxicity of Aloe vera (a medicinal plant). Drug and Chemical Toxicology 35: 32-25.
- Obidike I, Oluwakayinsola S (2013) Herbal medicines in the 21st century. Screening of Herbal medicines for potential toxicities. Intechopen 62-88.
- Okagu IU, Ndefo JN, Agbo MO (2021) Trado-Medical uses Chemical Constituents and Biological Activities of Newbouldia laevis (Bignoniaceae): A Review. Pharm Sci 1-52.
- Osigwe CC, Akah PA, Nworu CS (2017) Biochemical and Haematological Effects of the Leaf Extract of Newbouldia laevis in Alloxan-Induced Diabetic Rats. Journal of Biosciences and Medicines 5: 18-36.
- Lorke D (1983) A New Approach to Practical Acute Toxicity Testing. Arch Toxicol 54: 275–87.
- Langlois-Klassen D, Kipp W, Jhangri GS, Rubaale T (2007) Use of traditional herbal medicine by AIDS patients in Kabarole district, Western Uganda. American Tropical and Medical Hygiene 77: 757-763.
- Yuan X, Chapman RL, Wu Z (2011) Analytical methods for heavy metals in herbal medicines. Phytochemical Analysis 22:189–198.
- Saganuwan SA (2017) Toxicity studies of drugs and chemicals in animals: An overview. Bulg J Vet Med 20:291–318.
- Anaduaka E, Ogugua V, Simeon E (2013) Investigation of some important phytochemical, nutritional properties and toxicological potentials of Newbouldia laevis leaf and stem ethanol extracts. African Journal of Biotechnology 12:5941-5949.
- Marcus C, Karin L, Jain G, Matthias L, Jorns F, et al. (2003) Captive roe deer (Capreolus capreolus) select for low amount of tannic acid but not quebracho: Fluctuation of preference and potential benefit. Biochemistry and Molecular Biology 136:369-382.
- Ene AC, Onodagu VC, Ekwughaonu EO, Ndupu RO, Okoro NB, et al. (2022) Intraperitoneal and Oral Acute Toxicity Studies of Aqueous Leaf Extract of Chrysophyllum Albidum in Swiss Albino Rats. J Anal Bioanal Tech 10: 460.
- Ndarubu TA, Jigam AA, Olufunmilola AH, Famous OI, Damola AS, et al. (2020) Acute Toxicity Studies and Anti-plasmodial Potentials of Newbouldia laevis and Crateva adansonii in Plasmodium Berghei-infected Mice. Iranian Journal of Toxicology 14:93-104.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Ene AC, Aniche CB, Ajuzieogu GI, Elezua VC, Ezeifeka FC (2023) Acute Toxicity of Aqueous Leaf extract of Newbouldia laevis in Swiss Albino Mice. J Anal Bioanal Tech 14: 507.
Copyright: © 2023 Ene AC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 2153
- [From(publication date): 0-2018 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 1908
- PDF downloads: 245