Acute Stroke due to Cunninghamella bertholletiae orbital cellulitis - a case report and review of the literature
Received: 20-Jan-2022 / Manuscript No. JNID-22-51976 / Editor assigned: 22-Jan-2022 / PreQC No. JNID-22-51976(PQ) / Reviewed: 05-Feb-2022 / QC No. JNID-22-51976 / Revised: 10-Feb-2022 / Manuscript No. JNID-22-51976(R) / Published Date: 04-Mar-2022
Abstract
Introduction: We present a case with repeated cerebral infarction by thrombotic fungal mycelium (Cunninghamella bertholletiae) in the right and left pre-stenosed internal carotid artery.
Case: A 72-year-old female patient with a myelodysplastic syndrome presented with a right orbital swelling and a loss of vision. A computer tomography scan and magnetic resonance imaging were suspicious of orbital cellulitis. An orbital decompression was performed. After surgery, the patient developed a hemiparesis on the left side. A computer tomography revealed an internal carotid artery occlusion on the right side. A thrombectomy was performed successfully. The following days a central artery occlusion on the right eye was diagnosed. A lumbar puncture revealed a highly increased white blood count matching to the diagnosis of a meningoencephalitis. On the fourth day of treatment in the neurology department, the patient developed fixed and dilated pupils. A computer tomography showed new bilateral anterior cerebral artery infarctions and infarction of the entire territory of the left middle cerebral artery. Intensive care treatment was terminated. The autopsy found an orbital cellulitis with invasive mycosis caused by mould. Internal transcribed spacer (ITS) sequencing revealed Cunninghamella bertholletiae in the material obtained from the orbit and the left internal carotid artery. Retrospectively, sepsis of thrombotic fungal mycelium due to increased pathological coagulation repeatedly resulted in arterial embolism.
Conclusion: In immunosuppressed patients, rare pathogens can cause sepsis and septic complications. A calculated antifungal therapy should be considered in cryptic cases of meningoencephalitis.
Keywords
Cunninghamella bertholletiae; Septic embolism; Orbit; Sepsis; Stroke
Case presentation
Introduction: We present a case with repeated occurrence of cerebral infarction in the right and left pre-stenosed internal carotid artery due to thrombotic fungal mycelium (Cunninghamella bertholletiae).
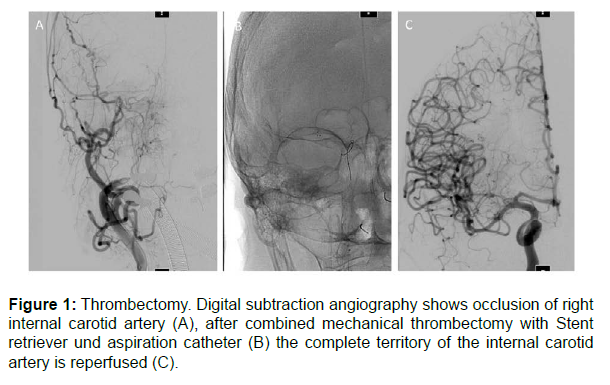
Case description: A 72-year-old female patient was admitted to the eye clinic due to right orbital swelling and a loss of vision. Medical history included a myelodysplastic syndrome with a blast excess and a low-grade T-cell lymphoma. With the suspected diagnosis of posterior ischemic optic neuropathy, a high dosed cortisone therapy was started. A computer tomography scan and magnetic resonance imaging of the orbit were suspicious of orbital cellulitis, and the patient was transferred to an ear, nose, and throat clinic, where orbital decompression was performed under antibiotic therapy with ampicillin and sulbactam. An hour after surgery, the patient presented with hemiparesis on the left side. A computer tomography revealed an internal carotid artery occlusion on the right side. The patient was transferred to the neurology department of the university hospital in Essen, Germany. At admission, the patient was awake with hemiplegia on the left side, a facial palsy on the left side, and pronounced swelling in the right orbital area. The laboratory tests revealed a pancytopenia with a white blood count of 2.38 /nL, a haemoglobin of 6.5 g/dL and a platelet count of 90 /nL. The C-reactive protein (CRP) was 7.3 mg/dL (normal range <0.5 mg/dL). Procalcitonin showed normal values. A thrombectomy was successfully performed in general anaesthesia (Figure 1).
Because of recent surgery and low platelet count, intravenous thrombolysis was not administered. The ventilated patient was transferred to the neurological intensive care unit. In the control imaging on the next day, only small infarct demarcation of the right rostral basal ganglia was detected. The ophthalmologist diagnosed a central artery occlusion of the right eye with a rock-hard bulb. Assuming a transorbital meningoencephalitis, a lumbar puncture was performed. There was a highly increase in leukocytes mostly granulocytes (1794 / μL) and in interleukin-6 (54631 pg/mL) in cerebrospinal fluid (CSF). The CRP increased to 29.6 mg/dL. A calculated antibiotic therapy with ceftriaxone, metronidazole and flucloxacillin was started. Blood and cerebrospinal fluid testing remained culturally negative. Serologically, there was no sign of syphilis and Lyme disease. The patient developed a septic shock, and weaning was at hold. Approximately 80 hours after admission, the patient suddenly presented with dilated and fixed pupils.
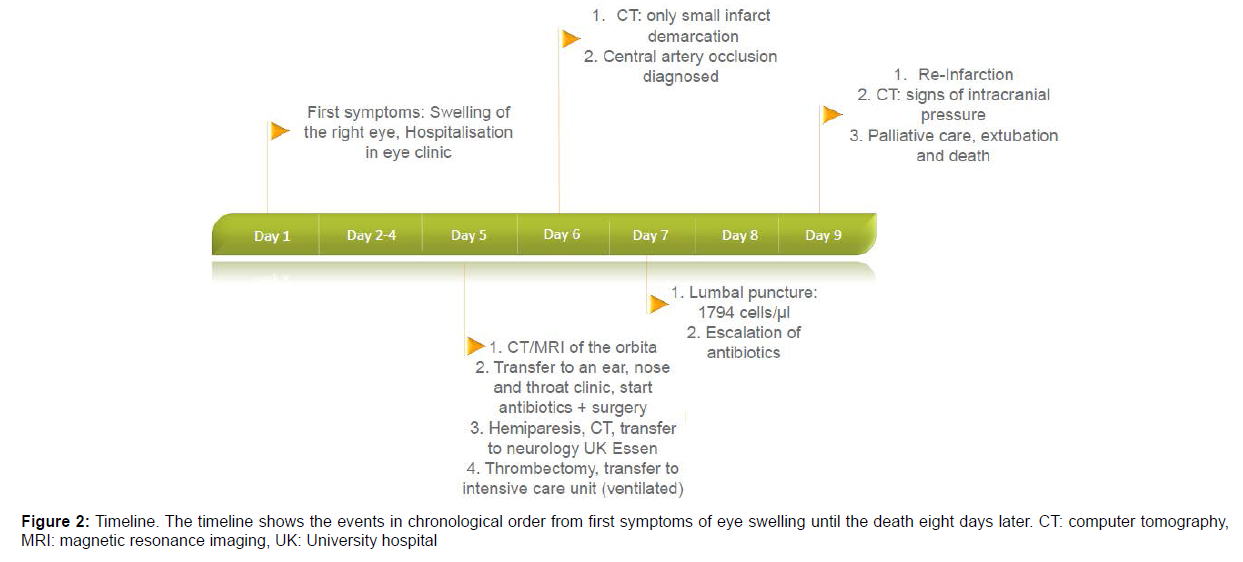
A computer tomography revealed new bilateral anterior cerebral artery infarctions and infarction of the entire territory of the left middle cerebral artery with clear signs of elevated intracranial pressure. Due to the poor prognosis, the intensive care treatment was terminated. The patient died 8 days after first symptoms of orbital swelling. (Figure 2) An autopsy was arranged to clarify the orbital process. The autopsy found an orbital cellulitis with invasive mycosis caused by mould. Internal transcribed spacer (ITS) sequencing revealed Cunninghamella bertholletiae in the material obtained from the orbit and the left internal carotid artery. In retrospect, sepsis of thrombotic fungal mycelium led to increased pathological coagulation and repeated arterial embolism in the right and left pre-stenosed internal carotid artery. This resulted in extensive bilateral infarcts with a consecutive increase in intracranial pressure.
Discussion
Cunninghamella bertholletiae (C. bertholletiae) is a worldwide occurring mould of the order Mucorales and the family Cunninghamellaceae. [1, 2] Within this family, C. bertholletiae is responsible for most human-pathogenic infections. Other human pathogenic Cunninghamellaceae are Cunninghamella elegans [3], Cunninghamella blakesleeana [4] and Cunninghamella echinulate [5].
Infections with Mucorales show a very variable incidence depending on geographical location and population [6]. Cunninghamellaceae infections account for about 5% of all Mucorales infections in Europe [7].
C. bertholletiae is an opportunistic pathogen. The main risk factor for an infection is therefore immunosuppression as in the context of an underlying haematological disease or post organ transplantation [6]. Very rarely immunocompetent patients fall ill [8]. The patient presented had a history of a blast excess due to a myelodysplastic syndrome and a low-grade T-cell lymphoma. In addition, the patient received a high dosed cortisone therapy due to the suspected diagnosis of posterior ischemic optic neuropathy. She therefore had risk factors for opportunistic infections.
The upper and lower respiratory tract is most frequently affected by an infection with C. bertholletiae via inhalation. Contamination of wounds occurs significantly less frequently [1]. Like other Mucorales, C. bertholletiae shows an angio-invasive behaviour, so that the pathogen can spread via the bloodstream and per continuitatem [1, 9]. Rhinoorbitocerebral infections account for 50% of infections in other Mucorales, but are less common in C. bertholletiae with 13% [1]. In this case, however, an orbital cerebral infection was present with histological evidence of fungal hyphae in the orbit, both internal carotid artery and leptomeningeal vessels. The lung showed no autoptic evidence of fungal hyphae.
Depending on the location of the invasive Mucorales infection, different symptoms may occur. While the pulmonary localization manifests itself by fever and respiratory symptoms. In rhino-orbitalcerebral disease, unilateral facial swelling, skin necrosis, proptosis, sinusitis and amaurosis may develop. An osseous destruction of the orbit as in the case presented here is possible. The invasion of the fungus of orbit, eye and brain can be visualized by CT and MRI morphology [1, 6].
The angio-invasive growth pattern of C. bertholletiae, which can lead to dissemination of the pathogen and thromboembolisation with infarction, has been described several times in the literature [10]. In this context, cerebral involvement also resulted in strokes as in the case presented here [11-13].
The following table provides an overview of previously described C. bertholletiae infections with proven or highly suspected* cerebral involvement. (Table 1)
| Reference | Age (years) Sex |
Underlying conditions | Initial symptoms | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Brennan 1983 [11] |
70 male |
Thalassemia minor Sideroblastic anaemia Iron overload |
Sinusitis Facial Cellulitis Facial Pain Unilateral decreased hearing |
Via culture | Died |
| Kolbeck 1985 [12] |
40 male |
Renal transplant nephrectomy after allograft rejection | Fever dyspnoea |
Via culture post mortem |
Died |
| Nimmo 1988 [14] |
19 female |
Morbus Wilson Liver transplant |
Fever | Via culture post mortem |
Died |
| Ortín 2004 [10] |
44 male |
T-Cell acute lymphoblastic leukaemia | Thoracic and substernal pain dyspnoea |
Via culture* post mortem |
Died |
| Righi 2008 [15] |
41 male |
Acute myeloid leukaemia | Fever Unilateral facial oedema |
Via culture* | Died |
| Mayayo 2009 [13] |
50 male |
T-Cell acute lymphoblastic leukaemia | Hemiparesis | Via culture post mortem |
Died |
| Mayayo 2009 [13] |
42 male |
Acute myeloid leukaemia | Fever Pleuritic chest pain |
Via culture post mortem |
Died |
| Su 2016 [16] |
13 female |
Acute lymphoblastic leukaemia | Cough Haemoptysis Dyspnoea |
Via polymerase-chain-reaction* | Died |
| Hiramoto 2020 [17] |
23 male |
Osteosarcoma | Fever Unilateral ptosis Loss of vision Unilateral mydriasis Disorientation Dyspnoea |
ITS-Sequencing* post mortem |
Died |
ITS sequencing was used to detect fungal hyphae of C. bertholletiae in the material obtained from the orbit and in the thrombotic material from the left internal carotid artery. During ITS sequencing, the ITS regions located within the eukaryotic rDNA are examined. The ITS regions show a high genetic polymorphism and can be used for species identification of for example Mucorales [18]. For the identification of C. bertholletiae, the 5.8 rDNA and ITS 2 region (LR1) (Figure 3) were examined by sequencing [19]. A polymerase chain reaction (PCR) product of 382 base pairs length was obtained. The product showed a sequence match of 90-96% with C. bertholletiae when searching with the National Center for Biotechnology Information Basic Local Alignment Search Tool (NCBI BLAST) (on August 5, 2020) [20]. (Figure 3)
Unlike Aspergillus or Candida spp., Mucorales cannot be identified by antigen detection. A commercial PCR is currently not available either. The detection can be done by cultural or histological evaluation. The evaluation of the morphology of Mucorales for genus or species identification is difficult. ITS sequencing represents an alternative [6].
The mortality rate of infections with C. bertholletiae is very high at 77% [1]. The global guideline for the diagnosis and management of mucormycosis issued by the European Confederation of Medical Mycology (ECCM) in cooperation with the Mycoses Study Group (MSG) and Education and Research Consortium (ERC) recommends rapid surgical debridement and antifungal therapy with liposomal amphotericin B 10 mg/kg bodyweight per day in suspected cases of infection with Mucorales and brain involvement. The material obtained during surgical debridement to control the disease can be examined6 histopathologically and microbiologically. Narrowing the spectrum of pathogens helps in the selection of antifungal therapy [6].
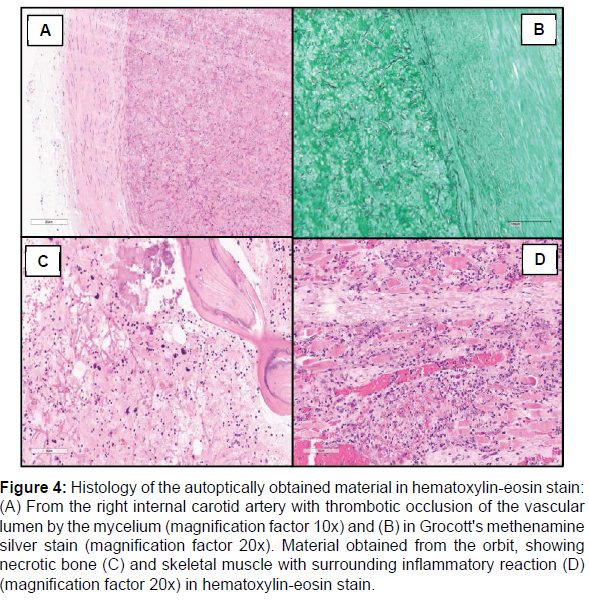
In conclusion, rare pathogens should be looked at very closely in immunosuppressed patients with an infection. If there are thromboembolic events in immunosuppressed patients with meningoencephalitis, mould like C. bertholletiae should be part of differential diagnostics. A calculated antifungal therapy should be considered in cryptic cases of meningoencephalitis without proof of classic pathogens. (Figure 4)
Figure 4: Histology of the autoptically obtained material in hematoxylin-eosin stain: (A) From the right internal carotid artery with thrombotic occlusion of the vascular lumen by the mycelium (magnification factor 10x) and (B) in Grocott's methenamine silver stain (magnification factor 20x). Material obtained from the orbit, showing necrotic bone (C) and skeletal muscle with surrounding inflammatory reaction (D) (magnification factor 20x) in hematoxylin-eosin stain.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Brennan RO, Crain BJ, Proctor AM, Durack DT, Phil MBD et al. (1983) Cunninghamella: a newly recognized cause of rhino cerebral mucormycosis. Am J Clin Pathol 80:98-102.
- Chen YC, Eisner JD, Kattar MM, Rassoulian-Barrett SL, Lafe K, et al. (2001) Polymorphic Internal Transcribed Spacer Region 1 DNA Sequences Identify Medically Important Yeasts. J Clin Microbiol 39: 4042-4051.
- Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, et al. (2019) Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis 19: 405-421.
- García-Rodríguez J, Quiles-Melero I, Humala-Barbier K, Monzon A, Cuenca-Estrella M, et al. (2012) Isolation of Cunninghamella blakesleeana in an immunodepressed patient. Mycoses 55:463- 465.
- Gomes MZ, Lewis RE, Kontoyiannis DP (2011) Mucormycosis caused by unusual mucormycetes, non-Rhizopus, -Mucor, and -Lichtheimia species. Clin Microbiol reviews 24:411- 445.
- Hiramoto R, Miyachi M, Nitta Y, Yoshida H, Kuwahara Y, et al. (2020) Detection of circulating fungal DNA by polymerase chain reaction in a fatal case of Cunninghamella bertholletiae infection. IDCases 20:e00760.
- Honda A, Kamei K, Unno H, Hiroshima K, Kuriyama T, et al.(1998) A murine model of zygomycosis by Cunninghamella bertholletiae. Mycopathologia 144:141-146.
- Kolbeck PC, Makhoul RG, Bollinger RR, Sanfilippo F (1985) Widely disseminated Cunninghamella mucormycosis in an adult renal transplant patient: case report and review of the literature. Am J Clin Pathol 83:747-753.
- Lemmer K, Losert H, Rickerts V, Just-Nübling G, Sander A, et al. (2002) Molekularbiologische Identifizierung von Cunninghamella spec. Mycoses 45:31-36.
- Mayayo E, Klock C, Goldani L (2011) Thyroid involvement in disseminated zygomycosis by Cunninghamella bertholletiae: 2 cases and literature review. Int J Surg Pathol 19:75-79.
- Nimmo GR, Whiting RF, Strong RW (1988) Disseminated mucormycosis due to Cunninghamella bertholletiae in a liver transplant recipient. Postgrad Med J 64: 82-84.
- Ortín X, Escoda L, Llorente A, Rodriguez R, Martínez S, et al. (2004) Cunninghamella Bertholletiae Infection (Mucormycosis) in a Patient with Acute T-cell Lymphoblastic leukemia. Leuk Lymphoma 45:617-620.
- Righi E, Giacomazzi CG, Lindstrom V, Albarello A, Soro O, et al. (2008) A case of Cunninghamella bertholettiae rhino-cerebral infection in a leukaemic patient and review of recent published studies. Mycopathologia 165:407- 410.
- Skiada A, Pagano L, Groll A, Zimmerli S, Dupont B, et al. (2011) Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect 17: 1859-1867.
- Su YY, Chang TY, Wang CJ, Jaing TH, Hsueh C, et al. (2016) Disseminated Cunninghamella bertholletiae Infection During Induction Chemotherapy in a Girl with High-Risk Acute Lymphoblastic Leukemia. Pediatr Neonatol 57: 531-534.
- Tadepalli K, Gupta PK, Asati DP, Biswas D, (2015) Onychomycosis due to Cunninghamella bertholletiae in an Immunocompetent Male from Central India. Case Rep Infect Dis :703240.
- Walther G, Pawłowska J, Alastruey-Izquierdo A, Wrzosek M, Rodriguez-Tudela JL, et al. (2013) DNA barcoding in Mucorales: an inventory of biodiversity. Persoonia 30:11-47.
- Yu J, Walther G, Van Diepeningen AD, Gerrits Van Den Ende AGH, Li R-Y, et al. (2014) DNA barcoding of clinically relevant Cunninghamella species. Medical Mycology 53(2):99-106.
- https://sites.duke.edu/vilgalyslab/rdna_primers_for_fungi/
- https://blast.ncbi.nlm.nih.gov/Blast.cgi
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Indexed at , Google Scholar , Crossref
Citation: Oster C, Steinborn JK, Blau T, Rath PM, Schmid KW, et al. (2022) Acute Stroke Due to Cunninghamella bertholletiae Orbital Cellulitis – A Case Report and Review of the Literature. J Neuroinfect Dis 13: 383.
Copyright: © 2022 Oster C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2346
- [From(publication date): 0-2022 - Feb 22, 2025]
- Breakdown by view type
- HTML page views: 1972
- PDF downloads: 374