Research Article Open Access
Accumulation of Highly Stable ΔFosB-Isoforms and Its Targets inside the Reward System of Chronic Drug Abusers - A Source of Dependence-Memory and High Relapse Rate?
Monika H Seltenhammer1, Ulrike Resch2, Martin Stichenwirth1, Jaqueline Seigner2, Christoph Reisinger CM1, Walter Vycudilik1, Christian Schöfer3, Rainer De Martin2, Johann Sölkner4 and Daniele U Risser1*1Department of Forensic Medicine, Medical University of Vienna, Sensengasse 2, A-1090 Vienna, Austria
2Department of Vascular Biology and Thrombosis Research, Center for Physiology and Pharmacology, Medical University of Vienna, Schwarzspanierstraße 17/I/III, A-1090 Vienna, Austria
3Division of Cell and developmental Biology, Center for Anatomy and Cell Biology, Medical University of Vienna, Schwarzspanierstraße 17/I, A-1090 Vienna, Austria
4H93200 Division of Livestock Sciences, University of Natural Resources and Life Sciences, Gregor-Mendel-Straße 33, A-1180 Vienna, Austria
- *Corresponding Author:
- Daniele U Risser
Department of Forensic Medicine
Medical University of Vienna, Sensengasse 2
A-1090 Vienna, Austria
Tel: +43-664-8001635500
Fax: +43-1-40160-935503
E-mail: daniele.risser@meduniwien.ac.at
Received date: June 14, 2016; Accepted date: September 22, 2016; Published date: October 01, 2016
Citation: Seltenhammer MH, Resch U, Stichenwirth M, Seigner J, Reisinger CM (2016) Accumulation of Highly Stable ΔFosB-Isoforms and Its Targets inside the Reward System of Chronic Drug Abusers - A Source of Dependence-Memory and High Relapse Rate?. J Addict Res Ther 7:297. doi:10.4172/2155-6105.1000297
Copyright: © 2016 Seltenhammer MH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Background: The ~33 kD transcription factor ΔFosB, a Fos-family protein and belonging to the immediate early genes (IEGs), is initiated in the acute phase as a response to a wide range of effects such as drugs, stress, and several external stimuli. ΔFosB forms heterodimers with Jun proteins to generate active activator protein-1 (AP-1) complexes. They bind to AP-1 sites in the promoter regions of many neural genes. To date, several downstream target genes for ΔFosB have been identified being involved in molecular pathways concerning addictive behavior, memory and learning. In answer to chronic stimuli, the rather unstable ~33 kD transcription factor ΔFosB is replaced by robust ~35-37 kD isoforms due to epigenetic splicing and different phosphorylation steps. The result is that these highly stable isoforms accumulate in the nucleus accumbens (NAc), a structure close to the hippocampus (HPC), playing a key role within the reward center of the brain. These stabilized ~35-37 kD ΔFosB derivatives linger in the brain for several weeks or longer even though the chronic stimulus has been removed – a fact that seems to be responsible for the development of sustained neuronal plasticity, (drug associated) long-term potentiation (LTP) and memory. In case of chronic drug abuse, the end result is addictive behavior and may be a crucial factor for high relapse rates.
Method: ΔFosB and cAMP response element binding protein (CREB), brain derived neurotrophic factor (BDNF), JunD, nuclear factor kappa B (NFκB) and cyclin-dependent kinase 5 (Cdk5) in both of the NAc and HPC of deceased chronic human opioid addicts were proven by immunohistochemistry even with a prolonged post-mortem interval (PMI) of 8.47 ± 2.61 days. Moreover accumulated ~35-37 kD ΔFosB isoforms could be detected in the NAc of the same samples by immunoblotting.
Results: All determined proteins showed a significant increased staining pattern in brain samples of chronic drug abusers in comparison non-drug users (p<0.05) according to Wilcoxon-Mann-Whitney-U Test. Further, accumulated ~35-37 kD ΔFosB isoforms were detectable in NAc samples of long-term drug addicts by immunoblotting in contrast to the control group, where no trace of any isoform was verifiable (p<0.05) according to Wilcoxon-Mann-Whitney-U Test.
Conclusion: Taken together with the results of already published functional in-vivo animal experiments, our findings provide additional evidence of the potential strong impact of ΔFosB on its downstream transcriptional targets, which are in turn responsible for sustainable effects and serious adaptations in the brain that lead to addictive behavior and dependence memory.
Keywords
Addiction; craving; Delta FosB (ΔFosB); Immediate early genes (IEGs); Nucleus accumbens (NAc); Hippocampus (HPC); Activator protein-1 (AP-1); cAMP response element binding protein (CREB); Brain derived neurotrophic factor (BDNF); Cyclin-dependent kinase 5 (Cdk5); Nuclear factor kappa B (NFκB); Jun-family proteins; Opioids; Relapse rate; Sustained neuroplasticity; Immunohistochemistry; Immunoblotting; Dependence memory
Abbreviations
AP-1: Activator Protein-1; BDNF: Brain Derived Neurotrophic Factor; CREB: cAMP Response Element Binding Protein; Cdk5: Cyclin-Dependent Kinase5; DAB: 3,3´- Diaminobenzidine; ECL: Enhanced Chemiluminiscence; FFPE tissue: Formalin-Fixed Paraffin-Embedded Tissue; GLM: General Linear Model; HIER: Heat-Induced Epitope Retrieval; HPC: Hippocampus; IEGs: Immediate Early Genes; LTP: Long-Term Potentiation; MSN: Medium Spiny Neurons; NAc: Nucleus Accumbens; NFκB: Nuclear Factor kappa B; PBS: Phosphate Buffered Saline; PMI: Post Mortem Interval; SD: Standard Deviation
Introduction
Addiction is defined as a chronic, recurring disease of the brain. It is characterized by compulsive, drug-craving, drug-seeking behavior and drug use, even when confronted with the harmful consequences [1]. However, it is in fact a complex phenomenon with severe physiological, psychological and social effects [1,2].
Irrespective of the noticeable decline in the demand for drugs in recent years, at least in Austria, the illegal use of these substances still remains a global affair [3,4]. Accompanied with rampant health problems such as blood-borne diseases (HIV, hepatitis B and C), it accounts for an increasing rate of morbidity, mortality and crime. Nevertheless people of younger ages in particular are disproportionately affected. In the World Drug Report 2015, the United Nations Office on Drugs and Crime (UNODC) estimated that, in 2013, about 246 million people (equivalent to 1 out of 20 people of the world’s 15 to 64 year olds) used illicit substances at least once a year. However, among all drug-dependent persons worldwide, an estimated 12 to 21 (midpoint: 16.5) million people are chronic opiate addicts with a relapse rate of >90% [4]. This in turn is a fact that leads to a vicious cycle of severe medical, social and other major problems.
There is increasing evidence that mechanisms facilitating addiction to drugs are provoked by sustained changes in gene expression [5]. In addition to the cAMP response element binding protein (CREB), nuclear factor kappa B (NFκB) and others, the transcription factor ΔFosB in particular is a remarkable molecular biological mediator. ΔFosB belongs to the Fos family proteins, encoded by one of so-called immediate early genes (IEGs). These genes are activated straight, transiently and rapidly in response to a variety of stimuli. ΔFosB is a component of activator protein-1 (AP-1), comprising of different combinations of Fos- and Jun-family proteins (c-Jun, JunB, JunD). This specific transcription factor plays a key role in the psychological phenomenon of addiction [6]. Chronic exposure to a range of stimuli, including drugs of abuse such as cocaine and morphine (opioids), stress, courses of treatment involving anti-psychotic or anti-depressant drugs, and certain types of lesions contribute to the induction of this transcription factor in specific brain regions, which are responsible for reward and emotional behaviors (the mesolimbic dopamine system). This system is one in which the nucleus accumbens (NAc) in particular, and the hippocampus (HPC) in a broader sense play a critical role [7-10].
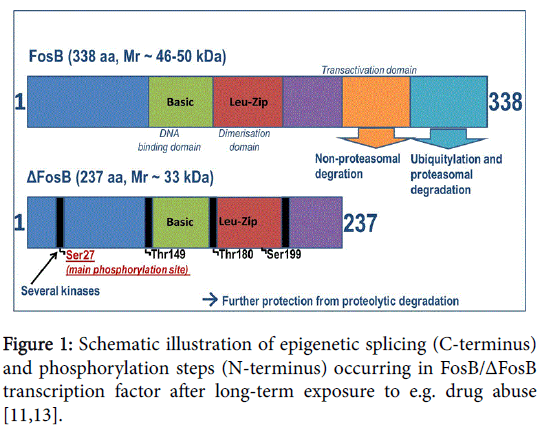
In contrast to all other Fos family proteins and IEGs, ΔFosB presents an unusually high degree of stability and therefore has a conspicuously longer half-life. The stimulation of the ~33 kD transcription factor ΔFosB in the acute phase and then its shift to stable ~35-37 kD isoforms due to chronic exposure to several inducements (stress, different drugs and other psychoactive substances) leads to a steady accumulation of highly stable ΔFosB isoforms. This can be explained by an intrinsic property of the molecule, i.e. the truncation of degron domains at the C-terminal present in full-length FosB [11], as well as a regulated process triggering phosphorylation mechanisms, as illustrated schematically in Figure 1 (Figure 1) [12,13]. This unique and, at the same time, insistent persistence of the accumulated ΔFosB in response to long-lasting exposure to drugs makes this transcription factor predestined to be a critical mediator driving this mechanistic pathway of addictive behavior and disposition to relapse. The latter is as a consequence of sustained neuronal plasticity; even after the stimulus has been removed.
Almost every major study to date comprising this subject has been involved animal experiments under specific conditions [6-10]. In turn, many of these experiments focused on explaining the impact of ΔFosB on behavioral responses to chronic cocaine exposure. In contrast, comparatively little attention has been paid to long-term opioid abuse, particularly in humans.
With this in mind, for the first time we conducted a study to detect the presence of accumulated ~35-37 kD ΔFosB isoforms in the NAc, and its targets cAMP response element binding protein (CREB), brain derived neurotrophic factor (BDNF), and cyclin-dependent kinase 5 (Cdk5), nuclear factor kappa B (NFκB), and ΔFosB´s partner JunD, in both NAc and HPC of chronic human opioid addicts with a prolonged postmortem interval (PMI) of 8.47 ± 2.61 days.
Materials and Method
Selection of cases and tissue sampling
The use of post-mortem human brain tissue samples of the nucleus accumbens (NAc) and hippocampus (HPC) was approved by the Ethical Committee of the Medical University of Vienna as required by Austrian law. We selected 30 cases, 15 of which were identified as drug related deaths (DRDs) with pronounced opioid abuse (opiate group) and 15 as non-drug-related deaths in the same age range (control group), as shown in Table 1 (Table 1). Subjects with a recent or past history of neurological disorders, or who had suffered head injuries, were excluded from the study. Forensic post-mortem and police reports relating to the deceased were retrieved from the database of the Department of Forensic Medicine of the Medical University of Vienna, in close cooperation with the Vienna Police Department. As usual, the corpses were stored at refrigeration temperature (4°C) until the autopsies were performed.
| Parameter | Opioid Group (n=15) | Control Group (n=15) |
|---|---|---|
| Age (years): Mean/Std. Deviation | 27.0/ ± 7.05 | 26.87/ ± 6.80 |
| Median | 26.0 | 24.0 |
| Range | 18.0 | 21.0 |
| p=0.96 | ||
| PMI (days): Mean/Std. Deviation | 8.47/ ± 2.61 | 9.33/ ± 3.87 |
| Median | 8.0 | 11.0 |
| Range | 9.0 | 10.0 |
| p=0.48 | ||
| Morphine (ng/g): Mean/Std. Deviation | 230.5/ ± 92.5 | 0.0/ ± 0.0 |
| Median | 196.5 | 0.0 |
| Range | 590.0 | 0.0 |
| p = 0.001 | ||
| Gender Distribution (F/M) | 2-13 | 4-11 |
| p=0.65 |
Table 1: Overview of medical histories (drug consumption) and demographics of donors of brain tissue samples with prolonged post-mortem
interval (PMI).
DRDs were defined according to edicts of the Austrian Federal Ministry of the Interior and the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), respectively [14,15]. All of the cases presented in this study underwent a standardized forensic autopsy involving the examination of all major organs.
The opiate group comprised 15 cases (2 females, 13 males). In this group, the ages ranged from 18 to 36 years with a mean age of 27.0 ± 7.05 years. The post-mortem interval (PMI) ranged from 4 to 13 days with a mean of 8.47 ± 2.61 days. All of the cases in this group were identified as illicit drug users, where death was imputed to morphine or heroin intoxication. According to clinical histories, all of the individuals were chronic opioid users.
The control group consisted of 15 cases (4 females, 11 males) with no history of any illicit drug dependence or major psychiatric issues and, consequently, in which the cause of death was not drug-related. In this group, the ages ranged from 18 to 39 years with a mean age of 26.87 ± 6.8 years; the PMI ranged from 4 to 14 days with a mean of 9.33 ± 3.87 days (Table 1).
Preparation of samples - Drug testing
All of the deceased underwent drug testing using two different, validated methods as described before [16-18]. The concentration of morphine was determined in the medulla oblongata, the cerebellum and, if available, in the blood, the hair and the urine. Peripheral blood and urine samples were routinely collected to perform a quantitative toxicological analysis using Gas Chromatography-Mass Spectrometry (GC-MS) and tested for 6-Monoacetylamorphine (6-MAM) as well as other psychoactive substances (e.g. cannabis, benzodiazepine, caffeine, etc.).
Preparation of samples – Immunohistochemistry, protein isolation and immunoblotting
For immunohistochemical analysis, brain tissue samples of NAc and HPC were fixed in 4% buffered formalin and embedded in paraffin (“FFPE tissue”). The blocks were sectioned at 2 μm, tissue sections were deparaffinized, and rehydrated. The highly sensitive, one-step, ready-to-use ImmPRESSTM Polymer Detection reagents system (ImmPRESS-AP and ImmPRESS-HRP; Vector Laboratories, Szabo- Scandic, Vienna, Austria) was used for FosB/ΔFosB, Ser27-ΔFosB (P- ΔFosB), Cdk5, CREB, BDNF, NfκB and JunD detection, employing the following primary antibodies, all of them diluted in Primary Antibody Diluent (Cedarlane®; Cedarlanelabs, Szabo-Scandic): polyclonal goat anti-FosB (1:200 dilution; Novus, USA, Catalog No. AF2214; THP Medical Products, Vienna, Austria); monoclonal rabbit anti-FosB (1:100 dilution; Pierce, USA, Catalog No. MA5-15056; THP); polyclonal rabbit anti-FosB (1:200 dilution; Bioss, USA, Catalog No. bs-1170R; THP); rabbit polyclonal anti-FosB Ser27 (1:200 dilution; Bioss, USA, Catalog No. bs-13198R; THP); rabbit monoclonal anti- Delta FosB (D3S8R) (1:100 dilution; Cell Signaling, Leiden, The Netherlands, Catalog No. 14695); monoclonal mouse anti-Cdk5 (clone DC17+DC43) (1:200 dilution; Thermo Scientific, Vienna, Austria); monoclonal mouse anti CREB-1 (clone D-12) (1:200 dilution; sc-377154, Santa Cruz Biotechnology Inc., Szabo-Scandic); polyclonal rabbit anti-BDNF (N-20) (1:200 dilution; sc-546, Santa Cruz); polyclonal rabbit anti-NfκB (1:100 dilution; Y021017 abmgood, THP); and polyclonal rabbit anti-JunD (329) (1:100 dilution; sc-74, Santa Cruz). As a negative control, the primary antibodies were replaced with phosphate buffered saline (PBS) in Primary Antibody Diluent (Cedarlane®; Cedarlanelabs, Szabo-Scandic). Before proceeding, “heatinduced epitope retrieval” (HIER) was performed to demask the antigen´s epitopes by means of Vector® Antigen Unmasking Solution, Citrate-based (Vector Laboratories, Szabo-Scandic) using a steamer (Braun Multi-Gourmet FS20, Braun Comp. Ltd., Kronberg im Taunus, Germany). After quenching endogenous horse radish peroxidase (HRP) with BLOXALLTM Endogenous Peroxidase and Alkaline Phosphatase Blocking solution (Vector Laboratories, Szabo-Scandic) for 20 min at room temperature, primary antibodies were incubated overnight at 4°C before antibody detection was carried out the day after. Immediately after detection and peroxidase localization via ImmPACT™ DAB chromogene (DAB; 3,3´-diaminobenzidine) giving a brown color (Vector Laboratories, Szabo-Scandic), a nuclear counterstain was performed with Vector® Hematoxilin QS Nuclear Counterstain (Modified Mayer´s Formula) (Vector Laboratories, Szabo-Scandic), providing a blue-violet color with crisp nuclear detail. Finally, slides were mounted with Vectashield® Mounting Medium (Vector Laboratories, Szabo-Scandic), an aqueous mounting medium. Immunohistochemically staining patterns were assessed as described previously [19]. Briefly, the presence or absence (qualification) of staining and the depth (semi-quantification) of color were noted. The depth of color was recorded as pale, medium, or dark according to how easily it was seen. The samples were then categorized as weak, moderate, or strong staining according to the following criteria: (a) strong (+++), dark staining of the nucleus and/or cells (fibres) in the case of BDNF respectively, that is easily visible with a low-power objective; (b) moderate (++), medium staining that is visible with a low-power objective; (c) weak (+), pale staining that is not easily seen under a low-power objective; and (d) negative (−), tissues that show none of the above. Specimens were analyzed by two independent observers, using light microscopy (Nikon Eclipse 800/Camera Nikon DsRi1); both of whom were blind to the groups.
For protein isolation prior to immunoblotting application, brain tissue samples (NAc only) were collected; partly immediately flash frozen and stored at -80°C until further procedures were carried out. Subsequently, the tissue (400 mg) was ultrasonically homogenized in an ice-cold (1.0-1.5 ml) lysis buffer (50 mM Tris-HCl pH 7.4; three tablets of Complete-Protease-Inhibitor/100 ml, Roche, Vienna, Austria; 1% Triton®X-114; 5 mM DTT; 10 mM sodium orthovanadate; Sigma- Aldrich, Vienna, Austria). Triton® X-114 served as an essential prerequisite to remove lipids by means of phase separation to enable further protein purification. Samples were subsequently boiled for ten minutes and centrifuged at full speed (16,000 g) for a further ten minutes at a temperature of 4°C. Supernatants were collected and stored at -80°C until further analysis. Immediately prior to gel separation, samples were subjected to a quantitative precipitation step with methanol and chloroform (Sigma-Aldrich), intended to purify and enrich proteins. After dissolving the samples in Laemmli-loading buffer, protein samples (30 μg per lane; previously determined via the Bradford assay; Bio-Rad company, Vienna, Austria) were then subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Transfected HEK-293 cells (ATCC No. CRL-1573; LGC Standards GmbH, Wesel, Germany) with human ΔFosB-vector (EX-A4302- Lv201-GS, GeneCopoeia, Inc.; THP) served as positive control (Supplementary Information). After blotting the proteins onto a nitrocellulose membrane (0.45 μm; Amersham Biosciences, Vienna, Austria), Ponceau-S-staining (Sigma-Aldrich) was carried out as an additional step to β-Actin or Tubulin detection to demonstrate a loading control, successful equal protein transfer and finally to serve as benchmark for semi-quantitative analysis of results. After being washed, the membrane was blocked with 5% skimmed milk (blocking buffer). Subsequently, primary antibody incubation was performed in the blocking buffer at 4°C overnight using a monoclonal mouse-anti- FosB antibody which recognizes ΔFosB isoforms [FosB (C11): sc-8013, Santa Cruz; raised against amino acids 75-150, mapping at the Nterminus of FosB of human origin] in a 1:100 dilution. Another primary antibody was used to detect ΔFosB isoforms: polyclonal rabbit-anti-panFRA (c-Fos: sc-253, Santa Cruz) in a 1:400 dilution. Finally, protein bands were visualized by enhanced chemiluminiscence (ECL) (Western Bright Chemiluminescenz Substrate Sirius, Biozym Art 541021; Biozym Comp., Vienna, Austria). Thereafter, signals were analyzed by performing a semi-quantitative analysis, where each Ponceau-S staining served as reference point, according to the following criteria: (a) strong (+++), signal that is easily visible after a short substrate exposure; (b) moderate (++), signal that is visible with after a medium substrate exposure; (c) weak (+), signal that is not easily seen after extended substrate exposure; and (d) negative (−), no signal detectable even after extended substrate exposure.
In addition to the method mentioned above, a further part of the FFPE tissue samples of both groups (case and control) was subjected to another (modified) immunoblotting procedure (Supplementary Information; Suppl. Figure 1), where both ΔFosB and P- ΔFosB could be detected in samples of the drug group, as shown in an example figure (Suppl. Figure 1).
Statistical evaluation
SAS®9.4 was used to conduct a statistical analysis [20]. Demographic concordance of opiate and control groups was tested with Student´s-t test and Chi-squared test.
Immunohistochemistry as well as immunoblotting data were analyzed using the NPAR1WAY procedure; the different immunoreactions (ΔFosB/DeltaFosB; Ser25FosB/P ΔFosB; Cdk5; CREB; BDNF; NFκB; and JunD) between drug users and control group were compared by Wicoxon-Mann-Whitney U test.
Data were expressed as means ± standard deviations (SD), median and range. Results were significant at p<0.05.
Results
Drug testing
Of the 30 individuals included in this study, autopsy and clinical files from the hospital were studied to determine the drug consumption-history and health status of the deceased, and to confirm the characteristics of each group. As shown in Table 1, the morphine concentration in the CNS (medulla oblongata) of cases in the opiate group was 50-640 ng/g (mean 230.5 ± 92.5 ng/g), whereas in the control group morphine concentration was negligible (p ≤ 0.001) (Table 1).
Detection of stable ΔFosB-isoforms in human brain tissue samples of chronic opioid abusers by means of immunohistochemistry and immunoblotting
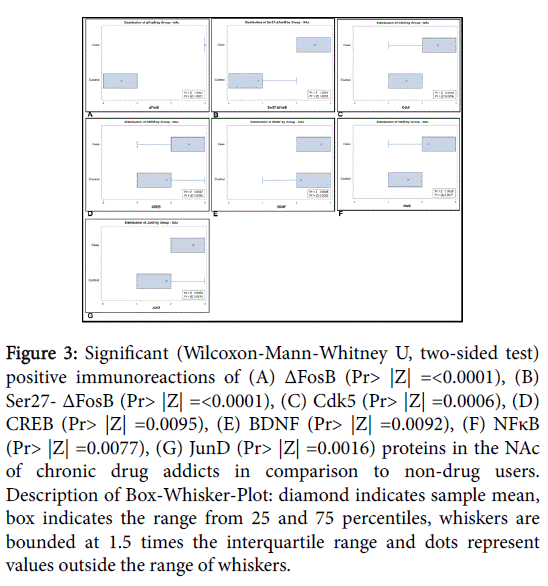
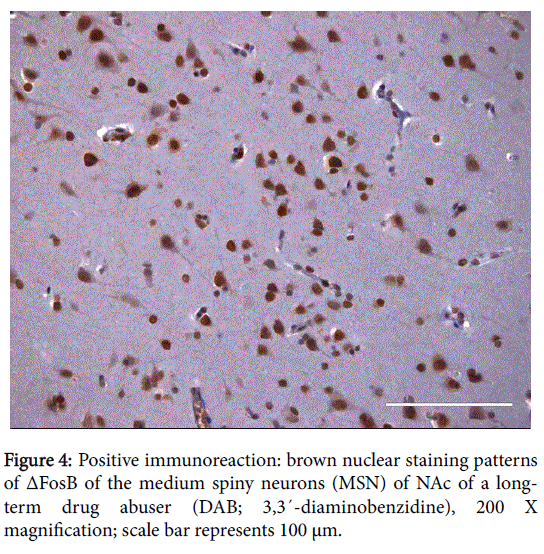
The positive expression of ΔFosB, Ser27-ΔFosB (P- ΔFosB), Cdk5, CREB, BDNF, NfκB and JunD verified via immunohistochemistry were significantly higher in brain tissues of chronic drug abusers (both NAc and HPC) than in tissue samples of non-drug users (Figures 2 and 3); also presented in sample illustrations of ΔFosB immunoreactions (Figures 4-6).
Figure 2: Significant (Wilcoxon-Mann-Whitney U, two-sided test) positive immunoreactions of (A) ΔFosB (Pr> |Z| =<0.0001), (B) Ser27- ΔFosB (Pr> |Z| =<0.0001), (C) Cdk5 (Pr> |Z| =0.0089), (D) CREB (Pr> |Z| =0.0825), (E) BDNF (Pr> |Z| =0.0003), (F) NFκB (Pr> |Z| =0.0240), (G) JunD (Pr> |Z| =<0.0001) proteins in the HPC of chronic drug addicts in comparison to non-drug users. Description of Box-Whisker-Plot: diamond indicates sample mean, box indicates the range from 25 and 75 percentiles, whiskers are bounded at 1.5 times the interquartile range and dots represent values outside the range of whiskers.
Figure 3: Significant (Wilcoxon-Mann-Whitney U, two-sided test) positive immunoreactions of (A) ΔFosB (Pr> |Z| =<0.0001), (B) Ser27- ΔFosB (Pr> |Z| =<0.0001), (C) Cdk5 (Pr> |Z| =0.0006), (D) CREB (Pr> |Z| =0.0095), (E) BDNF (Pr> |Z| =0.0092), (F) NFκB (Pr> |Z| =0.0077), (G) JunD (Pr> |Z| =0.0016) proteins in the NAc of chronic drug addicts in comparison to non-drug users. Description of Box-Whisker-Plot: diamond indicates sample mean, box indicates the range from 25 and 75 percentiles, whiskers are bounded at 1.5 times the interquartile range and dots represent values outside the range of whiskers.
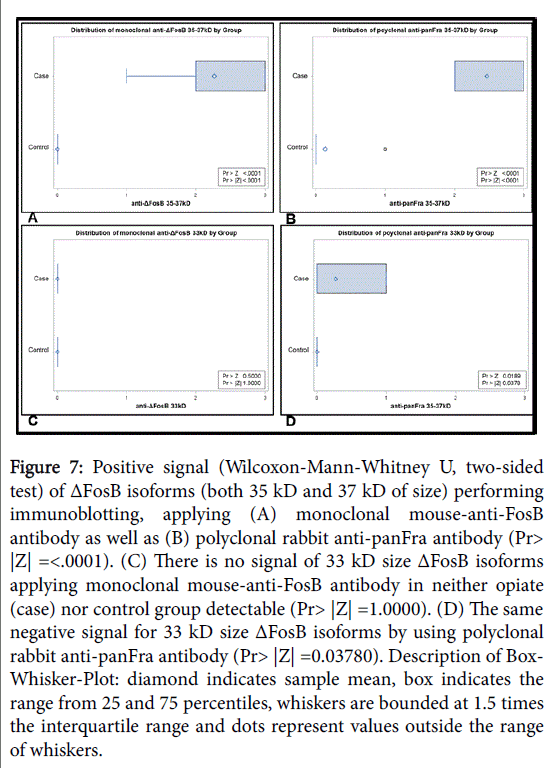
Furthermore, the expression of ΔFosB in post-mortem NAc tissue samples was proven by means of immunoblotting of chronic opioid addicts and compared this with the tissue of control individuals: Stable ΔFosB isoforms with a size of 35 kD and 37 kD were detected in the NAc of subjects with chronic drug abuse (n=15), even after a PMI of 8.47 ± 2.61 days (Table 1), with both the polyclonal and the monoclonal antibody. However, the comparatively unstable 33 kD ΔFosB isoforms were below the level of detection in both of the groups. In contrast, none of either ΔFosB isoforms could be found in postmortem samples (PMI 9.33 ± 3.87 days) of the control group (n=15) (p ≤ 0.05), even after extended substrate exposure as shown in Figure 7.
Figure 7: Positive signal (Wilcoxon-Mann-Whitney U, two-sided test) of ΔFosB isoforms (both 35 kD and 37 kD of size) performing immunoblotting, applying (A) monoclonal mouse-anti-FosB antibody as well as (B) polyclonal rabbit anti-panFra antibody (Pr> |Z| =<.0001). (C) There is no signal of 33 kD size ΔFosB isoforms applying monoclonal mouse-anti-FosB antibody in neither opiate (case) nor control group detectable (Pr> |Z| =1.0000). (D) The same negative signal for 33 kD size ΔFosB isoforms by using polyclonal rabbit anti-panFra antibody (Pr> |Z| =0.03780). Description of Box- Whisker-Plot: diamond indicates sample mean, box indicates the range from 25 and 75 percentiles, whiskers are bounded at 1.5 times the interquartile range and dots represent values outside the range of whiskers.
Discussion
The underlying biological process of drug addiction, namely the impact of repeated drug intake on the human brain, implies specific adaptations of individual neurons. This, in turn, alters the mode of operation of affected neurons, which again alters the way in which specific neural circuits’ function, within which these neurons usually operate. In combination, these effects lead to a specific behavioral complex: dependence, tolerance, sensitization, and craving. This defines the status of addition, as already published by Koob and Le Moal [1] and Wise [2].
As repeatedly confirmed by previous studies such as Lynch [21] and Robison et al. [22], the accumulation of stable 35-37 kD ΔFosB isoforms in the NAc as a consequence of chronic exposure to different stimuli, including drugs of abuse such as cocaine or morphine, appears to be responsible for sustained behavioral changes, inducing addiction or “drug-sickness”, as well as sustained neuroplasticity. Besides NAc, recent findings of Eagle et al. underscore the important role of IEGs and particularly ΔFosB inside HPC regarding learning and memory formation [23].
Although we assumed that ΔFosB might be a comparatively stable transcription factor, with an approximate half-life of 10 h in PC12 cells, as revealed by Ulery et al. [12], our recent results have considerably exceeded our expectations, especially with regard immunoblotting results.
Based on a study Chen et al. where the authors describe the consistently regulation of cyclin-dependent kinase 5 (Cdk5) by ΔFosB in the HPC of mice, we were able to demonstrate an increased immunoreactivity of Cdk5 in both NAc and HPC of chronic drug abusers [24]. As a result, the evidence of the fact that Cdk5 is one of the downstream target genes for ΔFosB could be proven by our findings. Toward CREB as a constitutively expressed transcription factor which is regulated by its phosphorylation and its relationship to ΔFosB, growing evidence suggests that CREB has a notable role in the molecular mechanisms of certain aspects of addiction [25-28]. According to a study of Kaste et al. chronic nicotine administration evoked accumbal ΔFosB induction, which is supposed to involve the induction of behavioral induction? In their study, the authors demonstrate that morphine increased CREB phosphorylation in the dopaminergic cell body areas in nicotine-exposed mice [27]. However, besides increased Cdk5 immunoreactivty, there was a clear CREB staining pattern in our opiate group detectable. Additionally, BDNF signaling seems to be critically involved in the regulation of neuronal and behavior related plasticity [29]. BDNF is one of the target genes of CREB and it is both an upstream activator and a downstream target of CREB-mediated signaling [30]. According to Kivinummi et al. BDNF levels were first detected 4 weeks after the cessation of drug treatment and therefore could be related to the long-lasting neuronal changes underlying addiction [28]. Based on their results, similar findings of elevated BDNF staining patterns could be generated in our study. Another important factor being involved in chronic drug abuse is NFκB, as many of the detrimental effects on the NAc and HPC appear to be also mediated by NFκB signaling [31]. Even in this case we could show distinct elevated immunoreactivity in the brain samples of chronic drug addicts.
According to experimental studies in rodent models, chronic exposures to various stimuli trigger a steady accumulation of the longlived isoform in distinct rat brain regions [12,32], but only a transient up-regulation of the expression of ΔFosB mRNA, as reported by Alibhai et al. [33]. However, recent findings by Teyssier et al. [34] clearly show that the ΔFosB pathway in brain tissue of depressed patients was chronically activated at the mRNA level, suggesting that a more complex genetic response may be involved, at least in the case of humans. Independent of these findings, both Dietz et al. [35] and Robison et al. [22] were able to detect stable ΔFosB isoforms in NAc of human tissue samples of patients with schizophrenia in the former and of cocaine-dependent individuals in the latter. However, postmortem intervals did not exceed more than one to two days for any of those examinations. It must therefore be conceded that our study has some limitations, particularly with regard to protein quality due to potential postmortem changes, given a PMI of 8.47 ± 2.61 days in the opiate and 9.33 ± 3.87 in the control group, respectively. To address this question, we significantly revised our method in respect of protein purification by adding supplementary purification steps, such as by using Triton® X-114 instead of Triton® X-100, although both belong to the group of detergents. One advantage of the former over the latter is the lower cloud point of 23°C in the case of Triton® X-114 versus 63-69°C in the case of Triton® X-100. This might be a critical challenge when working with either unstable or in our case stressed and potential degraded proteins due to prolonged PMIs. In general, detergents are essential to remove lipids by phase separation to enable further protein purification. Given the elevated levels of lipid-rich proteins in the brain tissue, protein-lipid-interactions might easily impair results by providing incorrect molecular weights. This means that additional purification steps are essential. In order to promote the stringent conditions, in our study a further quantitative precipitation step of the proteins was applied prior to immunoblotting analysis. Only then was it possible to detect stable 35-37 kD ΔFosB isoforms in the human NAc samples of chronic opioid abusers with prolonged PMIs. Finally, to verify our results, two different primary antibodies were applied independently to detect our target protein: (i) a polyclonal rabbit-antipanFRA and (ii) a monoclonal mouse-anti-FosB antibody. As demonstrated, both antibodies detected the target protein in the same (correct) size in brain tissue samples of the opiate group. Due to inevitable protein degradation, signals of the detection with the monoclonal antibody were rather weak in comparison to the polyclonal antibody. The same demotion mechanisms are responsible that neither β-Actin nor Tubulin detection as an internal and loading control was possible in a constitutive and reliable way, either. Therefore, only Ponceau-S staining alternatively served as a reliable loading-control and correct protein transfer onto the nitrocellulose membrane, and as reference for semi-quantitative analysis of the blotting results [36,37].
Another limitation which should be mentioned here is that only semi-quantitative and no quantitative analyses were possible at this stage of examination given the extent of protein degradation as a result of proteases over a prolonged PMI. However, it was not possible to reduce PMIs due to statutory regulations governing the release of subjects. Furthermore, functional analyses of potential downstream targets of ΔFosB and their regulatory mechanisms only constitute part of the work which still needs to be done.
Despite the extensive amount of literature on ΔFosB for cocaine action and, to a lesser extent, for morphine administration, most of these studies were conducted on rodents. However, in our study we were able for the first time to detect highly stable 35-37 kD ΔFosB isoforms in post-mortem human tissue samples of the NAc of chronic opioid addicts via immunoblotting. In conclusion, given the prolonged PMIs on the one hand, and elements of uncertainty in comparison with distinct projectable animal experiments under standardized laboratory conditions on the other, our remarkable findings add additional weight to view that this phosphorylated transcription factor is of major importance in the field of neuronal plasticity, affecting learning and memory, as well as addiction and relapse.
This fact should be taken into consideration when thinking about establishing and interpreting sensitive biomarkers as well as when developing novel therapeutic strategies for psychological disorders and drug addiction.
Acknowledgement
We thank Prof. Mario Herrera-Marschitz, BNI and ICBM Medical Faculty, University of Chile, and Dr. Lidija Sölkner, Austrian Breast & Colorectal Cancer Study Group (ABCSG) Vienna, for their assistance in writing and critically reviewing this manuscript. We also thank Renate Dinkelbach and Brita Leyrer, both Department of Forensic Medicine, Medical University of Vienna, and Sigurd Krieger, Department of Clinical Pathology, Medical University of Vienna, for their perfect technical advice. This work was supported by the Vienna Mayoral Medical-Scientific Fund Grant No. 2137. Furthermore, the authors declare that there are no competing financial interests.
References
- Koob GF, Le Moal M (1997) Drug abuse: Hedonic homeostatic dysregulation. Science 278: 52-58.
- Wise RA (1996) Neurobiology of addiction. CurrOpinNeurobiol 6: 243-251
- Weigl A, Anzenberger J, Busch M, Horvath I, Türscherl E (2015) National Report on the Drug Situation in Austria 2015 - “BerichtzurDrogensituation 2015 in Österreich”. ÖBIG, Ministry of Health and Women´s Affairs, GÖG.
- UNODC (2015) World Drug Report (United Nations Publication, Sales No. E.15.XI.6).
- Robison AJ, Nestler EJ (2011) Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci 12: 623-637.
- Nestler EJ, Kelz MB, Chen J (1999) ΔFosB: A molecular mediator of long-term neural and behavioral plasticity. Brain Res 835: 10-17.
- Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, et al. (1994) Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron 13: 1235-1244.
- Andersson M, Westin JE, Cenci MA (2003) Time course of striatal ΔFosB-like immunoreactivity and prodynorphin mRNA levels after discontinuation of chronic dopaminomimetic treatment. Eur JNeurosci 17:661-666.
- Peakman MC, Colby C, Perrotti LI, Tekumalla P, Carle T, et al. (2003) Inducible, brain region-specific expression of a dominant negative mutant of c-Jun in transgenic mice decreases sensitivity to cocaine. Brain Res 970:73-86.
- Zachariou V, Bolanos CA, Selley DE, Theobald D, Cassidy MP, et al. (2006) An essential role for ΔFosB in the nucleus accumbens in morphine action. Nat Neurosci 9: 205-211.
- Carle TL, Ohnishi YN, Ohnishi YH, Alibhai IN, Wilkinson MB, et al. (2007) Proteasome-dependent and -independent mechanisms for FosB destabilization: Identification of FosBdegron domains and implications for ΔFosB stability. Euro J Neurosci25:3009-3019.
- Ulery PG, Rudenko G, Nestler EJ (2006) Regulation of ΔFosB stability by phosphorylation. J Neurosci 26: 5131-5142.
- Ulery-Reynolds PG, Castillo MA, Vialou V, Russo SJ, Nestler EJ (2009) Phosphorylation of ΔFosB mediates its stability in vivo. Neuroscience 158: 369-372.
- Federal Ministry of Internal Affairs. Drug report 1994 (1995) Vienna, Austria: Federal Ministry of the Interior.
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) (2006) Collection and analysis of information on drug-related deaths, EMCDDA Scientific Report [online]. Lisbon, Portugal: Union Monitoring Centre for Drugs and Drug Addiction, 2001.
- Vycudilik W (1988) Comparative morphine determination in parts of the brain using combined gas chromatography/mass spectrometry. A possibility for assessing survival time.ZRechtsmed99: 263-272.
- Kintz P, Mangin P (1995) Simultaneous determination of opiates, cocaine and major metabolites of cocaine in human hair by gas chromotography/mass spectrometry (GC/MS). Forensic SciInt 73: 93-100.
- Seltenhammer MH, Marchart K, Paula P, Kordina N, Klupp N, et al. (2013) Micromorphological changes in cardiac tissue of drug-related deaths with emphasis on chronic illicit opioid abuse. Addiction:1287-1295.
- Giordano C, Vinet J Curia G, Biagini G (2015) Repeated 6Hz corneal stimulation progressively increases FosB/ΔFosB levels in the lateral amygdala and induces seizure generalization to the hippocampus. PloS One 10: e0141221.
- SAS Institute Inc. 2013. Base SAS® 9.4 Procedures guide: Statistical procedures, Second Edition. Cary, NC: SAS Institute Inc.
- Lynch MA (2004) Long-term potentiation and memory. Physiol Rev 84:87-136.
- Robison AJ, Vialou V, Mazei-Robison M, Feng J, Kourrich S, et al. (2013) Behavioral and structural responses to chronic cocaine require a feed forward loop involving ΔFosB and calcium/calmodulin-dependent protein kinase II in the nucleus accumbens shell. JNeurosci33:4295-4307.
- Eagle AL, Gajewski PA, Yang M, Kechner ME, Al Masraf BS, et al. (2015) Experience-dependent induction of hippocampal ΔFosBcontrols learning. J Neurosci 35: 13773-13783.
- Chen J, Zhang Y, Kelz MB, Steffen C, Ang ES, et al. (2000) Induction of cyclin-dependent kinase 5 in the hippocampus by chronic electroconvulsive seizures: Role of ΔFosB. J Neurosci 20: 8965-8971.
- Marttila K, Raattamaa H, Ahtee L (2006) Effects of chronic nicotine administration and its withdrawal on striatal FosB/ΔFosB and c-Fos expression in rats and mice. Neuropharmacology 51: 44-51.
- Marttila D, Piepponen TP, Kiianmaa K, Ahtee L (2007) AccumbalFosB/ΔFosBimmunoreactivity and conditioned place preference in alcohol-preferring AA rats and alcohol-avoiding ANA rats treated repeatedly with cocaine. Brain Res 1160:82-90.
- Kaste K, Kivinummi T, Piepponen TP, Kiianmaa K, Ahtee L (2009) Differences in basal and morphine-induced FosB/ΔFosB and pCREBimmunoreactivities in dopaminergic brain regions of alcohol-preferring AA and alcohol-avoiding ANA rats. PharmacolBiochemBehav 92: 655-662.
- Kivinummi T, Kaste K, Rantamäki T, Castrén E, Ahtee L (2011) Alterations in BDNF and phospho-CREB levels following chronic oral nicotine treatment and its withdrawal in dopaminergic brain areas of mice. NeurosciLett 491: 108-112.
- Lipsky RH, Marini AM (2007) Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Ann N Y AcadSci 1122: 130-143.
- Marco EM, Granstrem O, Moreno R, Liorente R, Adriani W, et al. (2007) Subchronic nicotine exposure in adolescence induces long-term effects on hippocampal and striatal cannabinoid-CB1 and mu-opioid receptors in rats. Eur J Pharmacol 557:37-43.
- McClintick JN, Xuei X, Tischfield JA, Goate A, Foroud T, et al. (2013) Stress-response pathways are altered in the hippocampus of chronic alcoholics. Alcohol 47: 505-515.
- Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, et al. (2004) Induction of ΔFosB in reward-related brain structures after chronic stress. J Neurosci 24: 10594-10602.
- Alibhai IN, Green TA, Potashkin JA, Nestler EJ (2007) Regulation of fosB and ΔfosB mRNA expression: In vivo and in vitro studies. Brain Res 1143: 22-33.
- Teyssier JR, Ragot S, Chauvet-Gélinier JC, Trojak B, Bonin B (2011) Activation of a ΔFOSB dependent gene expression pattern in the dorsolateral prefrontal cortex of patients with major depressive disorder. J AffectDisord133:174-178.
- Dietz DM, Kennedy PJ, Sun H, Maze I, Gancarz AM, et al. (2014) ΔFosB induction in prefrontal cortex by antipsychotic drugs is associated with negative behavioral outcomes. Neuropsychopharmacology 39: 538-544.
- Cooke SF, Bliss TV (2006) Plasticity in the human central nervous system. Brain 129: 1659-1673.
- White AO, Kramár EA (2016) BDNF rescues BAF53b-dependent synaptic plasticity and cocaine-associated memory in the nucleus accumbens. Nat Com mun 7: 11725.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Relapse prevention
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 17659
- [From(publication date):
October-2016 - Dec 04, 2024] - Breakdown by view type
- HTML page views : 16660
- PDF downloads : 999