Achalasia after Sleeve Gastrectomy: A Surgical Challenge
Received: 05-Nov-2020 / Accepted Date: 20-Nov-2020 / Published Date: 27-Nov-2020 DOI: 10.4172/2165-7904.1000415
Abstract
The prevalence of achalasia in the morbid obesity population is low. However, there havebeen cases of patients diagnosed with both conditions, including some diagnosed with achalasia after undergoing bariatric surgery. Even though the surgical management of both conditions has been well studied separately, there is no consensus of the treatment when these conditions coexist. We describe the case of a patient with history of a Laparoscopic Sleeve Gastrectomy who was later on diagnosed with achalasia and successfully treated with Laparoscopic Heller Myotomy.
Keywords: Morbid obesity, Bariatric surgery, Sleeve gastrectomy
Introduction
Achalasia is an esophageal motility disorder characterized by absence of esophagealperistalsis and failure of the lower esophageal sphincter (LES) to relax during swallowing [1].Prevalence of achalasia in the general population is around 10 cases per 100,000 individuals [2].Even when studies report a higher prevalence of esophageal motility disorders in obese patientscompared to cohorts with normal body mass index, achalasia and morbid obesity are twoconditions that do not classically occur together [3,4]. For this reason, there are no establishedsurgical guidelines as to the treatment for patients who present with achalasia and morbidobesity. It is well known that Laparoscopic Heller Myotomy (LHM) and partial fundoplication isthe standard surgical approach for achalasia in non-obese population [5]. There is also evidencethat bariatric surgery is the most effective treatment for morbid obesity; being sleevegastrectomy and Roux-en-Y gastric bypass (RYGB) the most frequently performed bariatricoperations [6]. However, there is little information regarding how to treat achalasia after bariatricsurgery. Cases of achalasia treatment after RYGB have been described in literature, but there isscarce information about treating achalasia after sleeve gastrectomy.
Case Report
We present the case of a 41-year-old female patient who presented to the emergencydepartment with chief complaint of persistent dysphagia to solids and liquids. The patient had ahistory of morbid obesity and had undergone Laparoscopic Sleeve Gastrectomy (LSG) in 2018,at an outside institution. She reported having dysphagia to solids and liquids since her earlytwenties,but had not been evaluated previously for this complaint. Our patient also reportedepisodes of post-prandial nausea and vomiting with associated epigastric discomfort. She alsohad a surgical history of abdominoplasty and bilateral breast implants performed a few monthsafter LSG. There were no significant findings on physical exam.
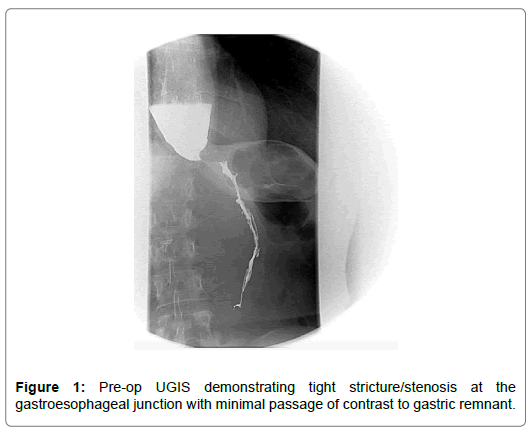
Preoperatively, contrast esophagogram demonstrated a standing column of oral contrastat the distal esophagus secondary to either a tight stenosis or beaking (Figure 1). An upperendoscopy was performed and revealed a dilated esophagus. The scope was advanced into thestomach without stricture or mucosal lesions identified. On manometry, elevated resting LESpressures were reported with absent relaxation during swallowing. It also revealed esophagealbody aperistalsis with esophageal pressurization, consistent with Type 2 achalasia.
After the above findings, the patient was scheduled for Laparoscopic Heller myotomy at ourinstitution. Upon entering the abdominal cavity via laparoscopy, paraesophageal membranes andadhesions from previous surgery were dissected. Gastroesophageal (GE) fat pad removed forbetter GE junction visualization, and distal mediastinal esophagus mobilized into the abdomen.
Heller myotomy was performed anteriorly, 5cm cephalad and 3cm caudal to GE junction,making sure that complete division of circular muscle fibers was done. Intraoperative endoscopywas performed to confirm adequate muscle division and no air leak. Upper GI series performedon postoperative day one showed slow esophageal emptying through smooth tapered narroweddistal esophagus and no evidence of leakage. There were no postoperative complications and thepatient was discharged on postoperative day two, tolerating liquid diet. Patient has been followedfor six months after surgery and no postoperative complications have been reported. Duringfollow up, Eckardt Symptom Score (ESS) has been used for post-surgical outcome evaluation.At six months after surgery, patient has maintained stable weight, reports only occasional chestpain and dysphagia, and denies any regurgitation episodes, corresponding to ESS of 2/12.
Discussion
There have been few cases of achalasia after bariatric surgery described in the literature,where most cases have been after RYGB. Masrur et al. described the first Robotic Hellermyotomy for achalasia after Laparoscopic RYGB with successful results [7]. Similarly, Bouleset al. reported eight patients who underwent Heller Myotomy (HM) to treat achalasia diagnosedafter RYGB. Six of the eight patients who underwent HM achieved resolution of achalasiasymptoms at a mean time of 1.6 months and remained asymptomatic for the total follow-up of 36months [8]. Casas et al. reached similar conclusions, indicating that LHM is an effective and safetherapy in patients with RYGB anatomy and achalasia [9].
While most of the cases in literature report patients treated for achalasia after bariatricsurgery, Ramos et al. suggested that morbidly obese patients with achalasia, should be treated forboth diseases during a single surgery [10]. Fisichella and colleagues suggested a concomitantLHM and laparoscopic RYGB as an approach that can provide excellent relief of symptoms andadequate control of reflux while promoting weight loss in obese patients with achalasia [11]. Incontrast, Crafts et al. concluded that patients who undergo surgical intervention for achalasiabefore having bariatric surgery tend to have better outcomes compared to those where achalasiais treated after bariatric surgery [12].
There have been only two cases reported in the literature of patients treated surgically forachalasia diagnosed after undergoing LSG, which were addressed in the systematic review ofCrafts et al. One of the patients was treated with LHM and conversion to RYGB, with initialimprovement of dysphagia that later recurred, and gradual weight loss of 17.5 kg in 6 months[12,13]. The second case involved a patient treated with LHM alone, but never achieved clinicalremission postoperatively and was later diagnosed with gastric stenosis requiring endoscopicdilation [12]. In the latter, it is difficult to evaluate the outcome of LHM, given the presence ofan additional pathology (gastric stenosis), which could have been responsible for the patient’sinitial clinical presentation and symptoms.
Our patient’s follow up included evaluation of symptoms six months after surgery usingEckardt Symptoms Score (ESS). ESS is widely used in both clinical and research settings as agold standard for measuring achalasia symptom severity [14]. It consists of a self-report scaleevaluating weight loss, dysphagia, chest pain and regurgitation, in which patient grades eachitem from 0 to 3 according to frequency. A patient is considered to be in clinical remission ifhe/she is assigned to be a stage 0-I or having less than three points out of twelve [15].
To our knowledge, this would be the first case described in the literature of a patient whoachieved clinical remission after undergoing LHM to treat achalasia diagnosed after LSG.
However, our patient’s history of dysphagia prior to bariatric surgery raises the suspicion of nondiagnosedachalasia prior LSG. This case highlights the importance of esophageal testing beforebariatric procedures in patients with esophageal dysmotility symptoms. Also, despite our patienthaving remained on clinical remission after 6 months of intervention, further comparative studiesneed to be performed before establishing clear guidelines to manage patients diagnosed withachalasia after bariatric surgery
Conclusion
We present a case of a patient diagnosed with achalasia after LSG, who was successfullytreated with LHM. Despite there being no consensus regarding the gold standard to treatachalasia after bariatric surgery, LHM seems to be a reasonable surgical option to treat achalasiaafter LSG. In selected patients, LHM can lead to resolution of dysphagia without adding otherwell known complications associated with RYGB.
References
- Koshy SS, Nostrant TT (1997) Pathophysiology and endoscopic/balloon treatment of esophageal motility disorders. Surgical Clinics of North America 77: 971–992.
- Sadowski D, Ackah F, Jiang B, Svenson L (2010) Achalasia: Incidence, prevalence and survival. A population-based study. Neurogastroenterology and Motility 22: 256-261.
- Jaffin BW, Knoepflmacher P, Greenstein R (1999) High prevalence of asymptomatic esophageal motility disorders among morbidly obese patients. Obes Surg 9: 390-395.
- Oviedo RJ, Sofiak CW, Dixon BM (2016) Achalasia: A case report on its effect during surgical decision making for laparoscopic sleeve gastrectomy in the young morbidly obese patient. Int J Surg Case Rep 26: 4â€6.
- Casas M, Schlottmann F, Herbella F, Buxhoeveden R, Patti M (2019) Esophageal achalasia after Roux-en-Y gastric bypass for morbid obesity. Updates Surg 71: 631-635.
- Kissler H, Settmacher U (2013) Bariatric surgery to treat obesity. Seminars in nephrology 33: 75-89.
- Masrur M, Gonzalez L, Giulianotti P (2016) Robotic heller myotomy for achalasia after laparoscopic Roux-en-Y gastric bypass. A case report & review of literature. Surgery for Obesity and Related Diseases 12: 1755-1757.
- Boules M, Corcelles R, Zelisko A, Batayyah E, Froylich D, et al. (2016) Achalasia after bariatric surgery. Journal of Laparoendoscopic & Advanced Surgical Techniques 26: 428-432.
- Casas M, Schlottmann F, Herbella F, Buxhoeveden R, Patti M (2019) Esophageal achalasia after Roux-en-Y gastric bypass for morbid obesity. Updates in Surgery 71: 631-635.
- Ramos A, Murakami A, Lanzarini E, Neto M, Galvão M (2008) Achalasia and laparoscopic gastric bypass. Surgery for Obesity and Related Diseases 5: 132-134.
- Fisichella P, Orthopoulos G, Holmstrom A, Patti M (2015) The surgical management of achalasia in the morbid obese patient. Journal of Gastrointestinal Surgery 19: 1139-1143.
- Crafts T, Lyo V, Rajdev P, Wood S (2020) Treatment of achalasia in the bariatric surgery population: a systematic review and single-institution experience. Surgical Endoscopy.
- Oh HB, Tang SW, Shabbir A (2014) Laparoscopic Heller’s cardiomyotomy & Roux-En-Y gastric bypass for missed achalasia diagnosed after laparoscopic sleeve gastrectomy. Surgery for Obesity and Related Diseases 10: 1002-1004.
- Taft TH, Carlson DA, Triggs J, Craft J, Starkey K, et al. (2018) Evaluating the reliability and construct validity of the Eckardt symptom score as a measure of achalasia severity. Neurogastroenteroly and Motily 30: e13287.
- Eckardt VF, Aignherr C, Bernhard G (1992) Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology 103: 1732-1738.
Citation: Rosa KXCDL, Montalvo AG, Domínguez AS (2020) Achalasia after Sleeve Gastrectomy: A Surgical Challenge. J Obes Weight Loss Ther 10: 415. DOI: 10.4172/2165-7904.1000415
Copyright: © 2020 Rosa KXCL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3221
- [From(publication date): 0-2020 - Jul 02, 2025]
- Breakdown by view type
- HTML page views: 2397
- PDF downloads: 824