Actinotignum schaalii Pilonidal Sinus: Case Report and Review of A. schaalii Soft Tissue Infections
Received: 27-Apr-2018 / Accepted Date: 14-May-2018 / Published Date: 18-May-2018 DOI: 10.4172/2332-0877.1000362
Abstract
A. schaallii has been recently recognised as an emerging uropathogen but its role in skin and soft tissue infections is less well-characterised. We describe an unusual case of A. schaallii pilonidal sinus infection and review the literature for skin and soft tissue infections involving this organism. A. schaallii soft tissue infections tend to involve the groin, breast or perineum and are sensitive to penicillins but are usually resistant to metronidazole, clotrimoxizole and ciprofloxacin.
Keywords: Actinotignum schaalii; Pilonidal sinus; Soft tissue infections
Introduction
Pilonidal disease is a relatively common condition affecting an estimated 26 per 100,000 patients and with a spectrum of presentations [1]. It may vary from an acute presentation associated with an abscess to chronically inflamed sinuses with persistent drainage. Although several theories exist on the cause of pilonidal sinus it has become accepted that the condition is acquired and is associated with loose hairs which may act as a foreign body and ultimately form pits with or without secondary infection [2,3]. Commonly identified bacteria in pilonidal disease are mainly anaerobic but also aerobic or facultative anaerobic organisms are found. These organisms are mainly Bacteroides spp., Escherichia coli, Proteus spp., and Pseudomonas spp. [4]. Interestingly, Staphylococcus spp., are not commonly found in these abscesses [5]. In this report we describe a case of a pilonidal sinus infection caused by Actinotignum schaalii (formerly Actinobaculum schaalii) and review our cases and the literature relating to skin and soft tissue infections with this organism.
Case Report
A 19 year old male student presented to our institute’s emergency department with a left superior buttock abscess within the natal cleft that had been increasing in size over the past 4 months, painful on lying down and unresponsive to two courses of oral antibiotics (unknown) given by his GP. He had no systemic features of infection otherwise. The patient had no relevant medical history and was not known to have inflammatory bowel disease nor did he have a family history of such. He was a non-smoker and non-drinker. Examination showed a fluctuant mass at the ‘6 o’clock’ area with numerous pustules around discharging pus. A large palpable collection over the left superior buttock extended from the abscess. Per rectum examination was tender at the ‘6’oclock’ point of purulent discharge. The patient was diagnosed with complex pilonidal abscess with other differentials being hidradenitis suppurativa, perianal abscess and perianal fistula.
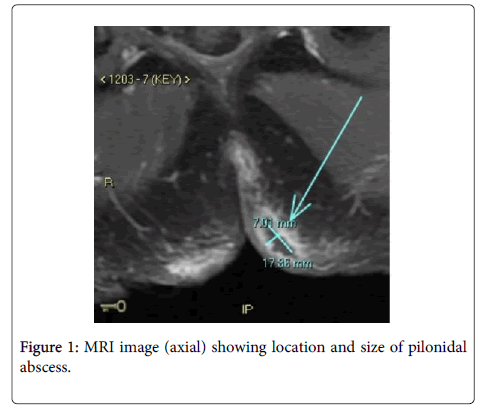
Magnetic resonance imaging was carried out to rule out anorectal fistulae. MRI pelvis (Figure 1) showed a perianal fistula at the 6 o'clock position that appeared to communicate with both left and right sided subcutaneous inflammatory changes with small subcutaneous abscesses noted measuring 17 × 7 mm. Several small collections were noted bilaterally, slightly larger on the left. The patient underwent an examination under anesthetic, incision and drainage of the pilonidal sinus with operative findings of purulent fluid expressed from three sinus openings (two sinus openings over the left superior buttock and one sinus opening over the right superior buttock) with four sinus openings over the midline lower back and natal cleft. All sinuses communicated via an extensive superficial cavity system lined with granulation tissue. Operative swab microscopy revealed a large number of polymorphs but no organisms were seen on gram stain. Culture grew mixed anaerobes on day 2 (gram positive bacilli, gram positive cocci and gram negative bacilli) but there was no growth in CO2. On day 3 of culture it was noted that the Horse blood agar plate incubated in CO2 had moderate amount of pure growth of A. schaalii. This organism was identified using MALDI-TOF (Bruker Biotyper 3.1 database version 4.0.0.1, Bruker Daltonik Bremen, Germany). There are no formal guidelines for susceptibility testing or interpretation of MIC results for this organism: antimicrobial susceptibility testing was performed using direct colony suspension of 0.5 McFarland inoculated onto Meuller-Hinton Sheep Blood agar (Thermo Fisher Scientific, Australia). Etest gradient strips (bioMérieux, Marcy l’Etoile, France) were placed onto the media and incubated in 5% CO2 at 35-37°C for 24 h. MIC results are shown in Table 1. The abscess responded well to surgical treatment along with empiric treatment of a single dose of intravenous ceftriaxone 1 g and intravenous metronidazole 500 mg given post-operatively. The patient represented 2 months later with a recurrent pilonidal sinus. Incision and drainage was re-performed with wound culture revealing Staphylococcus aureus sensitive to flucloxacillin. He had an uneventful recovery with nil recurrence.
| Antibiotic | MIC (ug/mL) |
|---|---|
| Penicillin | 0.023 |
| Ceftriaxone | 0.047 |
| Metronidazole | >256 |
| Ciprofloxacin | 3.0 |
Table 1: Antimicrobial susceptibility results of A. schaalii isolate.
Literature Review
A broad search strategy was undertaken given the limited publications on Actinotignum schaalii using MEDLINE (from January 1946 to December 2017) and EMBASE (from January 1980-December 2017). The search was carried out using keywords: Actinotignum, Actinotignum schaalii, Actinobaculum, and Actinobaculum schaalii, and was limited to humans and English articles. There were 96 articles found in the search. The authors reviewed all of these articles and excluded those that were not related to abscesses, skin infections or soft tissue infections. There were 33 cases of A. schaalii related abscesses, skin or soft tissue infections. Two of these cases were pilonidal sinuses infections. There are two previous published cases of A. schaalii pilonidal sinus infection.
Laboratory information system review
Monash Health is a large tertiary referral health network which services over 2000 beds from five hospitals in Victoria, Australia. The MALDI-TOF MS was introduced into routine laboratory work in February 2014. We searched the Laboratory Information System in the Microbiology Laboratory for non-urine specimens including the word “Actinobaculum” or “Actinotignum” from February 2014 until July 2017.
Discussion
Actinotignum schaalii is a bacterium, first described in 1997, belonging to the genus that includes Actinotignum urinale, Actinotignum massiliense, Actinotignum sanguinis and Actinotignum suis [6-8]. It is likely part of the normal flora in the skin, genital and urinary tract region and is a difficult to culture Gram-positive bacillus that is a facultative anaerobe, non-motile, catalase negative bacterium that is increasingly recognized as a human pathogen [7-11]. Previously it had been difficult to identify and regularly dismissed as a contaminant or potentially overgrown by other organisms due to its slow growth [12,13]. However, with PCR techniques it appears that A. schaalii is much more common than thought. One study showed the prevalence of A. schaalii in urine was 16% and in those older than 60 years the prevalence increased to 22% by PCR [13]. Despite being a well-recognised uropathogen the number of cases of abscesses and skin or soft tissue infections attributed to this organism is limited.
In our literature review we have found 43 cases (including our institutional cases, Table 2) of A. schaalii abscesses, skin and soft tissue infections. The average age of patients with infection with A. schaalii is 39 years old with a slight predilection towards females 0.85:1 (M: F) [6,7,10,12,14-19]. This contrasts with it affecting mainly the elderly population >65 years old as an uropathogen [13,14]. Location of infection is mainly in the groin region (10/43), breast (8/43) or perineum (8/43) three of these cases (including our case) were pilonidal abscesses. This data is summarized in Table 2 below. This is consistent with the knowledge that A. schaalii is part of the normal flora in the skin, genital and urinary tract region [13]. In these 3 cases of pilonidal abscesses all were surgically drained with two cases having additional antibiotic treatment. One case of pilonidal abscess had a good outcome, one case was unknown, and our case had recurrence albeit with a different organism. It is hard to draw conclusions on whether A. schaalii pilonidal abscesses have better or worse outcomes than those with other organisms due to the small sample size. It is also known that pilonidal abscesses have high recurrence rates with a long-term study of mean follow-up 14.8 years citing a 22% long-term recurrence rate, thus whether the recurrence was due to the inherent nature of the disease is also a factor to consider [20]. Our institutional data (Table 3) also showed a sizeable proportion (5/12) of breast abscesses. Of these, 3 were managed with aspiration and antibiotics with good outcome. The remaining 2 were treated with either antibiotics only or with surgical intervention plus antibiotics due to necrotizing fasciitis. Generally, the management of breast abscesses can be incision and drainage, aspiration (± repeated) and/or antibiotics with oral antibiotics often trialed prior to incision or aspiration techniques [21-24]. Our institution methods are in line with current accepted practices with no strong evidence to suggest a particular technique is better than another [25]. In the limited number of patients in this subgroup of breast infections good outcomes were seen in all which may suggest that A. schaalii breast abscesses should be treated like any other breast abscess.
| Case | Age | Sex | Disease | Treatment | Secondary infections | Outcome | Resistant |
|---|---|---|---|---|---|---|---|
| 1 | 20 | F | Pilonidal sinus abscess | Surgical drainage | No | Unknown | Unknown [7] |
| 2 | 25 | M | Pilonidal sinus abscess | Surgical Drainage and Cloxacillin | No | Favourable | Resistant to metronidazole, Clindamycin [19] |
| 3 | 33 | M | Fournier’s Gangrene | Surgical debridement and Amoxicillin-clavulanate (changed to ciprofloxacin and metronidazole) | Escherichia coli, Proteus mirabilis, Bacteroides fragilis and Candida albicans | Favourable | Resistant to metronidazole [15] |
| 4 | - | - | Abdominal abscess | Unknown | Polymicrobes not specified | Unknown | Unknown [10] |
| 5 | - | - | Skin abscess | Surgical drainage | Polymicrobes not specified | Unknown | Unknown [10] |
| 6 | - | - | Upper jaw abscess | Unknown | Polymicrobes not specified | Unknown | Unknown [10] |
| 7 | - | - | Bladder abscess post- bladder stone extraction | Unknown | Polymicrobes not specified | Unknown | Unknown [10] |
| 8 | - | - | Scrotal abscess | Unknown | Polymicrobes not specified | Unknown | Unknown [10] |
| 9 | - | - | Renal pelvis abscess | Unknown | Polymicrobes not specified | Unknown | Unknown [10] |
| 10 | 25 | M | Hidradenitis suppurativa abscess (groin) | Surgical drainage and Clindamycin and Minocycline then addition of biological agent | Prevotella melaninogenica | Favourable | Resistant to metronidazole, Clindamycin [6] |
| 11 | 12 | M | Breast abscess | Surgical drainage | Bacteroides fragilis | Favourable | Resistant to Trimethoprim and Ciprofloxacin [6] |
| 12 | 22 | F | Vulvar abscess | Surgical drainage | Gemella haemolysans | Favourable | Resistant to Trimethoprim and Ciprofloxacin [7] |
| 13 | 23 | M | Abdominal wall abscess | Vancomycin lock | No | Unknown | Unknown [7] |
| 14 | 48 | F | Inguinal abscess, extensive cellulitis | Surgical drainage and Cloxacillin | No | Favourable | Resistant to metronidazole [19] |
| 15 | 34 | F | Breast abscess | Surgical drainage | No | Favourable | Unknown [7] |
| 16 | 17 | M | Malleolus abscess | Amoxicillin-clavulantate | Peptostreptococcus spp. | Favourable | Unknown [7] |
| 17 | 69 | M | Diabetic foot ulcer | Unknown | Finegoldia spp, Anaerococcus spp | Unknown | Unknown [7] |
| 18 | 54 | F | Abscess on sternotomy | Local povidon iodine | Acinetobacter spp, Helcococcus spp, Anaerococcus spp. | Unknown | Unknown [7] |
| 19 | 27 | F | Vaginal abscess | Surgical drainage | Actinomyces turicensis | Favourable | Unknown [7] |
| 20 | 77 | M | Perineal necrotizing cellulitis | Vancomycin and metronidazole | Unknown | Unknown | Unknown [14] |
| 21 | 24 | M | Groin abscess | Surgical treatment | No | Favourable | Resistant to ciprofloxacin [17] |
| 22 | 25 | M | Groin abscess | Surgical treatment and amoxicillin-clavulanic acid | Arcanobacterium pyogenes | Favourable | |
| 23 | 26 | M | Groin abscess | Surgical treatment and amoxicillin-clavulanic acid | Aerococcus spp. | Favourable | |
| 24 | 34 | M | Groin abscess | Surgical treatment | No | Favourable | |
| 25 | 80 | M | Groin abscess | Surgical and treatment with imipenem IV and pristinamycin then switched to linezolid | Enterococcus faecalis | Favourable | |
| 26 | 46 | F | Groin abscess | Surgical treatment and pristinamycin | No | Favourable | |
| 27 | 46 | M | Abdominal abscess | Surgical treatment and linezolid | No | Favourable | |
| 28 | 38 | F | Breast abscess and periductal mastitis | Aspiration and antibiotics (not specified) | Streptococcus constellatus | Multiple recurrences | Unknown [16] |
| 29 | 19 | F | Inguinal fold abscess | Surgical drainage | Fusobacterium spp, Prevotella spp | Favourable | Resistant to ciprofloxacin [12] |
| 30 | 42 | F | Breast abscess | Surgical drainage and amoxicillin clavulanate | Prevotella spp., Staphylococcus coagulase negative | Favourable | Resistant to ciprofloxacin [12] |
| 31 | 9mo | F | Intradural abscess with fistulation to the skin | Surgical drainage and penicillin, metronidazole | Non-hemolytic Streptococci | Unknown | Unknown [18] |
Table 2: Cases of Actinotignum schaalii Abscess, skin and soft tissue infection.
Review of the 43 cases shows A. schaalii generally to be associated with simple collections that can be treated mostly without complications. Occasionally, severe infection such as Fournier’s gangrene/necrotising fasciitis can be a result of this organism. Whether this is due to the combined pathogenicity of other organisms it can co-exist with needs to be further investigated. Generally, it has a good response to treatment which in this review was surgical, antibiotics, a combination of both, or in one case local povidone iodine. 29/43 cases had favourable outcomes, 2/43 had recurrences and the remainder had unknown outcomes. It appears that collections/ skin or soft tissue infections with A. schaalii appear to be very true table with no serious morbidity reported in the literature [6,7,10,12,14-19].
Although there are no formal guidelines for susceptibility testing for A. schaalii, the literature suggests that this organism usually appears susceptible to amoxicillin, ceftriaxone and vancomycin in vitro but resistant to ciprofloxacin, metronidazole & cotrimoxazole. With regards to antibiotic choice, 9 cases describe resistance with ciprofloxacin found to be ineffective in 5/9 cases, followed by 4/9 cases of metronidazole, 2/9 cases clindamycin and 2/9 cases trimethoprim. Therefore, for empiric treatment a penicillin is preferred otherwise ceftriaxone is also another effective choice [12,13,16,21,22]. As A. schaalii is a facultative anaerobe with potential resistance to metronidazole there may be lack of treatment efficacy if metronidazole is given alone. We suggest it is important to have prolonged anaerobic cultures for microbiological investigation of pilonidal sinus infections, MALDI-TOF to help with accurate identification of bacteria especially in metronidazole unresponsive cases and to also consider an A. schaalii infection. There are no established guidelines with regards to length of duration of antibiotic treatment for A. schaalii infections. With regards to pilonidal abscess current guidelines recommend a limited role for antibiotics unless there is underlying immunosuppression, systemic illness or significant cellulitis [2,23]. It is important to note that while studies and guidelines support antibiotics in limited settings, whether these guidelines also apply to A. schaalii infected pilonidal sinuses is yet uncertain. This may not be completely unreasonable in infections with Actinomyces spp. as they potentially be prolonged infections or have multiple recurrences [16].
A. schaalii is becoming increasingly recognized due to better methods of identifying this organism. This study shows that this organism is not only limited to being an uropathogen. Despite this, there are not many reported cases of A. schaalii infections in skin, soft tissue and as abscesses. More research is required to elucidate the virulence of this organism, and whether this is a result of co-infection with other organisms or due to its own pathogenicity.
Acknowledgement
We acknowledge the patient for willingness to collaborate and Leanne Tilson (Microbiology) for her assistance with microbiological testing. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Khanna A, Rombeau JL (2011) Pilonidal Disease. Clin Colon Rectal Surg 24: 46-53.
- Steele SR, Perry WC, Mills S, Buie WD (2013) Practice Parameters for the Management of Pilonidal Disease. Dis Colon Rectum 56: 1021-1027.
- Humphries AE, Duncan J (2010) Evaluation and Management of Pilonidal Disease. Surg Clin N Am 90: 113-124.
- Brook I (1989) Microbiology of infected pilonidal sinuses. J Clin Pathol 42: 1140-1142.
- Brook I, Anderson K, Controni G, Rodriguez (1980) Aerobic and Anearobic Bacteriology of Pilonidal Cyst Abscess in Children. Am J Dis Child 134: 679-680.
- Maraki S, Evangelou G, Stafylaki D, Scoulica E (2017) Actinotignum schaalii subcutaneous abscesses in a patient with hidradenitis suppurativa: Case report and literature review. Anaerobe 43: 43-46.
- Prigent G, Perillaud C, Amara M, Coutard A, Blanc C, et al. (2016) Actinobaculum schaalii: A truly emerging pathogen?. New Microbe and New infect 11: 8-16.
- Lawson PA, Flasen E, Akervall E, Vandamme P, Collins M (1997) Characterization of some Actinomyces-like isolates from human clinical specimens: Reclassification of Actinomyces suis (Soltys and Spratling) as Actinobaculum suis comb. nov. and Description of Actinobaculum schaalii sp. nov. Int  J Syst Bacteriol 47: 899-903.
- Stevens R, Taylor P (2014) Actinobaculum schaalii-A review of cases and the development of a phenotypic screen. Pathol 46: S101.
- Tschudin-Sutter S, Frei R, Weisser M, Goldenberger D, Widmer AF (2011) Actinobaculum schaalii- invasive pathogen or innocent bystander? A restrospective observational study. BMC Infectious Disease 11: 289.
- Olsen AB, Andersen PK, Bank S, Soby KM, Lund L, et al. (2013) Actinobaculum schaalii, a commensal of the urogenital area. BJU Int 112: 394-397.
- Beguelin C, Genne D, Varca A, Tritten Ml, Siegrist HH, et al. (2011) Actinobaculum schaalii: clinical observation of 20 cases. Clin Microbiol Infect 17: 1027-1031.
- Cattoir V (2012) Actinobaculum schaalii: Review of an emerging uropathogen. J Infect 64: 260-267.
- Gomez E, Gustafson DR, Rosenblatt Je, Patel R (2011) Actinobaculum Bacteremia: a report of 12 cases. J Clin Microbiol 49: 4311-4313.
- Bempt IV, Trappen SV, Cleenwerck I, Vos PD, Camps K, et al. (2011) Actinobaculum schaalii causing Fournier’s gangrene. J Clin Microbiol 49: 2369-2371.
- Bing AU, Loh SF, Morris T, Hughes H, Dixon JM, et al. (2015) Actinomyces Species isolated from breast infections. J Clin Microbiol 53: 3247-3255.
- Â Lotte L, Lotte R, Durand M, Degand N, Ambrosetti D, et al. (2016) Infections related to Actinotignum schaalii (formerly Actinobaculum schaalii): a 3 year prospective observational study on 50 cases. Clin Microbiol Infect 22: 388-390.
- Reinhard M, Prag J, Kemp M, Andresen K, Klemmensen B, et al. (2005) Ten cases of Actinobaculum schaalii infection: Clinical relevance bacterial identification, and antibiotic susceptibility. J Clin Microbiol 43: 5305-5308.
- Tena D, Fernandez C, Lago MR, Arias M, Medina MJ, et al. (2014) Skin and soft-tissue infections caused by Actinobaculum schaalii: Report of two cases and literature review. Anaerobe 28: 95-97.
- Doll D, Krueger CM, Schrank S, Dettmann H, Petersen S, et al. (2007) Timeline of recurrence after primary and secondary pilonidal sinu surgery. Dis Colon Rectum 50: 1928-1934.
- Nielsen HL, Soby KM, Christensen J, Prag J (2010) Actinobaculum schaalii: A common cause of urinary tract infection in the elderly population. Bacteriological and clinical characteristics. Scand J Infect Dis 42: 43-47.
- Mavros MN, Mitsikostas PK, Alexiou VG, Peppas G, Falagas ME (2013) Antimicrobials as an adjunct to pilonidal disease surgery: a systematic review of the literature. Eur J Clin Microbiol Infect Dis 32: 851-858.
- Cattoir V, Varca A, Greub G, Prod'hom G, Legrand P, et al. (2010) In vitro susceptibility of Actinobaculum schaalii to 12 antimicrobial agents and molecular analysis of fluoroquinolone resistance. J Antimicrob Chemother 65: 2514-2517.
- Irusen H, Rohwer AC, Steyn DW, Young T (2015) Treatments for breast abscesses in breastfeeding women. Cochrane Database Syst Rev.
Citation: Tana D, Grahamb M, Fong P, Hasan Z, Loh SF (2018) Actinotignum schaalii Pilonidal Sinus: Case Report and Review of A. schaalii Soft Tissue Infections. J Infect Dis Ther 6: 362. DOI: 10.4172/2332-0877.1000362
Copyright: © 2018 Tana D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5811
- [From(publication date): 0-2018 - Apr 28, 2025]
- Breakdown by view type
- HTML page views: 4967
- PDF downloads: 844