Achilles Tendons Total Rupture, Open Surgical Treatment with PRF Augmentation: Clinical, Morphological and Functional Evaluation
Received: 15-May-2017 / Accepted Date: 26-May-2017 / Published Date: 02-Jun-2017 DOI: 10.4172/2329-910X.1000236
Abstract
Background: Changes in tendon structure after its rupture may also affect the likelihood of re-injury or strain injury to the musculotendinous complex of the plantar flexor.
Purpose: The aim of our study is to investigate ankle biomechanical properties and clinical features of acute rupture of the Achilles tendon treated with open suture and PRF augmentation.
Study design: Cohort study (NRS).
Methods: This study has been conducted on twenty patients, divided in two groups; one group underwent conventional open repair of the Achilles tendon using the Krackow technique only, while the other group underwent surgery with Platelet-Rich-Fibrin (PRF) augmentation. We performed a clinical evaluation at 3 months, 6 months, and after 1 year. Morphological and functional tendons properties were analyzed by ultrasound and Gait Analysis.
Results: The results obtained by the ultrasonographic assessment showed in the PRF group a statistically significant increasing of treated tendons the antero-posterior diameter, medio-lateral diameter. Statistically significant differences between the two groups have emerged in gait analysis performed with regard to network (Wnet). Furthermore, the network of healthy side and treated side was significantly different only in the group of patients not treated with PRF. Statistically significant values have emerged through the analysis of Pearson’s correlation between the diameter of the ultrasound treated tendon and the network, walking speed, swing speed and stride period.
Conclusion: Treatment with suture and PRF shows significant morphological modifications and functional improvements compared to the results achieved with Achilles tendon suture without PRF.
Keywords: Gait analysis; Achilles tendon; Growth factors; Biologic healing enhancement; General sports trauma; Imaging; Diagnostic ultrasound
13825Introduction
Our study focuses on acute Achilles tendon rupture (ATR), one of the most common tendon injuries. Our aim is to assess if PRF could improve surgical treatment both in clinical and morphological outcomes in non-randomized cohort study (NRS) with historical control group [1,2]. Similar study was performed by Sanchez M et al. [3], the authors performed a ultrasounds evaluation of patients treated for Achilles tendon rupture, comparing open surgical treatment with perf vs those treated with open surgical treatment without PRF, concluding that the operative management of tendons combined with the application of autologous PRF may present new possibilities for enhanced healing and functional recovery.
Achilles tendon rupture is more common in males than females, in middle age, and in people practicing sports [4,5]. Achille’s tendon, for its morphology, function and anatomy, is often implicated in pathologic processes that could bring it to an acute rupture. The tenocytes, because of their slow turnover, have a low tolerance to overuse activity. Moreover, the anatomy of Achilles tendons is characterized by a poor vascularization supply in its middle section [6]; these features can be cause of disorganizing regenerative process and changing in physiological structure of the tendon after chronic inflammation due to chronic overuse or overload activity, and can lead to a tissue degeneration or furthermore to tendon rupture [6,7]. Different treatments are feasible to treat ATR, including: open surgical, percutaneous and conservative treatment [8]. Conservative treatment is suitable as a treatment for patients clinically instable, in order to avoid the complications of surgery [9]. Open surgical treatment is often the best choice to prevent re-rupture and to obtain the best functional recovery [10]. The literature reports the Krackow open suture as the treatment of choice in young and adult patients with a high functional demand, this kind of surgical procedure allows the achievement of the best construct and has the lowest rate of re-rupture [11]. Finally, percutaneous treatment appears to be a good alternative in several cases [12].
In this context, the PRF is the object of several studies testing its efficacy as a medical device that could improve the treatment of ATR. Platelets contain storage pools of growth factors including PDGF, TGFClinical β and VEGF, as well as cytokines including proteins such as PF4 and CD40L. Moreover, there are a large number of animal models and in vitro studies describing PRF effectiveness to stimulate the cellular proliferation and the healing process [13,14]. Additionally, the presence of the growth factors related to platelets and/or fibrin is very useful in open surgical treatment of tendons injury. Infact, in this case it can be implanted directly on the tendon lesion, allowing a stable concentration of growth factors and supporting the tissue healing [3,15,16].
Normally, the anatomical structures provide the elasticity required in order to absorb and release energy, eventually increasing the efficiency of motion in activities like walking, running, and jumping [17,18]. Stiffness may be an important factor influencing injury and reinjury as well; in fact it has been observed that many muscle and tendon injuries occur when the joint is in the mid-range and not in an overextended position. When tissues are too stiff, they cannot absorb sufficient energy during loading, consequently they are more suitable to be injured [19-21].
Our study has been carried out to investigate the efficiency of PRF in a population of patients in which an open surgery is strictly indicated. In particular our aim was to investigate ankle biomechanical properties and clinical features of acute rupture of the Achilles tendon treated with open suture and PRF augmentation.
Materials and Methods
This non randomized control study (NRS) was composed of twenty patients that were divided into two groups of ten subjects each, assigned to two different treatments; the historical control group was treated with Krackow suture only, while the second group, the study group, was treated with the same surgical technique with the addition of a platelet rich fibrin (PRF) affixed in the surgical site. The inclusion criteria were completion of clinical gait analysis and treatment by suture techniques from Krackow, with or without PRF augmentation, at our orthopedics and traumatology department, from 2010 to 2014. Participants were excluded if they had a history of previous or contralateral ATR, breaks in the myotendinous junction, tendonitis in the contralateral AT, peripheral neuropathy of the lower limb, and neurologic and/or rheumatologic conditions that can result in gait alterations.
The study group was composed of patients treated for Achilles tendon rupture at our institution. They all accepted the PRF treatment and met our inclusion criteria. The historical control group has been selected from our patients archive. In this group, we included patients treated for Achilles tendon rupture that were followed-up and met the same inclusion criteria as the study group. The period of study went from March 2012 to September 2013. The enrolment of patients were performed by the authors involved in this study with a collective decision on subject eligibility.
Furthermore, the protocol of our study sample selection presents strict parameters in order to constitute a study sample as homogenous as possible. The purpose is to represent the population most subject to ATR and for which is more recommended the surgical treatment. Besides that, our protocol wants to eliminate all possible ambiguous elements within the morphological and functional results. Therefore, patients older than 50 years and younger than 25 years were excluded. We also excluded all patients with systemic pathologies, with a BMI>30 and never practicing sport or practicing sports at competitive level. Last, given the fact that just one female patient met our inclusion criteria, she was excluded as well in order to balance gender groups. In regards to the historical group, we selected 10 patients that met our including criteria and with the same follow-up and rehabilitation protocol (Table 1).
| Group | Mean | Min | Max | DS |
|---|---|---|---|---|
| Age NO PRF | 39.6 | 31 | 50 | 7.5 |
| Weight NO PRF | 83.8 | 70 | 100 | 12.4 |
| High NO PRF | 1.73 | 1.65 | 1.82 | 0.06 |
| BMI NO PRF | 24.14 | 20.83 | 27.3 | 3.3 |
| Age PRF | 34.9 | 18 | 50 | 9.37 |
| Weight PRF | 85.00 | 74 | 97 | 7.9 |
| High PRF | 1.78 | 1.65 | 1.9 | 0.1 |
| BMIPRF | 23.22 | 20.3 | 27.44 | 2.71 |
Abbreviation: PRF:Platelet Rich Fibrin.
Table 1: Demographic and anthropometric data.
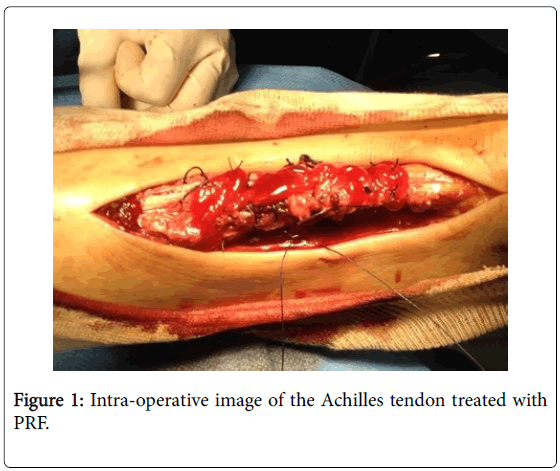
Patients treated with PRF were subjected to a sample of blood of 8 ml with a particular tube, which contains sodium citrate, to inhibit the coagulation cascade, and a gel. This gel allows the separation, during the centrifugation process, of red blood cells, that settle on the bottom of the tube (deep layer of waste), and white blood cells and platelets, that on the other hand form the so called “buffy coat” (middle layer) resting on the gel and on the surface of the non-cellular components of plasma. These were subjected to centrifugation at 3000 rpm for ten minutes. The protocol of this study uses specific jellifying agents, such as calcium gluconate and batroxobin, an enzyme that cleaves the fibrinopeptide in order to induce fibrin polymerization. The final product shows a density of fibrin matrix, which is strong enough for an application as fibrin glue. In all cases a postero-medial incision was performed. The Krakow end-to-end suturing technique was performed for all the patients enrolled in the study (Figure 1) [11].
All patients, in the post-surgical period, underwent to the same standard rehabilitation protocol, Divided into the following three phases: three weeks of immobilization of the ankle with brace in equinus and no load; a gradual weight bearing is allowed after 60 days, last, after 3-4 weeks all patients started an active rehabilitation protocol instructed and supervised by a physical therapist. All patients have been examined based on clinic, morphological and functional aspects. We monitored the complications, we measured calf diameter, weight, BMI and ROM. The passive Rom of the ankle was clinically assessed using a goniometer while a clinician pulled in full plantar and in full dorsal flexion the ankle of the patient checked, the same author performed all measurement. We also provided a self-questionnaire at 6 months and 1 year. We performed an ultrasound examination at each control in order to assess the variation in tendon diameters and area. After 6 months and 1 year of follow-up, we eventually performed a gait analysis. All data have been statistically compared and correlated. All patients underwent standardized ultrasound evaluation by an experienced musculoskeletal radiologist blinded to surgical procedure (Logic 400 MD, GE Medical Systems, with 7.5-12.0 MHz linear array transducer). Patients were examined in the prone position with the affected foot hanging over the end of the examination table. For each subject, ultrasound evaluation used as primary outcome, were performed at both Achilles tendons, the tendon was scanned both in transverse and longitudinal axis, medio-lateral diameter (MLD) and its antero-posterior diameters (APD) were measured [3]. The gait analysis has been performed in a standard gait laboratory which is equipped with eight infrared cameras (ELITE system BTS, Milan, Italy) and two Kistler platforms (Kistler Instruments, Winterthur, Switzerland). Retroreflective spherical markers were placed over prominent bone landmarks to determine the joint centers and segment axis [22]. Each participant was instructed to walk barefoot at their self-selected speed along a level surface approximately 10 m in length; three valid trials have been acquired for each subject and the mean value has been considered for time/distance, kinematic and kinetic data throughout the analysis. A valid trial is defined as one in which subjects struck the force platforms without adjusting their stride length. A moment-angle graph of the ankle during the stance phase of gait cycle was drawn, and the mechanical work (W) produced by the internal forces was calculated as the area under the curve [23-25]. All kinematic and kinetic data were acquired and digitized with a sampling rate of 100 Hz.
Statistical analysis
Data will be processed using SPSS 20.0. The sample size was estimated as Cohen’s d index that shows a large effect size in all variables related to ultrasounds controls at 6th month and a medium effect size. The cohen’s d values comparison of difference between treated tendons into the two groups were: A-P diameter difference at 6th month choen’s d=7.3, M-L diameter difference 6th month choen’s d=6.9, A-P diameter difference at 1 year choen’s d=0.67, M-L diameter difference 1y choen’s d=0. The cohen’s d value comparison of difference between health tendon and treated tendon into the two groups was: AP diameter difference at 6th month choen’s d=1,5, M-L diameter difference 6th month choen’s d=0.8, A-P diameter difference at 1 year choen’s d=0.48, M-L diameter difference 1 year choen’s d=0.77. Mann- Whitney U-test for nonparametric data was used to estimate the differences between the groups. Pearson’s correlation analysis was performed in order to estimate relation between ultrasound parameters and biomechanical data.
Results
We enrolled patients selecting subjects between 20-50 years old, male and practicing not agonistic sport routinely, therefore the sample resulted homogenous for age, gender and level of activity. No statistically significant difference has been noted for results obtained from clinical evaluations, such as, passive range of motion of dorsiflexion, plantar flexion and calf muscle diameter (Table 2).
| Clinical Evaluations | PRF | No PRF | ||
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| 6th month | 1 year | 6th month | 1 year | |
| ROM plantar-flexion(°) | 38.25(3.25) | 36.1(4.9) | 38.75 (7.5) | 38.2 (7.0) |
| ROM dorsal-flexion(°) | 14.2(2.5) | 17.7(5.4) | 16.25(2.5) | 18.7(2.5) |
| Circumference calf treated side(cm) | 36.75 (0.7) | 38.3 (2.6) | 34.3 (2.5) | 36.9(3.9) |
| ΔCircumf. Calf treated side/not treated side (cm) | 2.5(0.5) | 1.6(0.6) | 2.5(1.2) | 1.8(0.8) |
| VAS-Fa | 8.9(1.2) | 10.7(4.3) | 10.7(4.3) | 13.1(6.01) |
| VISA_A | 72.20(20.6) | 80.5 (11.06) | 63.20 (21.5) | 82.2 (13) |
| Atrs | 79 (13.1) | 20.7 (10.05) | 48.75 (36.5) | 24.8(17.7) |
| Sf12 ment | 56.8(2.5) | 53.2(4.8) | 50.1(5.4) | 47.6(11) |
| Sf12ph | 50.2(7.5) | 53.6(3.2) | 47.02 (10.3) | 47.4(9.3) |
Abbreviation: ROM:Range of Movement, PRF: Platelet Rich Fibrin.
Table 2: Clinical measurements.
Ultrasound follow-up exam at 6th months showed that APD, MLD and were significantly higher in the PRF group compared to the control group. Two parameters, the differences between the two groups and between treated and non-treated side regarding the medio-lateral diameter within the same group, were statistically significant (p <0.005) (Table 3).
| Follow-up | Test and p-value | A-P diam treated side | M-Ldiam treated side | Δ A-P diam | ΔL-M diam |
|---|---|---|---|---|---|
| 6th month | PRF : Mean, (SD) | 1.4 | 2.03 | 0.94 | 0.6 |
| 0.21 | (0.21) | (0.14) | (0,32) | ||
| NO PRF : Mean, (SD) | 1.19 | 1.7 | 0.7 | 0.4 | |
| 0.1 | (0.07) | (0.16) | (0.05) | ||
| Mann-WhitneyU-test | 82.00 | 88.000 | 4.000 | ||
| p-value | 0.15* | 0.03* | 0.02* | <0.001* | |
| 1 year | PRF: Mean, (SD) | 1.18 | 1.6 | 0.7 | 0.6 |
| 0.24 | (0.21) | (0.03) | (0.32) | ||
| NO PRF: Mean, (SD) | 1.04 | 1.6 | 0.6 | 0.4 | |
| 0.17 | (0.28) | (0.29) | (0.18) | ||
| Mann-Whitney U-test | 62.500 | 53,000 | 73.00 | ||
| p-value | 0.352 | 0.796 | 0.630 | 0.089 |
Abbreviation: A-P diam, antero-posterior diameter
M-L diam, medio-lateral diameter
Δ A-P diam, side to side differences
Table 3: Ultrasound measurements.
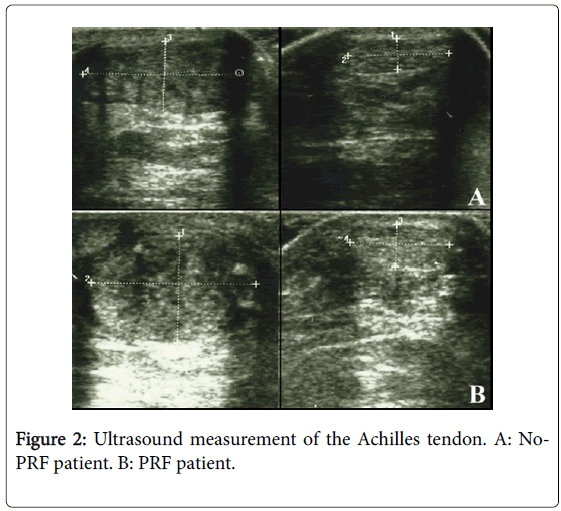
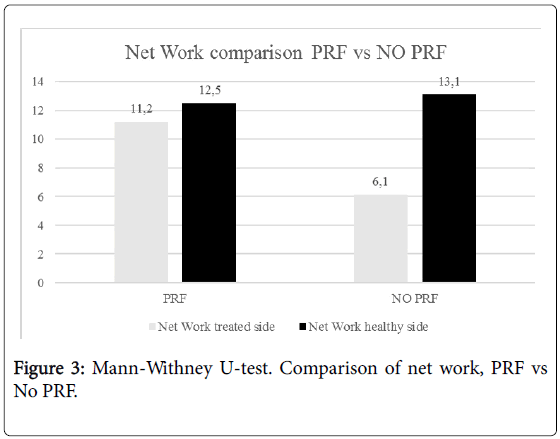
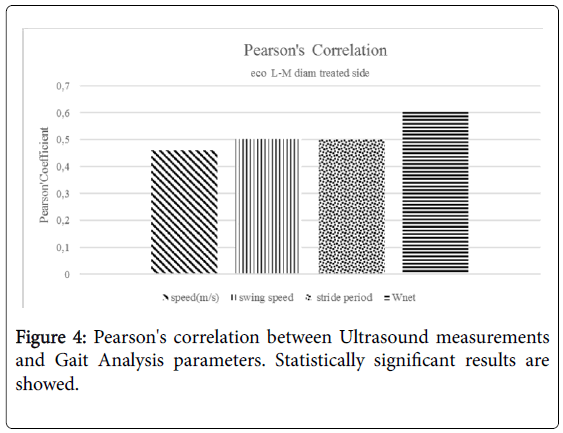
Besides, the echogenicity of the tendons of the group treated with PRF appeared more homogeneous than in the group not treated with PRF (Figure 2). No statistically significant difference has been highlighted at one year between the two groups. No statistically significant difference has been noted in the parameters of space and time resulting from the gait analysis at 6 months and 1 year performing Mann-Whitney U-test. Statistically significant differences have emerged from the analysis of the work of the treated side of the ankle during walking at 6 months. These differences were markedly more significant between the network of the treated side between the two groups (p<0.001) (Figure 3). No statistically significant differences emerged for the same parameters between the two healthy sides. Instead, a statistically significant difference was found between network healthy side and treated side (p=0.05), in the group of patients not treated with PRF (Figure 3). No difference, however, was detected in the group treated with PRF for these values. The values of stiffness of the ankle were 0.06 ± 0.01 and 0.05 ± 0.01 in non-PRF group and in PRF group respectively. Statistically significant correlations have emerged through the analysis of Pearson’s correlation between the mediolateral diameter of the treated tendon and network (p=0,005), walking speed (p=0.041), swing speed (p=0.024) and stride period (p=0.024) (Figure 4).
Discussion
The variation of Achilles tendon size is an important parameter in terms of correlation with the functional outcome of the patient. Achilles tendon hypertrophy is normally correlated with a weakness of the tendon itself. However, although the size of the Achilles tendon in the group of patients treated with PRF increases transiently at 6 month at a greater level than in the group not treated with PRF, this appears to be correlated with better results. The main parameters of the cycle time of the ankle-joint angle can be influenced by a number of factors, both functional and structural. In the literature there are numerous studies concerning the effect of the walking speed, and it is shown that as the speed increases, the dynamic stiffness and the net work increase significantly [23]. Other potentially relevant factors are sex and age, Gabriel et al. [26] found a gender-specific dynamic joint stiffness, with higher values in male (0.0844 Nm/kg/Degree) than in female (0.0691 Nm/kg/Degree). This value is in accordance with our results. It is important to recognize that the work done by the muscles is determined not only by the activity of actin myosin bridges within the sarcomere, but also by the elasticity of tendons. Like all biological tissues, tendons and ligaments can be stretched [20]. In the Achilles tendon, the elongation can occur as a consequence of an increase of the articular angle due to both a passive and active shortening of the muscle fibers. The elongation of the tendon and the elastic return reduce the mechanical work required for the muscle, and allows the muscle bundles to operate at speeds more favorable for the production of a work of greater effectiveness [17-21]. The utilization of the energy released due to the elastic deformation increases the effectiveness of muscle work (in terms of energy expenditure) during the cyclic movements as ambulation(19). The high speed of elastic recoil of the tendons and other elastic structures in series with the muscle bundles prevents the muscle of having to work at high speeds, which would be unfavorable and harmful [20,21]. The correlation with the biomechanical parameters in terms of net work product (Wnet) was in favor of patients in the PRF group. This correlation suggests that, contrary to what is observed in musculoskeletal ultrasound, where an increase in the size of the tendon is ascribed to a condition of tendinopathy, in this group of patients, however, the increase in size has a positive effect on the level of elasticity (viscoelastic property) of the tendon. Overall, it should be considered that, in terms of energy, the values of the side of the lesion in patients treated with PRF are similar to the values of the contralateral healthy side of the two groups, while the same values do not match in the group not treated with PRF. The positive correlation highlighted by statistical analysis between the medio-lateral diameter in the transverse axial scan of the tendon and the speed of the step supports the hypothesis that, only in this case, an increase of the size of the tendon is a positive factor for the performance of ambulation. It can be assumed that the increase in volume is not bound to imbibitions phenomena linked to inflammatory tissue-degenerate, as normal in injured tendon, but rather to a likely increase in the content of extracellular matrix components such as collagen and elastin [16]. The limitations of this study are to be ascribed to the low sample size, a short follow-up (one year) and the absence of a real cytological and histological evaluation of the tendons subjected to surgical treatment.
Conclusion
Our study shows that the operative treatment combined with PRF augmentation could represent a possibility to allow morphological modifications and functional improvements in the short and mediumterm period. Overall, our study suggests a biological effect of PRF itself. Additional studies are therefore needed to further investigate the usefulness of PRF during Achilles tendon surgery.
References
- Kaniki N, Willits K, Mohtadi NG, Fung V, Bryant D (2014) A retrospective comparative study with historical control to determine the effectiveness of platelet-rich plasma as part of nonoperative treatment of acute achilles tendon rupture. Arthroscopy30: 1139-1145.
- McHugh MP, Connolly DA, Eston RG, Kremenic IJ, Nicholas SJ, et al. (1999) The role of passive muscle stiffness in symptoms of exercise-induced muscle damage. Am J Sports Med.27: 594–599.
- Sánchez M, Anitua E, Azofra J, AndÃa I, Padilla S, et al. (2007) Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med35:245-251.
- Maffulli N, Waterston SW, Squair J, Reaper J, Douglas AS (1999) Changing incidence of Achilles tendon rupture in Scotland: a 15-year study. Clin J Sport Med 9: 157-160.
- Lantto I, Heikkinen J, Flinkkilä T, Ohtonen P, Leppilahti J (2015) Epidemiology of Achilles tendon ruptures: Increasing incidence over a 33-year period. Scand J Med Sci Sports25: e133-138.
- Carr AJ, Norris SH (1989) The blood supply of the calcaneal tendon. J Bone Joint Surg Br 71: 100-101.
- Cho NS, Hwang JH, Lee YT, Chae SW (2011) Tendinosis-like histologic and molecular changes of the Achilles tendon to repetitive stress: a pilot study in rats. ClinOrthopRelat Res469: 3172-3180.
- Wilkins R, Bisson LJ (2012) Operative versus nonoperative management of acute Achilles tendon ruptures: a quantitative systematic review of randomized controlled trials. Am J Sports Med40: 2154-2160.
- Jiang N, Wang B, Chen A, Dong F, Yu B (2012) Operative versus non operative treatment for acute Achilles tendon rupture: a meta-analysis based on current evidence. Int Orthop 36:765–773.
- Khan RJ, Carey Smith RL (2010) Surgical interventions for treating acute Achilles tendon ruptures. Cochrane Database Syst Rev 9: 1-8.
- Krackow KA, Thomas SC, Jones LC (1988) Ligament-tendon fixation: analysis of a new stitch and comparison with standard techniques. Orthopedics 11: 909-917.
- McMahon SE, Smith TO, Hing CB (2011) A meta-analysis of randomised controlled trials comparing conventional to minimally invasive approaches for repair of an Achilles tendon rupture. Foot Ankle Surg 17:211–217.
- Anitua E, Sanchez M, Nurden AT, Zalduendo M, de la Fuente M, et al. (2006) Autologous fibrin matrices: a potential source of biological mediators that modulate tendon cell activities. J Biomed Mater Res A77: 285-293.
- Dohan Ehrenfest DM, Rasmusson L, Albrektsson T (2009) Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol27: 158-167.
- Dietrich F, L Duré G, P Klein C, F Bampi V, V Padoin A, et al. (2015) Platelet-Rich Fibrin Promotes an Accelerated Healing of Achilles Tendon When Compared to Platelet-Rich Plasma in Rat. World J PlastSurg4: 101-119.
- Fernández-Sarmiento JA, DomÃnguez JM, Granados MM, Morgaz J, Navarrete R, et al. (2013) Histological study of the influence of plasma rich in growth factors (PRGF) on the healing of divided Achilles tendons in sheep. J Bone Joint Surg Am 95: 246-255.
- Ishikawa M, Komi PV, Grey MJ, Lepola V, Bruggemann GP (2005) Muscle-tendon interaction and elastic energy usage in human walking. J Appl Physiol 99:603–608.
- Fukunaga T, Kubo K, Kawakami Y, Fukashiro S, Kanehisa H, et al. (2001) In vivo behavior of human muscle tendon during walking. Proc Biol Sci 268:229–233.
- Lichtwark GA, Wilson AM (2007) Is Achilles tendon compliance optimized for maximum muscle efficiency during locomotion. J Biomech 40:1768–1775.
- Lichtwark GA, Bougouliasa K, Wilson AM (2007) Muscle fascicle and series elastic element length changes along the length of the human gastrocnemius during walking and running. J Biomech 40:157–1645.
- Kubo K, Kanehisa H, Takeshita D, Kawakami Y, Fukashiro S, et al. (2000) In vivo dynamics of human medial gastrocnemius muscle-tendon complex during stretch shortening cycle exercise. Acta Physiol Scand 170:127–135.
- Davis RB, De Luca PA (1996) Gait characterization via dynamic joint stiffness. Gait Posture4:224–231.
- Hansen AH, Childress DS, Miff SC, Gard SA, Mesplay KP (2004) The human ankle during walking: implications for design of biomimetic ankle prostheses. J Biomech37: 1467–1474.
- Frigo C, Crenna P, Jensen LM (1996) Moment-angle relationship at lower limb joints during human walking at different velocities. J Electromyogr Kinesiol 6:177–190.
- Crenna P, Frigo C (2011) Dynamics of the ankle joint analyzed through moment-angle loops during human walking: gender and age effects. Hum Movement Sci30:1185–1198.
- Gabriel RC, Abrantes J, Granata K, Bulas-Cruz J, Melo-Pinto P, et al. (2008) Dynamic joint stiffness of the ankle during walking: gender-related differences. Phys Ther Sport 9: 16–24.
Citation: Valeo M, Gurzì M, Alviti F, Di Giorgio L, Di Martino L, et al. (2017) Achilles Tendons Total Rupture, Open Surgical Treatment with PRF Augmentation: Clinical, Morphological and Functional Evaluation. Clin Res Foot Ankle 5: 236. DOI: 10.4172/2329-910X.1000236
Copyright: © 2017 Valeo M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5297
- [From(publication date): 0-2017 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 4329
- PDF downloads: 968