Research Article Open Access
Acute Cocaine Differentially Induces PKA Phosphorylation Substrates in Male and Female Rats
Wei-Lun Sun1, Luyi Zhou2, Arbi Nazarian1, Vanya Quinones- Jenab1,3 and Shirzad Jenab1,3*1Department of Psychology and Biopsychology and Behavioral Neuroscience, City University of New York, New York 10065, USA
2Biological Sciences and Biology, PhD Programs, City University of New York, New York, USA
3Biopsychology and Behavioral Neuroscience and Biology PhD Programs, City University of New York, New York, USA
- Corresponding Author:
- Dr. Shirzad Jenab
Department of Psychology
Hunter College of the City University
of New York, New York 10065, USA
Tel: +1-212-772-5732
Fax: +1-212-772-4619
E-mail: sjenab@hunter.cuny.edu
Received date: June 15, 2015 Accepted date: July 30, 2015 Published date: August 06, 2015
Citation: Sun WL, Nazarian A, Jenab S, Zhou L, Jenaba VQ (2015) Acute Cocaine Differentially Induces PKA Phosphorylation Substrates in Male and Female Rats. J Addict Res Ther 6:236. doi:10.4172/2155-6105.1000236
Copyright: © 2015 Sun WL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Background: Sex differences in intracellular dopamine pathways may contribute to the known sex differences in psychomotor responses to cocaine and differential development of dependence. This study aimed to determine whether there are sex differences in the activation of the extracellular signal-regulated kinases (ERK1/2, or p44/p42 MAPK) and PKA phosphorylation-dependent substrates in the nucleus accumbens (NAcc) of male and female rats at baseline or after acute cocaine administration.
Methods: 60-day-old male and female Fischer rats were injected with saline or cocaine (30 mg/kg) and sacrificed 5, 15, 30, 45 or 90 minutes later. Total locomotor activity, Stereotypic, rearing, and ambulatory behaviors was measured for 90 minutes using a two-frame automated Photobeam Activity
Results: Similar to our previous findings, total locomotor activities were higher in female rats after this single cocaine administration. Females had higher levels of phosphorylated PKA substrates after cocaine administration, and this change lasted longer and had a greater magnitude than in cocaine treated male rats. Furthermore, although cocaine administration increased the phosphorylation of ERK proteins, there were no sex differences in p-ERK protein levels either at baseline or after acute cocaine administration.
Conclusion: Taken together, these findings suggest that sex differences in basal and cocaine-induced alterations in PKA signaling activity in the NAcc may contribute to sex differences in psychomotor responses to cocaine. However, not all the components of the DA-intracellular signaling pathway maybe heightened in female rats as ERK phosphorylation patterns did not differ between the sexes.
Keywords
Cocaine; Sex differences; ERK; PKA; Nucleus accumbens; Females
Introduction
Cocaine use among women has steadily increased, rising to approximately 30% of users in the United States. As researchers pay more attention to hormonal effects on drug abuse, it is becoming apparent that men and women react differently to cocaine. Overall, women are more vulnerable to some aspects of cocaine abuse, such as being more sensitive to the addictive properties of cocaine, experiencing more nervousness after intermittent administration of cocaine, taking longer to feel its subjective effects, reporting less euphoria, and having more severe cravings in response to cocaine-associated cues [1-9]. Women also increase their rate of cocaine consumption more rapidly than do men, and once addicted it is more difficult for them to quit [9,10]. Likewise, after abstinence, women use cocaine for longer periods than do men [11,12].
Similar to humans, female rodents also show exaggerated and more robust psychomotor responses to cocaine than do males [13-16]. Females also more quickly develop cocaine-induced conditioned place preference (CPP) with lower doses and more readily acquire cocaine self-administration [17-20]. Taken together, human and animal studies suggest that sex-specific differences exist at all stages of cocaine abuse including induction, maintenance, and relapse.
Sex differences in the mesocorticolimbic dopamine (DA) system- -a regulator of cocaine’s psychomotor and rewarding effects have been demonstrated [21-24]. As recently reviewed by Becker and Hu [9], there are sex differences in the levels of DA receptors in the striatum, in the efficacy of DA antagonists and agonists to block DA receptors, and in cocaine-induced accumbal DA release/reuptake. The sexually dimorphic pattern in DA system activation after cocaine treatment is postulated to be correlated with sex differences in cocaine-induced DAmediated intracellular responses.
Behavioral studies have shown a positive association between protein kinase A (PKA) signaling changes and behavioral responses after cocaine administration [25,26]. Specifically, administration of a PKA activator or a PKA inhibitor enhances or dampens, respectively, acute cocaine-mediated locomotor behavior and modulates the cocaine-induced CPP [27,28]. PKA activation also potentiates behavioral sensitization after chronic cocaine administration [28,29]. Moreover, intra-accumbens injection of PKA activator in rats increases cocaine self-administration. By contrast, intra-accumbens infusion of PKA inhibitor decreases cocaine self-administration [30]. It is therefore feasible that sex differences in cocaine’s modulation of the DA-PKA signaling pathway in the NAcc may contribute to sex differences in the initiation and development of the rewarding properties of cocaine. Indeed, three recent studies have found sexually dimorphic responses to cocaine in the DA-PKA signaling pathway.
Nazarian et al. [22] demonstrated that PKA protein levels in the NAcc are higher in females both before and after cocaine administration; Lynch et al. [31] showed that protein levels of DA- and cAMP-regulated phosphoprotein of Mr 32 kDa (DARPP-32) are also higher in females after cocaine administration and Zhou et al. [32] found basal and cocaine-induced sex differences in the PKA-DARPP-32 cascade. Together, these studies suggest that sex differences both at baseline and after cocaine-induced alterations in the DA-PKA signaling pathways in the NAcc may contribute to patterns of sexual dimorphism in the initiation and development of addictive responses to cocaine.
The intracellular cascade of extracellular signal-regulated kinases (ERK1/2, or p44/p42 MAPK) -- one of several mitogen-activated protein kinases (MAPK) - has been postulated to be involved in psychostimulant addiction. In mice, acute cocaine treatment induces a rapid and transient increase in the ERK protein phosphorylation (p-ERK) in the NAcc, which can be abolished by pretreatment with a DA-D1 receptor antagonist [33-35]. Behaviorally, the cocaineinduced hyper-locomotor activity and the development/expression of sensitization and CPP are attenuated by treatment with ERK inhibitors [36-39]. Recent studies on ERK mutant mice indicated that, owing to the hyper-phosphorylation of ERK protein, mutated mice show stronger psychostimulant (e.g., cocaine) induced CPP and behavioral sensitization than do the wild-type controls [40,41].
However, it is not known if the stronger and longer-lasting cocaine responses in females (including CPP and psychomotor activation) are related to a disregulation of the ERK-mediated intracellular responses. Cyclic AMP response element binding protein (CREB) is a transcription factor that is involved in modulating the psychomotor and addictive properties of cocaine [42]. According to one report, enhanced activation of ERK enables and mediates cocaine-induced CREB phosphorylation in the NAcc [36]. However, others have found that cocaine-induced ERK and CREB phosphorylation are dissociated in many brain regions [43,44]. After cocaine administration, pCREB levels in the NAcc are induced for a longer time in male rats, but the magnitude of change in pCREB levels is higher in female rats [22]. Because pCREB is a substrate of the ERK pathway, we hypothesized that p-ERK activation closely follows the previously reported observation for pCREB and that differences in basal or cocaine-induced alterations in ERK levels have a sexually dimorphic pattern. The aim of this study was to test this hypothesis by measuring basal and cocaine-induced ERK proteins and p-(Ser/Thr)-PKA substrates in male and female rats.
Methods
Animals
60-day-old male and female Fischer rats (Charles River, Raleigh, NC) were individually housed in Plexiglas chambers (20 × 20 × 41 cm) layered with beta chips. Rats were given free access to food and water and maintained on a 12 hour light/dark cycle (lights on at 9:00 AM). All rats were weighed, handled, and intraperitoneally injected daily with saline for 5 consecutive days prior to testing. For behavioral testing, 10 rats per group were used. For protein analysis, 4 rats per group were used.
Repeated vaginal lavage attenuates cocaine-induced activity, abolishes estrous cycle effects, and establishes CPP in female rats, thus possibly increasing DA-mediated responses [45]. Therefore, as noted by Walker et al. [45], the use of lavaged female rats could skew female behavioral responses when making side-by-side comparisons with male rats. For this reason, females were randomly assigned to experimental groups without regard to their estrous cycle. Animal care and use was in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication 85-23, Bethesda, MD) and approved by the Hunter Institution Animal Care Use Committee.
Materials
Cocaine hydrochloride and p-ERK primary antibody were purchased from Sigma Chemical Co. (St. Louis, MO). Phospho-(Ser/ Thr) PKA substrate-specific antibody measures the levels of substrate phosphorylation by PKA, a class of kinases referred to as Arg-directed kinases or AGC-family kinases that share a substrate specificity characterized by Arg at position -3 relative to the phosphorylated Ser or Thr. This antibody detects phosphorylation by PKA to substrates that contain a phospho-serine/threonine residue with arginine at the 3rd position (Cell Signaling Technologies; Beverly, MA). Phospho-(Ser/ Thr) PKA antibody substrates include Apaf-1, CAMKII, Caspase-10, Caspase-2, CREB, and NMDAR1, among others (see Cell Signaling #9621). Alpha--tubulin antibody was purchased from Santa Cruz Technologies (Santa Cruz, CA). All appropriate secondary antibodies were purchased from Amersham Pharmacia (Piscataway, NJ).
Cocaine administration
Cocaine solutions were prepared on the day of testing by dissolving the drug in physiological saline (0.9%). On the day of testing, rats were injected (i.p.) with saline or cocaine (30 mg/kg) and sacrificed 5, 15, 30, 45 or 90 minutes later. Rats were returned to their home cages after drug treatment.
Behavioral measurements
All behavioral measurements were conducted in the rat’s home cage. Behavioral activity was measured only for the male and female groups sacrificed at 90 minutes after drug treatment. Total locomotor activity was measured for 90 minutes using a two-frame automated Photobeam Activity System (San Diego Instruments; San Diego, CA). Total activity was determined by total counts of any photobeam interruptions in the lower or upper frame. Stereotypic, rearing, and ambulatory behaviors for these rats were previously reported [22].
Tissue collection
After decapitation (following a brief 20 second exposure to CO2), their brains were removed, flash frozen in 2-methylbutane (-40°C), and stored at -80°C until used. The NAcc was dissected from coronal sections (1 mm thick) using a matrix (ASI Instruments; Warren, MI). The NAcc (including both shell and core) was dissected from coronal sections ranging from 1.70 to 1 mm anterior to the bregma (1 mm thick) by using a brain matrix. Tissue was homogenized with the use of a Polytron handheld homogenizer (Kinematica; Luzern, Switzerland) in homogenizing buffer [HEPES 7.9 (20 mM), KCl (10 mM), EDTA (1 mM), NP40 (0.2%), glycerol (10%), NAccl (200 mM), pepstatin, leupeptin, DTT (1 M), aprotenin, PMSF (100 mM), NaF (50 mM), and Na3VO4 (1 mM)]. Total protein content was determined with use of a Bradford kit (Bio-Rad Laboratories; Hercules, CA). Samples were stored at -80°C until used.
Western blot analysis
Protein samples (40 μg) were boiled in Lammeli buffer containing 5% β-mercaptoethanol and loaded onto 10% SDS-PAGE. Gels were electrophoresed, transferred to nitrocellulose membranes, and blocked for 60 minutes with 5% nonfat dry milk in tris-buffer-saline-tween (TBST, pH = 7.4) at room temperature. Membranes were probed overnight at 4°C with phospho- (Ser/Thr) PKA substrate antibody (1:500) and p-ERK antibody (1:1000). After three washes with TBST, membranes were then incubated with their appropriate secondary antibody (1:1000) for 60 minutes at room temperature, followed by three more washes with TBST. Antibody binding was detected using an enhanced chemiluminescence kit (Amersham Pharmacia; Piscataway, NJ). Resulting films were scanned and quantified with a computer densitometer and ImageQuant software (Molecular Dynamics; Sunnyvale, CA).
To compare sex differences in the protein levels, samples from saline-treated male and female rats were loaded onto the same gel. Within each sex, to determine the time course of changes in protein levels after treatment, all saline- treated samples (5 min - 90 min), or cocaine-treated (5 min - 90 min) samples with saline control (5 min), were run on the same gel. Four sets of gels were run for each determinant. To normalize band intensity to protein levels, membranes were re-probed with α−tubulin antibody (1:1000).
Statistical analysis
The time-course of locomotor counts was analyzed using three-way repeated measures analysis of variance (ANOVAs) for the variables sex (male vs. female), drug (saline or cocaine), and time (18 five minute time blocks for acute cocaine/saline). In addition, two-way ANOVAs (sex × drug) were used to determine differences of accumulative locomotor counts. For post hoc analysis, LSD tests were conducted when appropriate.
All protein levels are expressed as a ratio to α-tubulin levels. Data are presented as mean ± SEM. Within sex, one-way ANOVAs followed by post-hoc LSD analysis were used to determine differences during the time course. For comparison between sexes in cocaine-treated rats, percentage changes of protein levels between the cocaine-treated group and the average of protein levels in saline controls of the same sex were used. This approach was necessary because male and female samples were run in different gels. Independent t-test was used to evaluate any sex difference at each specific time point after cocaine administration. Statistical significance was considered to be p < 0.05 for all analyses.
Results
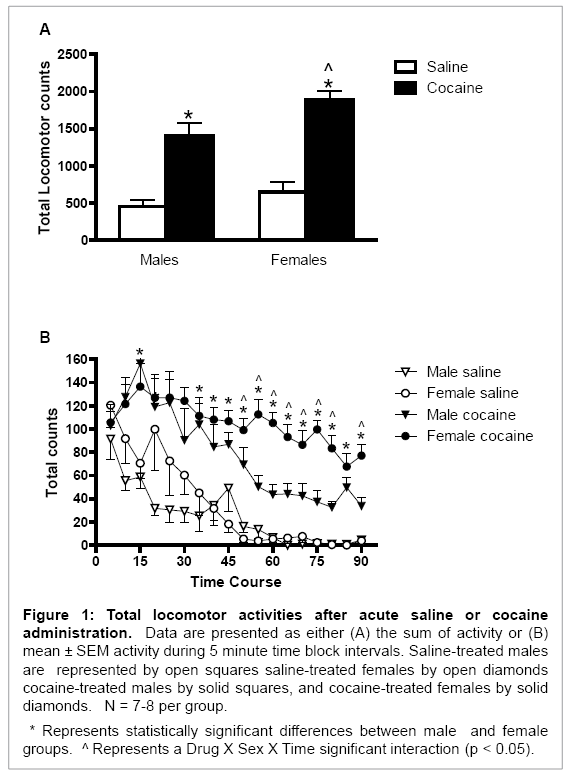
Overall, cocaine increased locomotor activity in both male and female rats (Drug main effect: F (1, 26) = 73.04, p < 0.001; Figure 1A). Female rats had higher cocaine-induced total locomotor activity [Sex main effect: F (1, 26) = 7.19, p < 0.02; Figure 1A] and longer-lasting total locomotor activity than did male rats [Drug × Sex × Time interaction: F (17, 468) = 2.32, p < 0.01; Figure 1B]. This behavioral activity was significantly higher during the final 40 minutes (time intervals 11-18) of the testing session in female rats (p < 0.05 for all comparisons; Figure 1B).
Figure 1: Total locomotor activities after acute saline or cocaine administration. Data are presented as either (A) the sum of activity or (B) mean ± SEM activity during 5 minute time block intervals. Saline-treated males are represented by open squares saline-treated females by open diamonds cocaine-treated males by solid squares, and cocaine-treated females by solid diamonds. N = 7-8 per group.
* Represents statistically significant differences between male and female groups. ^ Represents a Drug X Sex X Time significant interaction (p < 0.05).
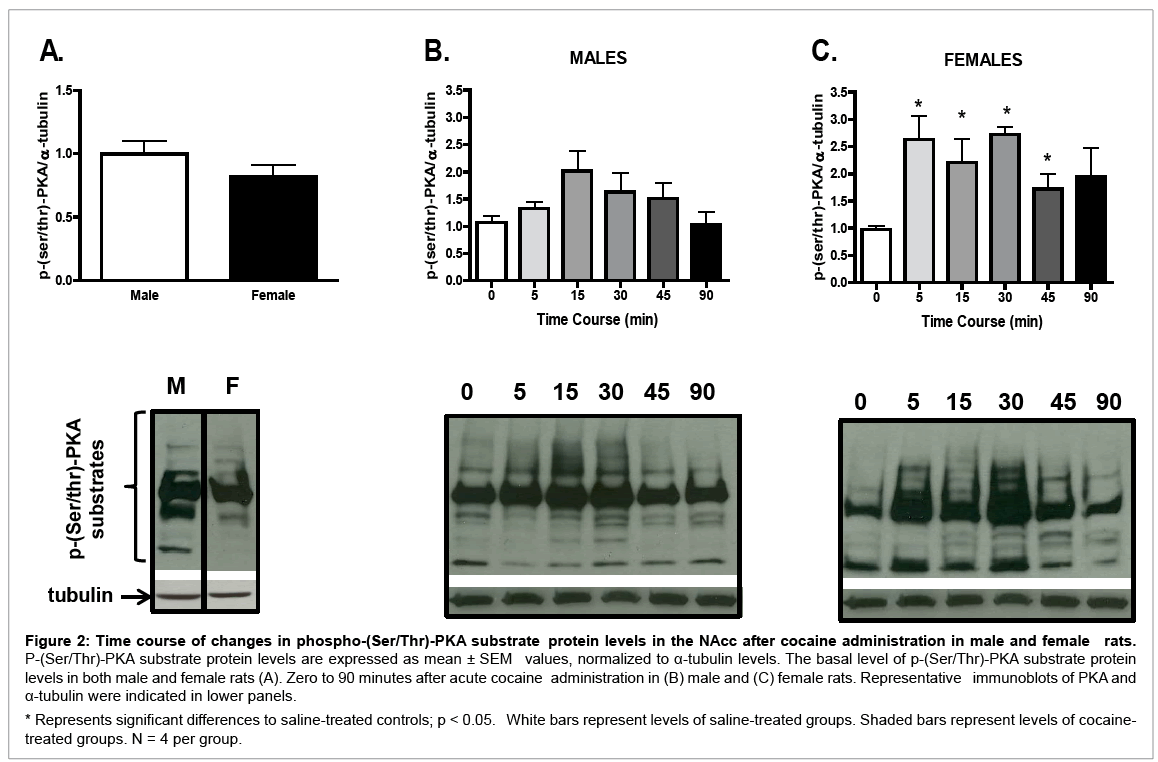
Although no sex differences in basal p-(Ser/Thr)-PKA substrate protein levels were found (Figure 2A), a sexually dimorphic temporal pattern in p-(Ser/Thr)-PKA substrate protein levels was observed after cocaine treatment. In male rats (Figure 2B), although acute cocaine induced p-(Ser/Thr)-PKA substrate protein levels in the NAcc, oneway ANOVA failed to reach statistical significance (F (5, 23) = 2.01, p = 0.13), as compared with the saline control (p < 0.05). In contrast, in female rats (Figure 2C), a significant time effect was seen after cocaine administration (F (5, 23) = 2.83, p < 0.05); p-(Ser/Thr)-PKA substrate protein levels were increased from 5 to 30 minutes after cocaine treatment as compared with saline controls (p < 0.05 for all comparisons). Sex differences were observed in overall levels of p-(Ser/ Thr)-PKA substrate protein (Table 1); female rats had higher p-(Ser/ Thr)-PKA substrate protein levels than males regardless of the time after cocaine injection (t (1, 38) = 2.98, p < 0.01). In addition, 5 and 30 minutes after cocaine administration, p-(Ser/Thr)-PKA substrate protein levels in females were significantly higher than in males (t (1, 6) = 2.77, p < 0.05; and t (1, 6) = 2.65, p < 0.05, respectively; Table 1).
Figure 2: Time course of changes in phospho-(Ser/Thr)-PKA substrate protein levels in the NAcc after cocaine administration in male and female rats. P-(Ser/Thr)-PKA substrate protein levels are expressed as mean ± SEM values, normalized to α-tubulin levels. The basal level of p-(Ser/Thr)-PKA substrate protein levels in both male and female rats (A). Zero to 90 minutes after acute cocaine administration in (B) male and (C) female rats. Representative immunoblots of PKA and α-tubulin were indicated in lower panels.
* Represents significant differences to saline-treated controls; p < 0.05. White bars represent levels of saline-treated groups. Shaded bars represent levels of cocainetreated groups. N = 4 per group.
| 5 min | 15 min | 30 min | 45 min | 90 min | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | |
| p-PKA substrate | 24.5 ± 11.9 | 136.8 ± 38.7* | 89.1 ± 33.8 | 77.7 ± 38.9 | 53.2 ± 31.6 | 144.3 ± 13.5* | 41.2 ± 27.3 | 54.5 ± 24.7 | -3.3 ± 22.2 | 74.4 ± 47.4 |
| p-ERK | 173.2 ± 34.9 | 231.4 ± 43.7 | 129.8 ± 4.6 | 155.6 ± 36.2 | 164.6 ± 19.3 | 207.0 ± 28.5 | 96.8 ± 15.1 | 100.3 ± 12.1 | 112.7 ± 15.5 | 94.2 ± 22.7 |
Data are presented as % change of protein levels ± SEM as compared to 5 min saline controls of the same sex.
* And bolded represents statistically significant differences between sexes. (N = 4 per group).
Table 1: Percentage change of p-(Ser/Thr)-PKA- substrate signaling and p-ERK protein levels in NAc.
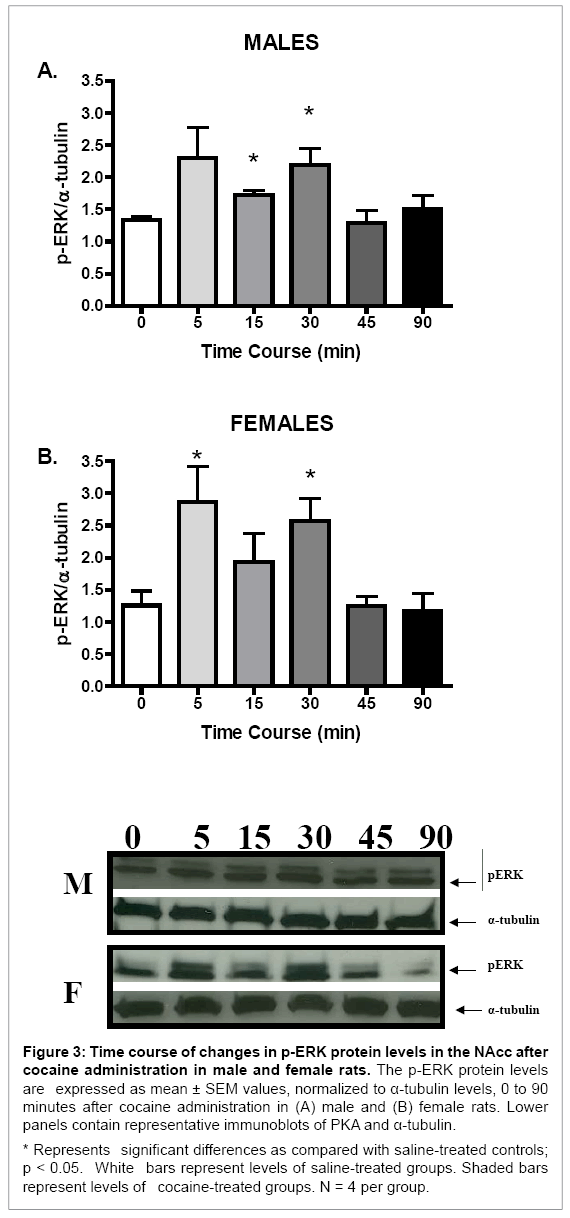
Although no sex differences in basal or cocaine-induced protein levels of p-ERK were seen (data not shown), p-ERK protein levels in the NAcc of both sexes were increased after acute cocaine administration (F (5, 23) = 3.02, p < 0.05, and F (5, 23) = 4.25, p < 0.01, for males and females, respectively; Figure 3). Furthermore, the temporal pattern of p-ERK induction after cocaine administration was similar for males and females. In both sexes, p-ERK protein levels were significantly increased 5 and 30 minutes after cocaine administration as compared with their respective saline controls (p < 0.05 for all comparisons; Figure 3). No sex differences in the magnitude of change of p-ERK protein levels after cocaine administration were observed (Table 1).
Figure 3: Time course of changes in p-ERK protein levels in the NAcc after cocaine administration in male and female rats. The p-ERK protein levels are expressed as mean ± SEM values, normalized to α-tubulin levels, 0 to 90 minutes after cocaine administration in (A) male and (B) female rats. Lower panels contain representative immunoblots of PKA and α-tubulin.
* Represents significant differences as compared with saline-treated controls; p < 0.05. White bars represent levels of saline-treated groups. Shaded bars represent levels of cocaine-treated groups. N = 4 per group.
Discussion
This study further extends observations by Nazarian et al., Zhou et al., and Lynch et al. [22,31,32] by demonstrating that female rats have a functionally augmented PKA signaling transduction pathway. We report here that the phosphorylation of PKA substrates was induced differentially after cocaine administration. Cocaine may modulate ERK pathway signaling through the activation of DA-D1 as well as NMDA receptors [46]; e.g., inhibition of NMDA reduces cocaine-induced ERK activation [47]. The fact that neither the ERK pattern of phosphorylation nor the levels of activation differed between the sexes suggests that (1) cocaine’s effects on calcium-NMDA dependent mechanisms are not sexually dimorphic and/or (2) not all components of the females’ DAintracellular signaling pathway are heightened.
No sex differences in basal phosphorylation patterns of substrates for PKA were observed. However, after cocaine administration, female rats had overall faster (activation after 5 minutes of cocaine administration), longer (males have activation lasting only up to 15 minutes, whereas females have activation lasting up to 30 minutes), and higher levels of PKA phosphorylation of its substrates (females have a higher percentage change in p-(Ser/Thr)-PKA substrate protein levels than do male rats). Nazarian et al. [22] showed that female rats have higher basal protein levels of PKA in the NAcc. Since the phosphorylation pattern of substrates for PKA before cocaine administration did not differ between the sexes, it suggests that the increases of basal PKA protein levels seen in the NAcc of females may lead to functional effects of the PKA pathway.
After acute cocaine administration, female rats have been shown consistently to have greater behavioral responses to cocaine [15,48]. Indeed, we found in this study that female rats exhibited longer-lasting and more robust psychomotor responses to cocaine. It is feasible that the augmented phosphorylation of PKA substrates in the NAcc of females may contribute to the observed increase in behavioral responses after cocaine administration. In fact, because many PKA substrates are also transcriptional factors known to be involved in modulating the psychostimulating and addictive properties of cocaine [22], a prolonged and more robust phosphorylation of PKA substrates may indeed provide more changes in protein activation and transcription in female rats. These changes, in turn, may further impel the differences in psychomotor responses between males and females.
Consistent with previous reports [34,39,43,46,49-51] after a single cocaine injection in male rats, a rapid and transient increase of p-ERK protein levels was observed. To our knowledge this is the first report that, as with males, p-ERK protein levels were also induced in the NAcc of female rats. Nazarian et al. [22] demonstrated that in female rats accumbal pCREB activation was more robust, but of shorter duration, than in male rats (in male rats pCREB protein levels increased 5 to 30 minutes after cocaine administration, whereas in female rats they increased only at 5 minutes after cocaine treatment). It has been postulated that enhanced activation of ERK enables and mediates cocaine-induced CREB phosphorylation in the NAcc (see review in [52]). For example, acute cocaine administration augments CREB phosphorylation in an ERK-dependent manner [53-56]. However, others have found that cocaine-induced ERK and CREB phosphorylation were dissociated in the NAcc [44]. Independent of ERK, CREB phosphorylation at the S133 residue is regulated by PKA in addition to several other kinases [44,57]. Thus, as postulated by Edwards et al. [44], in some brain areas PKA may be the primary kinase for CREB. Unlike p-CREB [22], in this study p-ERK demonstrated neither a sexually dimorphic temporal pattern of activation nor sex differences in the magnitude of cocaine-induced changes. On the other hand, the greater basal and cocaine-induced PKA levels in females may contribute to sex differences in quantified pCREB phosphorylation intensity, and PKA protein levels between sexes may also underlie the increases of phosphorylation of CREB after cocaine administration. In addition to pCREB, the phosphorylation of S845 GluA1 subunit (pGluA1), one of PKA-mediated phosphorylation substrates, is important for the cocaine-mediated behavioral response [58]. The enhanced pGluA1 has been demonstrated to increase of AMPA receptor-mediated excitatory current through the augmentation of GluA1 membrane insertion [59-61]. It is plausible that the differential pGluA1 induction in the NAcc is responsible for the behavioral sex difference after acute cocaine administration. Further, besides PKA, pGluA1 is highly regulated by other phosphatases including calcineurin. Previously, we have shown basal and cocaine-induced sex differences on calcineurin protein expression in the NAcc [32]. Taken together, these results indicate that pGluA1 is the potential read-out for the cellular mechanism underlying cocaine-induced behavioral difference in male and female rats. Nevertheless, the pGluA1 is our ongoing research to further decipher the molecular pathway contributing the sex dimorphism of cocaine addiction.
In summary, our results suggest that females have an elevated D1-PKA-mediated intracellular second messenger transduction as compared with male rats. Thus, data presented here further elucidate the neurobiological basis for sex differences in cocaine’s rewarding effects. Our results reinforce the recurrent postulate that many aspects of cocaine addiction and responses are gender specific. However, the extent to which sex differences in the activation of D1-PKAmediated intracellular second messenger transduction contribute to females’ greater vulnerability to some aspects of cocaine remains to be determined. Answers to these questions are needed to further understand drug abuse in both males and females and to improve treatment in a manner that will meet the needs of both sexes.
Acknowledgement
We are grateful to Dr. Patricia Stephens for her editorial comments. Supported by DA12136 and MD007599.
References
- Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ (1993) Gender differences in cocaine use and treatment response. J Subst Abuse Treat 10: 63-66.
- Chen K, Kandel D (2002) Relationship between extent of cocaine use and dependence among adolescents and adults in the United States. Drug Alcohol Depend 68: 65-85.
- Van Etten ML, Neumark YD, Anthony JC (1999) Male-female differences in the earliest stages of drug involvement. Addiction 94: 1413-1419.
- Kosten TR, Kosten TA, McDougle CJ, Hameedi FA, McCance EF, et al. (1996) Gender differences in response to intranasal cocaine administration to humans. Biol Psychiatry 39: 147-148.
- Van Etten ML, Anthony JC (2001) Male-female differences in transitions from first drug opportunity to first use: searching for subgroup variation by age, race, region, and urban status. J Womens Health Gend Based Med 10: 797-804.
- Lynch WJ, Roth ME, Carroll ME (2002) Biological basis of sex differences in drug abuse: Preclinical and clinical studies. Psychopharmacology (Berl) 164: 121-137.
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR (2004) Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend 74: 265-272.
- Najavits LM, Lester KM (2008) Gender differences in cocaine dependence. Drug Alcohol Depend 97: 190-194.
- Becker JB, Hu M (2008) Sex differences in drug abuse. Front Neuroendocrinol 29: 36-47.
- Brady KT, Randall CL (1999) Gender differences in substance use disorders. PsychiatrClin North Am 22: 241-252.
- Robbins SJ, Ehrman RN, Childress AR, O'Brien CP (1999) Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend 53: 223-230.
- Gallop RJ, Crits-Christoph P, Ten Have TR, Barber JP, Frank A, et al. (2007) Differential transitions between cocaine use and abstinence for men and women. J Consult ClinPsychol 75: 95-103.
- Van Haaren F, Meyer ME (1991) Sex differences in locomotor activity after acute and chronic cocaine administration. PharmacolBiochemBehav 39: 923-927.
- Schindler CW, Carmona GN (2002) Effects of dopamine agonists and antagonists on locomotor activity in male and female rats. PharmacolBiochemBehav 72: 857-863.
- Festa ED, Quinones-Jenab V (2004) Gonadal hormones provide the biological basis for sex differences in behavioral responses to cocaine. HormBehav 46: 509-519.
- Harrod SB, Booze RM, Welch M, Browning CE, Mactutus CF (2005) Acute and repeated intravenous cocaine-induced locomotor activity is altered as a function of sex and gonadectomy. PharmacolBiochemBehav 82: 170-181.
- Russo SJ, Festa ED, Fabian SJ, Gazi FM, Kraish M, et al. (2003) Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience 120: 523-533.
- Lynch WJ, Carroll ME (1999) Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 144: 77-82.
- Hu M, Crombag HS, Robinson TE, Becker JB (2004) Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology 29: 81-85.
- Jackson LR, Robinson TE, Becker JB (2006) Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology 31: 129-138.
- Koob GF (1992) Neural mechanisms of drug reinforcement. Ann N Y AcadSci 654: 171-191.
- Nazarian A, Sun WL, Zhou L, Kemen LM, Jenab S, et al. (2009) Sex differences in basal and cocaine-induced alterations in PKA and CREB proteins in the nucleus accumbens. Psychopharmacology (Berl) 203: 641-650.
- Festa ED, Russo SJ, Gazi FM, Niyomchai T, Kemen LM, et al. (2004) Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology 46: 672-687.
- Walker QD, Ray R, Kuhn CM (2006) Sex differences in neurochemical effects of dopaminergic drugs in rat striatum. Neuropsychopharmacology 31: 1193-1202.
- Hope BT, Crombag HS, Jedynak JP, Wise RA (2005) Neuroadaptations of total levels of adenylatecyclase, protein kinase A, tyrosine hydroxylase, cdk5 and neurofilaments in the nucleus accumbens and ventral tegmental area do not correlate with expression of sensitized or tolerant locomotor responses to cocaine. J Neurochem 92: 536-545.
- Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, Nestler EJ (1991) A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res 548: 100-110.
- Cervo L, Mukherjee S, Bertaglia A, Samanin R (1997) Protein kinases A and C are involved in the mechanisms underlying consolidation of cocaine place conditioning. Brain Res 775: 30-36.
- Miserendino MJ, Nestler EJ (1995) Behavioral sensitization to cocaine: Modulation by the cyclic AMP system in the nucleus accumbens. Brain Res 674: 299-306.
- Schroeder JA, Hummel M, Unterwald EM (2004) Repeated intracerebroventricularforskolin administration enhances behavioral sensitization to cocaine. Behav Brain Res 153:255-260.
- Self DW, Genova LM, Hope BT, Barnhart WJ, Spencer JJ, et al. (1998) Involvement of cAMP-dependent protein kinase in the nucleus accumbens in cocaine self-administration and relapse of cocaine-seeking behavior. J Neurosci 18: 1848-1859.
- Lynch WJ, Kiraly DD, Caldarone BJ, Picciotto MR, Taylor JR (2007) Effect of cocaine self-administration on striatal PKA-regulated signaling in male and female rats. Psychopharmacology (Berl) 191: 263-271.
- Zhou L, Nazarian A, Sun WL, Jenab S, Quinones-Jenab V (2009) Basal and cocaine-induced sex differences in the DARPP-32-mediated signaling pathway. Psychopharmacology (Berl) 203: 175-183.
- Radwanska K, Caboche J, Kaczmarek L (2005) Extracellular signal-regulated kinases (ERKs) modulate cocaine-induced gene expression in the mouse amygdala. Eur J Neurosci 22: 939-948.
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, et al. (2000) Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci 20: 8701-8709.
- Valjent E, Pagès C, Hervé D, Girault JA, Caboche J (2004) Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci 19: 1826-1836.
- Mattson BJ, Bossert JM, Simmons DE, Nozaki N, Nagarkar D, et al. (2005) Cocaine-induced CREB phosphorylation in nucleus accumbens of cocaine-sensitized rats is enabled by enhanced activation of extracellular signal-related kinase, but not protein kinase A. J Neurochem 95: 1481-1494.
- Miller CA, Marshall JF (2005) Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron 47: 873-884.
- Pierce RC, Pierce-Bancroft AF, Prasad BM (1999) Neurotrophin-3 contributes to the initiation of behavioral sensitization to cocaine by activating the Ras/Mitogen-activated protein kinase signal transduction cascade. J Neurosci 19: 8685-8695.
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, et al. (2005) Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. ProcNatlAcadSci USA 102: 491-496.
- Ferguson SM, Fasano S, Yang P, Brambilla R, Robinson TE (2006) Knockout of ERK1 enhances cocaine-evoked immediate early gene expression and behavioral plasticity. Neuropsychopharmacology 31: 2660-2668.
- Mazzucchelli C, Vantaggiato C, Ciamei A, Fasano S, Pakhotin P, et al. (2002) Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal- mediated learning and memory. Neuron 34:807-820.
- Carlezon WA Jr, Duman RS, Nestler EJ (2005) The many faces of CREB. Trends Neurosci 28: 436-445.
- Jenab S, Festa ED, Nazarian A, Wu HB, Sun WL, et al. (2005) Cocaine induction of ERK proteins in dorsal striatum of Fischer rats. Brain Res Mol Brain Res 142: 134-138.
- Edwards S, Graham DL, Bachtell RK, Self DW (2007) Region-specific tolerance to cocaine-regulated cAMP-dependent protein phosphorylation following chronic self-administration. Eur J Neurosci 25: 2201-2213.
- Walker QD, Nelson CJ, Smith D, Kuhn CM (2002) Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats. PharmacolBiochemBehav 73: 743-752.
- Sun WL, Zhou L, Hazim R, Quinones-Jenab V, Jenab S (2008) Effects of dopamine and NMDA receptors on cocaine-induced Fos expression in the striatum of Fischer rats. Brain Res 1243: 1-9.
- Jiao H, Zhang L, Gao F, Lou D, Zhang J, et al. (2007) Dopamine D(1) and D(3) receptors oppositely regulate NMDA- and cocaine-induced MAPK signaling via NMDA receptor phosphorylation. J Neurochem 103: 840-848.
- Walker QD, Cabassa J, Kaplan KA, Li ST, Haroon J, et al. (2001) Sex differences in cocaine-stimulated motor behavior: disparate effects of gonadectomy. Neuropsychopharmacology 25: 118-130.
- Corbillé AG, Valjent E, Marsicano G, Ledent C, Lutz B, et al. (2007) Role of cannabinoid type 1 receptors in locomotor activity and striatal signaling in response to psychostimulants. J Neurosci 27: 6937-6947.
- Sun WL, Zhou L, Hazim R, Quinones-Jenab V, Jenab S (2007) Effects of acute cocaine on ERK and DARPP-32 phosphorylation pathways in the caudate-putamen of Fischer rats. Brain Res 1178: 12-19.
- Zhang L, Lou D, Jiao H, Zhang D, Wang X, et al. (2004) Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors. J Neurosci 24: 3344-3354.
- Nestler EJ (2005) Is there a common molecular pathway for addiction? Nat Neurosci 8: 1445-1449.
- Brami-Cherrier K, Valjent E, Hervé D, Darragh J, Corvol JC, et al. (2005) Parsing molecular and behavioral effects of cocaine in mitogen- and stress-activated protein kinase-1-deficient mice. J Neurosci 25: 11444-11454.
- Kano T, Suzuki Y, Shibuya M, Kiuchi K, Hagiwara M (1995) Cocaine-induced CREB phosphorylation and c-Fos expression are suppressed in Parkinsonism model mice. Neuroreport 6: 2197-2200.
- Karasinska JM, George SR, Cheng R, O'Dowd BF (2005) Deletion of dopamine D1 and D3 receptors differentially affects spontaneous behaviour and cocaine-induced locomotor activity, reward and CREB phosphorylation. Eur J Neurosci 22:1741-1750.
- Walters CL, Kuo YC, Blendy JA (2003) Differential distribution of CREB in the mesolimbic dopamine reward pathway. J Neurochem 87: 1237-1244.
- Yamamoto KK, Gonzalez GA, Biggs WH 3rd, Montminy MR (1988) Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature 334: 494-498.
- Sun WL, Coleman NT, Zelek-Molik A, Barry SM, Whitfield TW Jr, et al. (2014) Relapse to cocaine-seeking after abstinence is regulated by cAMP-dependent protein kinase A in the prefrontal cortex. Addict Biol 19: 77-86.
- Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL (1996) Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron 16: 1179-1188.
- Banke TG, Bowie D, Lee H, Huganir RL, Schousboe A, et al. (2000) Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci 20: 89-102.
- Oh MC, Derkach VA, Guire ES, Soderling TR (2006) Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J BiolChem 281: 752-758.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Relapse prevention
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 14905
- [From(publication date):
September-2015 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 10495
- PDF downloads : 4410