Research Article Open Access
Abnormal Lipid Accumulation and Cluster Formation of Naive Peripheral Blood Mononuclear Cells: A Useful Tool for Early Detection of Central Nervous System Damage in Elderly
Serchisu Luca1, Peiretti Enrico2, Costaggiu Diego1, Barcellona Doris1, Caminiti Giulia2, Abete Claudia1, Fossarello Maurizio2 and Mandas Antonella1*1Department of Internal Medical Sciences and Public Health, University of Cagliari S.S. 554 bivio Sestu, 09042 Monserrato (CA), Italy
2Department of Surgical Sciences, Eye Clinic, University of Cagliari, Via Ospedale-09124 Cagliari (CA), Italy
- *Corresponding Author:
- Antonella Mandas
Internal Medical Sciences and Public Health Department
University of Cagliari, S.S. 554 bivio Sestu
09042 Monserrato (CA), Italy
Tel: 0039 070 675 4190
E-mail: amandas@unica.it
Received date: May 09, 2017; Accepted date: May 19, 2017; Published date: May 26, 2017
Citation: Serchisu L, Peiretti E, Costaggiu D, Barcellona D, Caminiti G, et al. (2017) Abnormal Lipid Accumulation and Cluster Formation of Naive Peripheral Blood Mononuclear Cells: A Useful Tool for Early Detection of Central Nervous System Damage in Elderly. J Alzheimers Dis Parkinsonism 7:328. doi:10.4172/2161-0460.1000328
Copyright: © 2017 Serchisu L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits nrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Background: There is a great need for new research models, which are simple and accessible diagnostic procedures, in order to monitor the progression of suspected age-related neurological disorders. Objective: To determine whether changes in neutral lipids (NLs), and in the ability to form clusters, represent early events occurring in freshly isolated (naïve) peripheral blood mononuclear cells (PBMCs) from aged subjects affected by different neurological disorders. Methods: We examined 192 subjects, ≥ 65 years old, attending the outpatient services of the geriatric care unit for geriatric check-up, analysing NLs by Oil Red O (ORO) staining method, and the cluster formation (CF) rate of PBMCs. Results: ORO score was higher in PBMCs from subjects with any type of dementia than in PBMCs from healthy controls (HC). ORO score did not differ significantly between Alzheimer's disease (AD), mixed dementia (MD) and vascular dementia (VD), but in mild cognitive impairment (MCI) it was significantly higher than in HC, and significantly lower than in AD, MD and VD. There was also significant inverse correlation between ORO staining and Mini Mental State Examination (MMSE). The percentage of ORO staining intensity, calculated as a percentage of positively stained total cell area, was significantly lower in PBMCs from HC than in those from MCI and dementia. Furthermore, PBMCs from demented patients and MCI groups tend to aggregate in vitro to form cellular clusters: CF rate showed a similar pattern to that of ORO staining. Subjects with dementia but not vision problems had lower ORO staining and CF scores than subjects with both eye disorders and dementia. Conclusion: We suggest that the presence of NLs in the cytoplasm of unstimulated PBMCs, combined with their potential tendency to form clusters, may represent a novel, non-invasive approach to detecting and monitoring neuronal injury in the early stages of disease.
Keywords
Dementia; Cognitive impairment; Neuronal injury; Neutral lipids; Oil Red o staining; Cluster formation; Blood mononuclear cells
Introduction
Age-related neurodegenerative disorders, characterized by progressive deterioration in cognitive ability and capacity for independent living, are frequently causes of disability and mortality in elderly (age 65 years and older) people [1]. Most of them are irreversible and effective treatment does not exist. This is mainly attributable to the scarcity of early detection methods and poor knowledge of the underlying pathogenic mechanisms [2]. This makes it important to find appropriate diagnostic methods for the primary care setting [1]. We previously reported that freshly isolated (naïve) peripheral blood mononuclear cells (PBMCs) from Alzheimer’s disease (AD) affected patients and some of their first-degree relatives are characterized by alterations in cholesterol ester (CE) metabolism, leading to an accumulation of neutral lipids (NLs) in their cytoplasm [3,4]. We suggested that changes in NL content in the PBMCs may reflect altered cholesterol metabolism in the brain, which in turn might contribute to AD pathogenesis. We utilized different methodological approaches to assess NL content, including solvent extraction, thin-layer chromatography (TLC), (14C) acetate labelling, Nile red and Oil Red O (ORO) staining: all of them revealed high levels of NLs in PBMCs from AD patients [3-5]. Among these techniques, ORO staining was the cheapest, fastest and easiest to perform, and was the one that required less material [3-5]. Therefore, we suggested this procedure as a useful tool for early AD detection in clinical practice [2,3]. During our studies, we also noted that PBMCs from AD have, contrary to age-matched healthy controls (HC), an increased tendency to in vitro spontaneous aggregation (cluster formation-CF), thus resembling PBMCs mitogenically activated by phytohemagglutinin (PHA) treatment [3,4]. We assumed that CF in unstimulated in vitro PBMCs might be a sign of their activation in vivo [2]. This observation raised the possibility that the assessment of the rate of unstimulated PBMCs clustering in vitro could be an adjuvant of ORO staining in discriminating between people considered to be normal and those who are or are at risk of being AD affected.
Beside AD, which is the most common cause of dementia in the elderly, accounting for 60-70% of all dementia cases [6,7], there are many other types of age-related dementia. These include the second most common dementia (around 20%), namely vascular dementia (VD), caused by brain blood supply problems, leading to significant accumulated damage to the brain tissue and its functioning [6]. Additionally, it is important to point out that there can be mixed dementia (MD: around 10% of cases), in which both AD and VD characters are present in the same individual. Other forms of dementia are less common (around 10% altogether) [7]. Since AD and VD share many common pathological, symptomatic and neurochemical features [8] there are valid reasons to believe that ORO staining and CF rate may also be useful in early VD and MD diagnosis. In the present study, we determined ORO staining and CF rate in freshly isolated PBMCs from HC and from subjects with AD, VD and MD. In addition, we included samples from individuals with mild cognitive impairment (MCI), which is considered to be an intermediate stage between the expected cognitive decline of normal aging and the more serious decline of dementia [9]. Our aim was to verify whether changes in NLs and in the CF rate of naïve PBMCs might signal a neurological disorder other than AD, and whether they occur during the early stages of the disease.
A number of similarities between AD, VD pathology and several distinct age-related ocular degenerations has also been described in AD patients, in particular cataract, diabetic retinopathy, glaucoma and age-related macular degeneration, all leading causes of vision loss and blindness in the elderly [10]. All these are complex disorders, multifactorial degenerations of Central Nervous System (CNS) tissue, in which age is a primary risk factor, and specific areas of the brain are damaged over time [10]. Therefore, to further validate ORO staining and CF rate as potential biomarkers of CNS injury, we extended the study to these common age-related eye disorders.
Materials and Methods
In this study, we determined ORO staining in 192 subjects 65 years or older. Demographic, clinical and comprehensive geriatric assessment (CGA) information was extrapolated from a previously computerized anonymous database, including 1,320 subjects attending the outpatient geriatric care unit services of the University of Cagliari for geriatric check-up from 2006 to 2014. During examination, either the patient or his/her legal guardian was requested to give written informed consent for these procedures, and also to state whether they agreed to anonymous use of data and blood samples for experimental purposes. The ethical committee of Cagliari University (Italy) approved this procedure. Detailed interviews about medical history and physical examinations were performed. Probable diagnosis of AD was made according to the criteria previously described in detail [11,12]. Diagnoses of possible/ probable VD were made according to the criteria previously described in detail [13]. According to research by Health Quality Ontario [14], neuroimaging evidence (computed tomography scan and/or nuclear magnetic resonance) supported differential diagnosis between AD and VD. MCI is a syndrome defined by clinical, cognitive and functional criteria. Like dementia, it may be due to one or more aetiologies and cannot be diagnosed by a laboratory test. It was determined by changes in cognition faculties compared to the person’s previous check-up results, impairment in one or more cognitive domains, preservation of independence in functional abilities and absence of dementia as previously suggested by Petersen [9]. Subjects without any form of dementia or MCI were taken as a control group (HC).
Dyslipidaemias, hypertension, diabetes, general atherosclerosis and the presence of the most common age-related eye problems were established by examining a careful and detailed medical history obtained by recording interviews, past medical problems and blood test results. Medical records underlining the findings described in the manuscript are deposited in the public repository of the geriatric unit of the University of Cagliari (Italy).
Comprehensive geriatric assessment (CGA)
Functional assessment:
• Activities of Daily Living Scale (ADL) were performed as previously described in detail [15]. The summed score is from 0 to 100; 100 indicating a patient fully independent in physical functioning, and 0 representing a totally dependent bed-ridden state.
• Instrumental Activities of Daily Living scale (IADL) were assessed as previously described in detail [16], a score of 8 indicating total autonomy, and 0, total dependence.
Comorbidities:
• Cumulative Illness Rating Scale (CIRS) was used to evaluate comorbidities as previously described in detail [17]. Severity of pathology in 14 categories was estimated assigning a scoring value from 1 (no impairment) to 5 (extremely severe) to each system. The final score, provided by the sum of the scores for each 14 categories, represents the CIRS-total (CIRS-T). CIRS-maximum organ impairment (CIRS-MI) reflects the highest degree of impairment assigned to one or more categories investigated in the CIRS (ranging 1-5). The total number of categories in which moderate or severe levels (grades 3-5) of disease are quoted (ranging 0-14) gives Comorbidity Index (CIRS-CI). Severity Index (CIRS-SI), reflects the overall severity of diseases and the average rating of 13 disease categories, excluding psychiatric and behavioural problems (ranging 1-5).
Cognitive and mood status:
• Mini-Mental State Examination (MMSE), corrected for age and education, assessed as previously described in detail, was used for evaluating cognitive status [18,19]. Thirty correct-answer points indicate cognitive deficit absence, while a score of zero indicates maximum cognitive deficit. A score of less than 24 out of 30 points suggests cognitive deficit.
• Geriatric Depression Scale (GDS), assessed as previously described in detail [20], was used to assess psychological state. Scores of 0-4 are considered normal, depending on age, education and medical complaints; 5-8 indicate mild depression; 9-11 indicate moderate depression; and 12-15 indicate severe depression.
ORO staining and CF
ORO staining was determined as previously described in detail [3]. Briefly, PBMCs were isolated by carefully layering 500 μl of anticoagulated whole blood onto the upper layer of an equal amount of Ficoll, which were put into 1.5 mL Eppendorf tubes. The tubes were then centrifuged at 5,000x g for 10 min. After centrifugation, PBMCs present as a white ring in the middle of the layer were taken out, with the help of micropipettes, and carefully plated in 6-well tissue culture plates. PBMCs were washed three times with phosphate-buffered saline (PBS), and fixed in 10% neutral-buffered formalin (10 min). Cells were then treated with isopropyl alcohol (60%), washed, stained with 1 ml of ORO working solution and incubated at room temperature for a few minutes. Haematoxylin counterstain was used to visualize the nucleus. ORO was then removed and cells washed 3 times with PBS, 3 min each. After ORO staining, cells were imaged using an inverted phase microscope fitted with a digital camera with magnification x400.
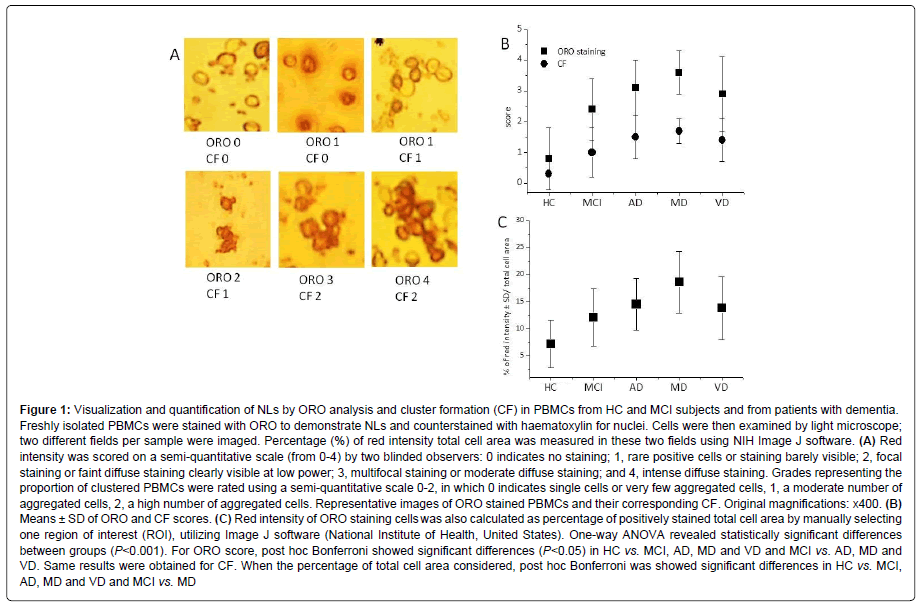
At least two different fields per sample were imaged and analysed. Red intensity was scored on a semi-quantitative scale (from 0-4) by two blinded observers: 0 indicates no staining; 1, rare positive cells or staining barely visible; 2, focal staining or faint diffuse staining clearly visible at low power; 3, multifocal staining or moderate diffuse staining; and 4, intense diffuse staining. Grades representing the proportion of clustered PBMCs were rated using a semi-quantitative scale 0-2, in which 0 indicates single cells or very few aggregated cells, 1, a moderate number of aggregated cells, 2, a high number of aggregated cells. Red intensity of ORO staining cells was also calculated as a percentage of positively stained total cell area by manually selecting one region of interest (ROI), utilizing Image J software (National Institutes of Health, United States).
Statistical Analysis
Quantitative variables were expressed as means ± standard deviation (M ± SD). Data were analysed statistically by the Student t-test for independent samples. All variables were submitted to a Kolmogorov- Smirnov (K-S) goodness of fit test (normal probability test). Since data were normally distributed, we used one way analysis of variance (ANOVA), followed by a Bonferroni post hoc test to compare differences in parameters between various types of dementia and ocular diseases. Chi-square (χ2) test was used for categorical data. Subsequently, we completed Spearman's Rank Correlation Coefficient to determine ORO staining, CF, MMSE and age correlations. Excel and XLSTAT 2014 were used as software for other analyses and graphing.
Results
This study includes 192 participants, of which 68 affected by different types of dementia (20 with AD, 14 with MD, and 34 with VD), 29 patients with MCI and 95 HC. We initially analysed age and CGA data. One-way ANOVA, followed by post-hoc Bonferroni test, showed that the average age between subjects with dementia, MCI and HC was not statistically different (Table 1). MMSE was significantly lower in demented patients than MCI and HC, and significantly higher in HC than in MCI (Table 1). GDS was significantly higher in demented patients than MCI and HC (Table 1). ADL were significantly lower in demented patients than MCI and HC (Table 1). IADL were significantly lower in demented patients than MCI and HC and significantly higher in HC than in MCI (Table 1). Other CGA parameters were not significantly different between demented patients, MCI and HC (Table 1). Furthermore, no significant differences were found about CGA parameters between AD, MD, and VD, except for CIRS-SI (Table 1). K-S test showed that, at the level of alpha significance=0.050, we cannot reject the null hypothesis of no difference between the empirical and theoretical cumulative distributions of CGA parameters in MCI, AD, MD, VD.
| Variables | All | HC | MCI | Dementia | AD | MD | VD |
|---|---|---|---|---|---|---|---|
| n | 192 | 95 | 29 | 68 | 20 | 14 | 34 |
| Gender (M/F) | 68/124 | 43/52 | 15/14 | 10/58 | 4/16 | 4/10 | 2/32 |
| Age, y | 77.7 ± 7.0 | 78.4 ± 6.8 | 77.2 ± 8.6 | 77.0 ± 6.3 | 77.5 ± 6.8 | 73.7 ± 3.6 | 78.1 ± 6.4 |
| MMSE, score | 23.4 ± 5.8 | 27.3 ± 1.7 | 25.6 ± 1.8 | 17.7 ± 5.2 | 18.2 ± 4.3 | 16.1 ± 7.1 | 18.1 ± 4.6 |
| GDS, score | 8.0 ± 4.3 | 6.3 ± 3.8 | 7.8 ± 4.4 | 10.6 ± 3.5 | 11.1 ± 2.8 | 10.4 ± 4.3 | 10.3 ± 3.6 |
| ADLs, score | 75.9 ± 19.4 | 79.0 ± 20.6 | 81.4 ± 9.3 | 71.0 ± 19.9 | 66.9 ± 17.1 | 70.2 ± 19.1 | 75.5 ± 22.7 |
| IADL, score | 4.3 ± 2.4 | 6.2 ± 1.0 | 5.9 ± 0.8 | 1.9 ± 1.7 | 1.6 ± 1.4 | 1.9 ± 1.5 | 2.1 ± 1.9 |
| CIRS-T, score | 33.3 ± 4.4 | 32.6 ± 5.0 | 32.7 ± 4.4 | 34.2 ± 3.5 | 35.1 ± 3.3 | 33.3 ± 4.0 | 34.1 ± 3.2 |
| CIRS-MI | 3.7 ± 0.7 | 3.6 ± 0.7 | 3.5 ± 0.6 | 3.8 ± 0.8 | 3.9 ± 0.7 | 3.8 ± 0.8 | 3.8 ± 0.7 |
| CIRS-SI | 2.3 ± 0.3 | 2.3 ± 0.4 | 2.3 ± 0.3 | 2.4 ± 0.3 | 2.5 ± 0.2 | 2.3 ± 0.3 | 2.3 ± 0.2 |
| CIRS-CI | 7.5 ± 2.0 | 7.4 ± 2.4 | 7.1 ± 2.0 | 7.7 ± 1.7 | 8.4 ± 1.5 | 7.5 ± 1.6 | 7.4 ± 1.7 |
| ORO score | 1.7 ± 1.5 | 0.7 ± 0.8 | 2.0 ± 1.1 | 3.1 ± 1.1 | 3.1 ± 0.8 | 3.3 ± 1.2 | 3.1 ± 1.3 |
| CF score | 0.7 ± 0.9 | 0.2 ± 0.5 | 0.7 ± 0.7 | 1.5 ± 0.8 | 1.6 ± 0.7 | 1.4 ± 0.9 | 1.4 ± 0.9 |
| % of total cell area | 10.7 ± 6.2 | 7.2 ± 4.5 | 12.2 ± 5.4 | 14.9 ± 5.8 | 14.6 ± 4.9 | 18.3 ± 5.9 | 13.8 ± 5.9 |
Results are shown as mean ± standard deviation (SD). Statistically significant differences are indicated in bold type
HC, MCI, Dementia
ANOVA: Age, P=0.406; MMSE, P<0.001; GDS, P<0.001; ADL, P=0.011; IADL, P<0.001; CIRS-T, P=0.064; CIRS-MI, P=0.103; CIRS-SI, P=0.175; CIRS-CI, P=0.411; ORO score, P<0.001; CF score, P<0.001; % of total cell area, P<0.001
Bonferroni post-hoc test:
MMSE, significant differences (P<0.05) in HC vs. MCI and Dementia and MCI vs. Dementia
GDS, significant differences (P<0.05) in Dementia vs. HC and MCI
ADL, significant differences (P<0.05) in Dementia vs. HC and MCI
IADL, significant differences (P<0.05) in HC vs. MCI and Dementia and MCI vs. Dementia
ORO score, significant differences (P<0.05) in HC vs. MCI and Dementia and MCI vs. Dementia
CF score, significant differences (P<0.05) in HC vs. MCI and Dementia and MCI vs. Dementia
% of total cell area, significant difference (P<0.05) in HC vs. MCI and Dementia
AD, MD, VD
ANOVA: Age, P= 0.075; MMSE, P=0.419; GDS, P=0.715; ADL, P=0.317; IADL, P=0.579; CIRS-T, P=0.307; CIRS-MI, P=0.873; CIRS-SI, P=0.005; CIRS-CI, P=0.087; ORO score, P=0.847; CF score, P=0.676; % of total cell area, P=0.046.
Bonferroni post-hoc test:
CIRS-SI, significant differences (P<0.05) in AD vs. MD and VD
% of total cell area, significant difference (P<0.05) in MD vs. VD
Abbreviations: HC: Healthy Controls; MCI: Mild Cognitive Impairment; AD: Alzheimer’s Disease; MD: Mixed Dementia; VD: Vascular Dementia; MMSE: Mini Mental State Examination; GDS: Geriatric Depression Scale; ADL: Activities of Daily Living; IADL: Instrumental Activities of Daily Living; CIRS: Cumulative Illness Rating Scale; CIRS MI: Maximum Impairment; CIRS-SI: Severity Index; CIRS-CI: Comorbidity Index; ORO score: Oil Red O Staining Intensity; CF score: Rate of Cluster Formation; % of total cell area: Percentage of Positively Stained Total Cell Area
Table 1: Comprehensive geriatric assessment parameters, ORO score, CF score and % of total cell area in controls, MCI and demented patients.
Next, we estimated NLs in PBMCs from all enrolled subjects (Table 1 and Figure 1A). Figure 1A shows microphotographs of representative cells assigned to scores 0-4, based on red intensity of lipid droplet accumulation in PBMCs.
The rate of ORO score was significantly higher in PBMCs from demented patients than in those from MCI and HC, and significantly lower in PBMCs from HC than in those from MCI (Table 1 and Figure 1B). The percentage of ORO staining intensity in total cell area was significantly lower in PBMCs from HC than in those from MCI and demented patients (Table 1 and Figure 1C).
ORO staining did not differ significantly between AD, MD and VD groups (Table 1), but, the percentage of ORO staining intensity in total cell area was significantly higher in PBMCs from MD than in those from VD (Table 1). Furthermore, when ANOVA between HC, MCI, AD, MD and VD groups, followed by post hoc Bonferroni, were performed, significant differences (P<0.05) were observed in HC vs. MCI, AD, MD and VD, and MCI vs. AD, MD and VD (Figure 1). Same results were observed for CF rate (Figure 1). When the total cell area percentage was considered, post hoc Bonferroni showed significant differences in HC vs. MCI, AD, MD and VD and MCI vs. MD (Figure 1).
Figure 1: Visualization and quantification of NLs by ORO analysis and cluster formation (CF) in PBMCs from HC and MCI subjects and from patients with dementia. Freshly isolated PBMCs were stained with ORO to demonstrate NLs and counterstained with haematoxylin for nuclei. Cells were then examined by light microscope; two different fields per sample were imaged. Percentage (%) of red intensity total cell area was measured in these two fields using NIH Image J software. (A) Red intensity was scored on a semi-quantitative scale (from 0-4) by two blinded observers: 0 indicates no staining; 1, rare positive cells or staining barely visible; 2, focal staining or faint diffuse staining clearly visible at low power; 3, multifocal staining or moderate diffuse staining; and 4, intense diffuse staining. Grades representing the proportion of clustered PBMCs were rated using a semi-quantitative scale 0-2, in which 0 indicates single cells or very few aggregated cells, 1, a moderate number of aggregated cells, 2, a high number of aggregated cells. Representative images of ORO stained PBMCs and their corresponding CF. Original magnifications: x400. (B) Means ± SD of ORO and CF scores. (C) Red intensity of ORO staining cells was also calculated as percentage of positively stained total cell area by manually selecting one region of interest (ROI), utilizing Image J software (National Institute of Health, United States). One-way ANOVA revealed statistically significant differences between groups (P<0.001). For ORO score, post hoc Bonferroni showed significant differences (P<0.05) in HC vs. MCI, AD, MD and VD and MCI vs. AD, MD and VD. Same results were obtained for CF. When the percentage of total cell area considered, post hoc Bonferroni was showed significant differences in HC vs. MCI, AD, MD and VD and MCI vs. MD
We also found that freshly isolated (unstimulated) PBMCs from demented patients and MCI groups tend to aggregate in vitro in low, medium, or high clusters (Figure 1A), hence resembling cultured PBMCs after mitogen activation with PHA [3,4]. By rating CF using a semi-quantitative scale 0-2, we found that CF follows a similar pattern to ORO staining (Table 1 and Figure 1B).
Since the groups were gender-imbalanced, we also analysed gender distribution by comparing age, CGA, ORO staining (ORO score and % of total cell area) and CF rate in all males and all females and in HC, demented and MCI males and females (Table 2). We found that MMSE score was significantly higher in males than in females, while GDS, ORO score, CF rate and total cell area percentage were significantly lower in males compared to females (Table 2). These changes are probably due to the greater prevalence of dementia in the female group rather than the male one (46.8% vs. 14.7%). There were no differences regarding other parameters considered. Regarding data for HC, we found that GDS, CIRS-T and CF scores were significantly lower in males compared to females (Table 2).
In MCI subjects, we found that GDS and IADL scores were significantly lower in males compared to females, while CIRS-T score was higher in males rather than females (Table 2). ORO score, CF rate and total cell area percentage do not differ regarding MCI in males and females, supporting the hypothesis that changes in these three parameters are present not only in full-blown dementia, but probably also in the early stages of the disease (Table 2).
No significant differences were found between demented men and women in regard to all CGA variables (Table 2). However, we observed, in demented females rather than demented males, a trend to reach greater ORO staining intensity (ORO score and % of total cell area) and CF rate, although only in CF rate it resulted statistically significant (Table 2). These changes are probably due to the imbalance in the number of males and females affected by dementia.
| n | All Males 68 |
All Females 124 |
HC Males 43 |
HC Females 52 |
MCI Males 15 |
MCI Females 14 |
Demented Males 10 |
Demented Females 58 |
|---|---|---|---|---|---|---|---|---|
| AGE | 78.6 ± 6.7 | 77.3 ± 7.0 | 77.9 ± 6.1 | 78.7 ± 7.3 | 79.3 ± 9.3 | 75.8 ± 7.6 | 80.2 ± 5.4 | 76.4 ± 6.3 |
| MMSE, score | 25.5 ± 4.7* | 22.3 ± 6.0 | 27.6 ± 1.8 | 27.0 ± 1.6 | 25.6 ± 2.0 | 25.6 ± 1.8 | 19.4 ± 5.1 | 17.4 ± 5.2 |
| GDS, score | 5.7 ± 3.7* | 9.3 ± 4.1 | 5.1 ± 3.5§ | 7.2 ± 3.8 | 4.9 ± 3.2≠ | 10.9 ± 3.2 | 9.6 ± 2.6 | 10.7 ± 3.7 |
| ADLs, score | 76.8 ± 22.0 | 75.4 ± 18.1 | 80.6 ± 25.4 | 77.8 ± 16.7 | 80.0 ± 8.8 | 82.9 ± 10.1 | 66.0 ± 19.6 | 72.3 ± 19.6 |
| IADL, score | 4.7 ± 2.4 | 4.1 ± 2.5 | 6.0 ± 1.3 | 6.3 ± 0.6 | 5.3 ± 0.7≠ | 6.4 ± 0.5 | 1.2 ± 1.5 | 2.0 ± 1.7 |
| CIRS-T, score | 32.8 ± 5.4 | 33.5 ± 3.8 | 29.8 ± 5.3§ | 34.1 ± 4.1 | 34.7 ± 4.0≠ | 30.7 ± 3.8 | 35.8 ± 4.2 | 33.7 ± 3.2 |
| CIRS-MI | 3.7 ± 0.7 | 3.6 ± 0.7 | 3.5 ± 0.6 | 3.6 ± 0.7 | 3.8 ± 0.4 | 3.2 ± 0.6 | 4.0 ± 0.8 | 3.8 ± 0.7 |
| CIRS-SI | 2.3 ± 0.4 | 2.3 ± 0.3 | 2.1 ± 0.4 | 2.4 ± 0.3 | 2.5 ± 0.3 | 2.1 ± 0.2 | 2.5 ± 0.4 | 2.3 ± 0.2 |
| CIRS-CI | 7.3 ± 2.2 | 7.6 ± 1.9 | 6.5 ± 2.3 | 7.9 ± 2.2 | 7.9 ± 2.0 | 6.3 ± 1.8 | 8.0 ± 1.8 | 7.7 ± 1.6 |
| ORO score | 1.3 ± 1.3* | 2.0 ± 1.5 | 0.7 ± 0.8 | 0.7 ± 0.8 | 2.1 ± 1.2 | 1.9 ± 1.2 | 2.6 ± 1.4 | 3.2 ± 1.1 |
| CF score | 0.4 ± 0.7* | 0.9 ± 0.9 | 0.1 ± 0.4§ | 0.3 ± 0.5 | 0.7 ± 0.7 | 0.7 ± 0.7 | 1.0 ± 0.9Ë? | 1.6 ± 0.8Ë? |
| % total cell area | 9.1 ± 5.5* | 11.6 ± 6.5 | 6.9 ± 3.8 | 7.5 ± 5.0 | 12.0 ± 4.5 | 12.4 ± 6.5 | 14.2±7.6 | 15.1 ± 5.5 |
Results are shown as mean ± standard deviation (SD). Statistically significant differences are indicated in bold type
t-test:
* males vs. females: MMSE, P=0.000; GDS, P=0.000; ORO score, P=0.001; CF score, P=0.000, % of total cell area, P=0.008
§ HC males vs. HC females: GDS, P=0.007; CIRS-T, P=0.006; CF score, P=0.037
≠ males with MCI vs. females with MCI GDS, P=0.000; IADL, P=0.000; CIRS-T, P=0.01
Ë? Demented males vs. Demented females: CF score, P=0.035
Abbreviations: HC: Healthy Controls; MCI: Mild Cognitive Impairment; MMSE: Mini Mental State Examination; GDS: Geriatric Depression Scale; ADL: Activities of Daily Living; IADL: Instrumental Activities of Daily Living; CIRS: Cumulative Illness Rating Scale; CIRS MI: Maximum Impairment; CIRS-SI: Severity Index; CIRS-CI: Comorbidity Index; ORO score: Oil Red O Staining Intensity; CF score: Rate of Cluster Formation; % of total cell area: Percentage of Positively Stained Total Cell Area.
Table 2: Base statistical gender distribution in our study population.
A number of studies have linked cardiovascular disease to cognitive impairment and dementia [21,22]. To exclude a possible confounding effect of cardiovascular disease on ORO staining and CF scores, we also estimated the impact of the most common cardiovascular risk factors (hypertension, atherosclerosis, dyslipidaemia, diabetes) on these parameters; as shown in Table 3, no association was found between ORO staining and CF variables and cardiovascular risk factors, thus indicating low probability that these risk factors may influence the levels of these variables.
| Participants (numbers) |
Dyslipidaemia No Yes |
Atherosclerosis No Yes |
Diabetes No Yes |
Hypertension No Yes |
|---|---|---|---|---|
| % cell area High Low χ2 P-value |
6545 5329 0.609 0.435 |
4070 3052 0.001 0.975 |
7139 5329 0.000 0.990 |
2882 1666 0.939 0.333 |
| ORO score High Low χ2 P-value |
6046 5828 2.354 0.125 |
3967 3155 0.011 0.915 |
7036 5432 0.219 0.640 |
2587 1967 0.060 0.807 |
| CF score High Low χ2 P-value |
6344 5530 0.679 0.410 |
4067 3055 0.089 0.765 |
7235 5233 0.774 0.379 |
2780 1768 0.735 0.391 |
Table 3: Relationship between cardiovascular risk factors and ORO staining and CF score.
To maximize the statistical power of the study, we merged data collected from the entire population (n=192) and performed Spearman correlation analysis between age and MMSE, age and ORO staining and between MMSE and ORO staining. Spearman's coefficient showed a moderate but significant correlation coefficient between MMSE and age (r=0.150, P=0.037). No significant correlation was observed between ORO score and % of total cell area and age (r=-0.078, P=0.284; r=-0.087, P=0.228, respectively). A significant and negative correlation is evident between the MMSE and ORO score (r=-0.699, P<0.001) and MMSE and % of total cell area (r=-0.586, P<0.001). In general, these results suggest that normal aging has little effect on the changes in ORO staining found in PBMCs from AD and MCI subjects.
Cataract, glaucoma, diabetic retinopathy and age-related macular degeneration are the four most common eye conditions among people over age 65 and share a number of striking similarities with AD, MD and VD [23,24]. Consequently, as a final point, we conducted separate analyses to examine whether and how these related eye problems may influence ORO staining and CF score.
In our study population, eye diseases were detected in 52 of the 192 enrolled subjects, giving prevalence of 27.1%; of these, 24 subjects suffered from cataract, 8 glaucoma, 8 age-related macular degeneration and 12 diabetic retinopathy. Eye disorders in relation to all types of cognitive impairment are summarized in Table 4. The small number of participants for each type of disease made it difficult to achieve statistical comparisons between subgroups. Participants were divided into two groups according to the presence or absence of eye disorders and subdivided in subgroups according to the absence of cognitive impairment or the presence of dementia or MCI (HC with or without eye disorders; MCI with or without eye disorders; demented patients with or without eye disorders). These groups were compared to CGA parameters and ORO staining and CF rate (Table 5). The t-test showed that, in the group of all patients with at least an eye disorder, GDS, CIRS-T and CIRS-CI scores were significantly higher than all subjects without eye disorders. In the group of cognitively unimpaired subjects with at least an eye disorder, GDS, CIRS-T, CIRS-SI and CIRS-CI scores were significantly higher and MMSE and IADL scores were significantly lower compared to cognitively unimpaired subjects without eye disorders. Demented patients with at least an eye disorder had GDS, CIRS-T, ORO and CF scores significantly higher compared to demented patients without eye disorders. Although failing to reach significance, the percentage of ORO staining of total cell area was lower in demented patients without eye disorders compared with demented patients with at least an eye disorder. T-test was not performed between MCI subjects with or without eye disorders given the small number of participants of the second group.
| Tot | HC | MCI | Dementia | AD | DV | DM | |
|---|---|---|---|---|---|---|---|
| No Eye Disorders | 140 | 65 | 25 | 50 | 16 | 24 | 10 |
| All Eye Disorders | 52 | 30 | 4 | 18 | 4 | 10 | 4 |
| Cataract | 24 | 10 | 2 | 12 | 2 | 8 | 2 |
| Age-related macular degeneration | 8 | 6 | 0 | 2 | 2 | 0 | 0 |
| Glaucoma | 8 | 8 | 0 | 0 | 0 | 0 | 0 |
| Diabetic retinopathy | 12 | 6 | 2 | 4 | 0 | 2 | 2 |
Abbreviations: HC: Healthy Controls; MCI: Mild Cognitive Impairment; AD: Alzheimer’s Disease; MD: Mixed Dementia; VD: Vascular Dementia χ2: No significant differences (P=0.167) were observed between HC, patients with Dementia, patients with MCI and patients with or without eye disorders.
Table 4: Eye disorders in relation to all types of cognitive impairment.
Discussion
In this study, we found that freshly isolated (naive) PBMCs from subjects with dementia accumulate NLs, as determined in ORO staining, irrespective of dementia type: ORO score in PBMCs from demented patients and MCI was significantly higher than in HC, and significantly lower in MCI compared to demented patients. Moreover, while no significant differences in ORO score and CF rate were found between AD, MD and VD groups, the percentage of ORO staining intensity in total cell area was significantly higher in PBMCs from MD than in those from VD. It has also been shown that ORO staining in all enrolled subjects was not age-correlated, suggesting that, in an elderly population, NL levels in PBMCs increase with cognitive impairment severity and are not influenced by aging. We also observed, in demented females, a tendency to reach greater ORO staining intensity (ORO score and % of total cell area) and CF rate, although only in CF rate it resulted statistically significant. This is probably a consequence of the imbalance in the number of dementia affected between genders, with a greater prevalence of dementia in the female group rather than the male one (46.8% vs. 14.7%). Interestingly, ORO score, % of total cell area and CF rate do not differ in MCI males and females, suggesting that changes in these three parameters are present not only in fullblown dementia, but probably also in the early stages of disease.
These results are in agreement with our earlier observations which showed that skin fibroblasts and PBMCs from patients with diagnosis of probable sporadic AD display an imbalance between free cholesterol and CE pools due to cytoplasmic NL accumulation, mainly CEs [3,4]. In addition, they seem to be consistent with other research, which either found altered lipid composition-cholesterol, in particular-in the brains of patients affected by different neurodegenerative diseases [1,25] or a potential link between diet-induced alterations in brain cholesterol metabolism and β-site AβPP-cleaving enzyme 1 (BACE1) overexpression, which represents an early event in AD development [26]. Dysregulation of cholesterol homeostasis in the brain has, in fact, been linked to chronic neurodegenerative disorders, including AD, Huntington’s disease, Parkinson’s disease, Niemann-Pick type C disease and Smith-Lemli Opitz syndrome, as well as to acute neuronal injuries such as stroke and brain trauma [1,25]. The first clear proof for a critical role of CEs in the pathogenesis of neurological disorders derives from observations by Huttunen and Kovacs [27], who focused their studies on acyl-coenzyme A:cholesterol acyltransferase (ACAT), an endoplasmic reticulum membrane-bound enzyme that catalyses the conversion of free cholesterol to CEs, which coalesce to form lipid droplets in the cytoplasm. They found that cells with elevated CE levels produced more amyloid-β (Aβ) peptides, which are crucially involved in AD as the main component of the amyloid plaques [28,29]. By contrast, cells that lacked CEs, but had elevated free cholesterol, produced almost no Aβ. The researchers also found that ACAT inhibitors CP-113,818 and CI 1011 administered to AD-affected mice significantly reduced amyloid plaques and rescued cognitive deficits.
| No Eye Disorders (140) M ± SD |
All Eye Disorders (52) M ± SD |
HC without Eye disorders (65) M ± SD |
HC with an Eye disorder (30) M ± SD |
MCI without Eye disorders (25) M ± SD |
MCI with an Eye disorder (4) M ± SD |
Dementia without Eye Disorders (50) M ± SD |
Dementia with Eye Disorders (18) M ± SD |
|
|---|---|---|---|---|---|---|---|---|
| Age, y | 77.9 ± 7.1 | 77.4 ± 6.7 | 78.0 ± 6.4 | 79.1 ± 7.5 | 78.8 ± 8.2 | 68 ± 4.1 | 77.2 ± 7.2 | 76.7 ± 2.7 |
| MMSE, score | 23.7 ± 5.8 | 23.4 ± 4.9 | 27.5 ± 1.7 | 26.7 ± 1.6** | 25.9 ± 1.9 | 24.0 ± 0 | 17.7 ± 5.6 | 17.7.0 ± 3.9 |
| GDS, score | 7.0 ± 4.1 | 10.7 ± 3.3* | 4.9 ± 3.5 | 9.2 ± 2.3** | 7.7 ± 4.4 | 8.5 ± 4.6 | 9.4 ± 3.3 | 13.7 ± 2.0# |
| ADLs, score | 75.9 ± 19.7 | 75.8 ± 19.0 | 79.5 ± 23.3 | 78.1 ± 15.5 | 79.9 ± 8.6 | 94.0 ± 4.0 | 71.3 ± 18.8 | 70.7 ± 21.8 |
| IADL, score | 4.3 ± 2.5 | 4.3 ± 2.3 | 6.5 ± 1.1 | 5.8 ± 0.4** | 5.8 ± 0.8 | 6.0 ± 1.1 | 1.8 ± 1.5 | 2.0 ± 1.9 |
| CIRS-T, score | 32.3 ± 4.6 | 35.7 ± 0.7* | 30.8 ± 5.4 | 35.7 ± 1.7** | 32.9 ± 4.6 | 31.0 ± 1.1 | 33.2 ± 3.5 | 36.3 ± 2.4# |
| CIRS-MI | 3.6 ± 0.7 | 3.7 ± 0.4 | 3.5 ± 0.6 | 3.7 ± 0.7 | 3.6 ± 0.6 | 3.0 ± 0.6 | 3.8 ± 0.8 | 3.9 ± 0.6 |
| CIRS-SI | 2.3 ± 0.3 | 2.5 ± 0.2 | 2.2 ± 0,4 | 2.5 ± 0.2** | 2.3 ± 0.3 | 2.1 ± 0.1 | 2.3 ± 0.3 | 2.5 ± 0.2 |
| CIRS-CI | 7.1 ± 2.0 | 8.4 ± 1.7* | 6.6 ± 2.4 | 8.7 ± 1.7** | 7.1 ± 2.1 | 7.5 ± 0.7 | 7.6 ± 1.6 | 8.1 ± 1.8 |
| ORO score | 1.8 ± 1.5 | 2.1 ± 1.6 | 0.7 ± 1.0 | 1.0 ± 1.2 | 2.3 ± 1.3 | 3.0 ± 1.3 | 2.9 ± 1.2 | 3.7 ± 0.5# |
| CF score | 1.0 ± 0.8 | 1.0 ± 0.9 | 0.3 ± 0.4 | 0.5 ± 0.6 | 1.0 ± 0.8 | 1.2 ± 0.8 | 1.3 ± 0.7 | 1.9 ± 0.3# |
| % of total cell area | 10.5 ± 6.0 | 11.2 ± 6.8 | 7.0 ± 4.0 | 7.7 ± 5.4 | 11.6 ± 4.5 | 15.7 ± 9.6 | 14.5 ± 6.1 | 16.1 ± 4.8 |
t test:
*No Eye Disorders vs. All Eye Disorders: GDS P<0.001; CIRS-T P<0.001; CIRS-CI P=0.003
**HC without Eye disorders vs. HC with an Eye disorder: MMSE P=0.032; GDS P<0.001; IADL P=0.001; CIRS-T P<0.001; CIRS-SI P=0.009; CIRS-CI P=0.005
MCI without Eye disorders vs. MCI with an Eye disorder: comparison not evaluated because of small number of Eye Disorders among MCI subjects
#Dementia without Eye Disorders vs. Dementia with Eye Disorders: GDS P<0.001; CIRS-T P=0.005; ORO score P=0.008; CF score P=0.002
Abbreviations: MMSE: Mini Mental State Examination; GDS: Geriatric Depression Scale; ADL: Activities of Daily Living; IADL: Instrumental Activities of Daily Living; CIRS: Cumulative Illness Rating Scale; CIRS MI: Maximum Impairment; CIRS-SI: Severity Index; CIRS-CI: Comorbidity Index; ORO score: Oil Red O Staining Intensity; CF score: Rate of Cluster Formation; % of total cell area: Percentage of Positively Stained Total Cell Area
Table 5: CGA parameters, ORO score, CF score and % of total cell area in relation to cognitive impairment and eye disorders.
Following that, Bryleva et al. [30], using a combined mouse-genetic and biochemical approach to evaluate the potential role of ACAT in AD, showed that ACAT1 gene ablation in triple AD transgenic mice leads to more than 60% reduction in full-length human Aβ precursor protein (AβPP), as well as in its proteolytic fragments, and ameliorates cognitive deficits. These exciting findings led them to hypothesize that ACAT inhibitors might be of therapeutic value in the treatment of cognitive impairment. Overall, these data clearly indicate a direct association between impaired cholesterol metabolisms in the brain during neurodegeneration; however, whether a common molecular mechanism links the neurodegenerative phenotype to altered brain cholesterol metabolism remains to be established. This is an important point, because if this link existed, it would mean that a common therapeutic approach for these diseases might be possible. Awareness of these problems and possible solutions to them is severely limited by the inaccessibility of direct examination of the living human brain; for this reason, potential surrogate tissues must be utilized. In this context, PBMCs appear particularly attractive because they seem to participate directly in neurodegenerative processes. PBMCs have been shown to share much of the non-synaptic biochemical environment of neurons, and contain the full complement of epigenetic enzymes and machinery that are found in both neurons and peripheral nucleated cells, as in most other tissues, as suggested by Arosio et al. [31]. The present results show that NL accumulation in PBMC cytoplasm is not restricted to AD, but may also occur in other diseases characterized by the deterioration of cognitive functions. These changes have also been observed in MCI, but with less magnitude and extent. The control subjects, cognitively healthy and free of any neurological or psychiatric illnesses, had minimal NL content changes in their PBMCs, as previously reported by us [2-4,32]. Interestingly, NL content was significantly correlated to clinical severity, as assessed by MMSE [2- 4,32]. These results led us to hypothesize that NL changes in PBMCs might reflect dynamic modifications in cholesterol homeostasis, which occur in the brain during early development of cognitive deficit [2- 4,32]. Another interesting finding of this study was that PBMCs from dementia and MCI groups, freshly isolated from buffy coat and plated in 6-well tissue culture dishes, merged together to form large aggregates or clusters, which are visible under light microscope. By rating cellclustering using a semi-quantitative scale ranging from 0 to 2 we found that it follows a pattern similar to ORO staining, with CF score being significantly lower in HC than in the other four groups (MCI, AD, MD and VD) and higher in AD, MD and VD than in MCI. The mechanism by which PBMCs from dementia and MCI subjects form clusters remains to be determined. However, since clusters observed in dementia and MCI cells resemble those seen in PHA-stimulated PBMCs [2,3], we assumed that cluster formation in the unstimulated cultures of PBMCs may reflect the fact that they pre-exist in in vivoactivated state, secondary to the release of inflammatory mediators, such as cytokines, by brain cells [2-4,31]. Studies have consistently documented the presence of sustained inflammatory responses, involving glial cells in the early stages of neurodegeneration [33]. It is generally agreed that brain injury results in the immediate recruitment of glial cells to the damaged area, where they become activated and secrete cytokines and chemokines [34-36]. It should be stressed that the term ‘glial cell activation’ is not specific, and may reflect different pathological states. Inflammatory plasma-protein levels (i.e., interleukin-1, interleukin-6 and a-1-antichymotrypsin) have been found to increase before clinical onset of dementia in patients with AD and VD. Detection of circulating cytokines has also been proposed as a potential biomarker for screening, diagnosis and monitoring of neurodegenerative diseases and other neurological pathologies [37,38] as well as a tool to evaluate the progression of brain inflammation associated with cerebrovascular disease. Taken together, these data indicate that profound alterations in cholesterol metabolism, associated with signals of enhanced inflammatory response, occur both in the periphery and in the CNS, in the early stages of many neurological disorders, but whether these changes are a cause or a consequence of neurodegeneration remains uncertain. In addition, no clear molecular mechanism has yet emerged that would directly relate alterations in cholesterol homeostasis and neuroinflammation in the brain to molecular changes occurring in peripheral cells. Based on the information currently available and that of the present study, we propose a plausible mechanism linking changes in cholesterol metabolism and brain neurodegenerative phenotype inflammation, and explaining how changes in NLs and in PBMC cluster formation may reflect these effects. There is ample evidence that neuronal function and survival is compromised by neuroinflammation, which may be initiated as the result of a variety of causes, including infection, traumatic brain injury, toxic metabolites and autoimmunity [33,36] and also by perturbed cholesterol homeostasis, which may occur as a consequence of genetic and/or environmental factors [2]. So, it may be argued that these two processes may be mutually dependent upon each other, indicating that altered cholesterol homeostasis may induce neuroinflammation and vice versa. In any case, this would lead to neuronal damage. Response to neuronal damage includes activation of resident immune cells (microglia), resulting in a phagocytic phenotype and in the release of inflammatory mediators, such as cytokines and chemokines, which could pass into the systemic circulation, leading to PBMC activation [36]. This activation may be responsible for the observed PBMC cluster formation in vitro. On the other hand, following brain injury, damaged cells may release free cholesterol, which is taken up by neighbouring neurons, where, at first, it is presumably esterified by ACAT1 and stored within cytoplasmic lipid droplets. If in excess, free cholesterol, which cannot pass the blood brain barrier (BBB), is hydroxylated in neurons to 24(S)- hydroxycholesterol (24-OHC) and transferred into the blood circulation. At this point it should be mentioned that 24-OHC is an oxygenated cholesterol derivative and, consequently, a potential inducer of LDL oxidation. Consistent with this, increased levels of circulating oxidized low-density lipoprotein (LDLox) were found in patients affected by neurological disorders [2,32]. Through a mechanism similar to that described for atherosclerosis, these LDLox, which can be recognized by receptors on the surface of PBMCs, release CEs, once internalized, transforming PBMCs into foam cells. Such a scenario may explain why subjects with neurological disorders are characterized by an accumulation of CEs in the cytoplasm of their PBMCs [2,32]. 24-OHC can be responsible for amplifying neuronal damage in AD by inducing oxidative stress, as well as net synthesis of Aβ1-42 by up-regulating expression levels of AβPP and b-secretase and its activity [39,40].
The retina contains a specialized type of glia, the Müller glia, not found anywhere else in the CNS. Like other glial cells of the CNS, Müller cells undergo reactive gliosis following acute retinal injury or chronic neuronal stress [41]. Growing evidence in clinical and experimental studies strongly suggests involvement of Muller glia activation in the pathogenesis of the most common age-related eye disorders, including cataract, glaucoma, diabetic retinopathy and age-related macular degeneration. In addition, high CE levels, which tend to become oxidized into oxysterols, have also been reported in several eye lesions in particular, drusen and in ageing Bruch’s membrane, which are all hallmark features of age-related macular degeneration [41-43]. We thus sought to determine whether unstimulated PBMCs derived from subjects with various age-related eye diseases have the capacity to self-assemble into clusters and to accumulate NLs. To do this, participants were divided into two groups, according to the presence or absence of eye disorders. Each group was further divided according to the absence of cognitive impairment or the presence of dementia or MCI (HC with or without eye disorders; MCI with or without eye disorders; demented patients with or without eye disorders). No significant difference in ORO and CF scores and percentage of cell area red intensity was observed between subjects with ocular disorders and those without ocular disorders. However, ORO and CF scores were significantly higher in demented patients with at least an eye disorder than demented patients without eye disorders, and, although failing to reach significance, the percentage of ORO staining of total cell area was lower in this second group compared to the first. These results indicate that eye damage may exacerbate NL accumulation and the rate of cell clustering observed in PBMCs of demented patients.
It has been previously established that MMSE is not well suited for identifying MCI, which raises the question whether it is an adequate "standard" to be used to assess cognitive performance. The aim of the present work was to characterize potential biological marker(s), which could be used either in early diagnosis or for prediction of the risk of severe neurological symptom development. Unlike other organ-based diseases, where diagnosis can be done rapidly by using biomarkersusually involving blood tests-there are no rapid, definitive biochemical markers to detect potential brain injury early, nor is there a gold standard for subject stratification by neurological injury severity, or for monitoring injury progression. In this context, it appears imperative to develop tools that might facilitate disease state assessment and new drug efficacy, in particular, tools that may help to predict the early stages of the disease and the long period of time that precedes the onset of manifest signs. In fact, an effective therapeutic approach is most likely to be successful in pre-manifest subjects who have not yet developed irreversible neurodegeneration. Here, we present a novel, non-invasive approach for early neuronal injury detection based on the presence of NLs in the cytoplasm of unstimulated- PBMCs, combined with their potential tendency to form clusters. This approach is the result of two independent lines of investigation: the first derives from the general problem of cholesterol metabolism in the CNS: a constant equilibrium between synthesis and degradation is maintained through cholesterol-to-24-OHC oxidation-a metabolite able to cross BBB-the blood circulation levels of which are taken as an index of brain cholesterol elimination [2,32]; the second, from the connection between inflammation and neurodegeneration: the immune response of the CNS is orchestrated by microglial cells, which, on activation, become phagocytes and secrete a wide range of inflammatory mediators, including cytokines and chemokines, growth factors, complement molecules and adhesion molecules [2,32]. Inflammatory response detectable in the periphery is considered an index of neurological disease progression [44].
Conclusion
In summary, our data demonstrate that: (i) NLs measurable in naïve PBMCs are shown to parallel a critical phase of neuronal loss in disease characterized by progressive brain damage; (ii) NLs and CF in PBMCs appear to be a valuable tool to discriminate pre-manifest subjects from neurological patients and might, therefore, be helpful to investigate subjects before the onset of neural symptoms. To our knowledge, this is the first evidence indicating that neurodegenerative processes could be monitored by testing the effects of compounds generated by altered CNS metabolism on PBMCs. To further validate these data and their clinical implications, larger cross-sectional and longitudinal studies are essential. Furthermore, discovering if ORO score and CF remain low in MCI patients who do not develop the overt disease could be very important. Further longitudinal studies in this matter will be necessary.
Acknowledgement
This paper is dedicated to the memory of Prof. Sandra Dessì.
References
- Rahman K (2007) Studies on free radicals, antioxidants and co-factors. Clin Interv Aging 2: 219-236.
- Mandas A, Dessì S (2014) Mononuclear cells in dementia. Clin Chim Acta 431: 278-287.
- Pani A, Mandas A, Diaz G, Abete C, Cocco PL, et al. (2009) Accumulation of neutral lipids in peripheral blood mononuclear cells as a distinctive trait of Alzheimer patients and asymptomatic subjects at risk of disease. BMC Med 7: 66.
- Mandas A, Abete C, Putzu PF, la Colla P, Dessì S, et al. (2012) Changes in cholesterol metabolism-related gene expression in peripheral blood mononuclear cells from Alzheimer patients. Lipids Health Dis 11: 39.
- Pani A, Dessì S, Diaz G, La Colla P, Abete C, et al. (2009) Altered cholesterol ester cycle in skin fibroblasts from patients with Alzheimer's disease. J Alzheimers Dis 18: 829-841.
- Fratiglioni L, De Ronchi D, Agüero-Torres H (1999) Worldwide prevalence and incidence of dementia. Drugs Aging 15: 365-375.
- Fratiglioni L, Rocca W (2001) Epidemiology of dementia. In Handbook of neuropsychology: Aging and dementia, Boller F, Cappa SF (eds). Elsevier Sci Publ, Amsterdam, pp: 193-215.
- Kalaria R (2002) Similarities between Alzheimer's disease and vascular dementia. J Neurol Sci 203-204: 29-34.
- Petersen RC (2003) Conceptual overview. In mild cognitive impairment: Aging to Alzheimer’s disease, Petersen RC. Oxford University Press, New York, pp: 1-14.
- Sivak JM (2013) The aging eye: Common degenerative mechanisms between the Alzheimer’s brain and retinal disease. Invest Ophthalmol Vis Sci 54: 871-880.
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, et al. (1984) Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology 34: 939-944.
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, et al. (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the national institute on aging and the Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7: 263-269.
- Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, et al. (1993) Vascular dementia: Diagnostic criteria for research studies. Report of the NINDSAIREN International Workshop. Neurology 43: 250-260.
- Health Quality Ontario (2014) The appropriate use of neuroimaging in the diagnostic work-up of dementia: an evidence-based analysis. Ont Health Technol Assess Ser 14: 1-64.
- Shah S, Vanclay F, Cooper B (1989) Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol 42: 703-709.
- Lawton MP, Brody EM (1969) Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 9: 179-186.
- Parmelee PA, Thuras PD, Katz IR, Lawton MP (1995) Validation of the cumulative illness rating scale in a geriatric residential population. J Am Geriatr Soc 43: 130-137.
- Folstein MF, Folstein SE, McHugh PR (1975) “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189-198.
- Measso G, Cavarzeran F, Zappalà G, Lebowitz BD, Crook TH, et al. (1993) The mini-mental state examination: Normative study of a random sample of Italian population. Dev Neuropsychol 9: 77-85.
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, et al. (1983) Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res 17: 37-49.
- Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, et al. (2006) Heart failure and risk of dementia and Alzheimer disease: A population-based cohort study. Arch Intern Med 166: 1003-1008.
- van Oijen M, de Jong FJ, Witteman JC, Hofman A, Koudstaal PJ, et al. (2007) Atherosclerosis and risk for dementia. Ann Neurol 61: 403-410.
- Rumney NJ (1998) The aging eye and vision appliances. Ophthalmic Physiol Opt 18: 191-196.
- Cronin-Golomb A, Corkin S, Rizzo JF, Cohen J, Growdon JH, et al. (1991) Visual dysfunction in Alzheimer's disease: Relation to normal aging. Ann Neurol 29: 41-52.
- Vance JE (2012) Dysregulation of cholesterol balance in the brain: Contribution to neurodegenerative diseases. Dis Model Mech 5: 746-755.
- Mastrocola R, Guglielmotto M, Medana C, Catalano MG, Cutrupi S, et al. (2011) Dysregulation of SREBP2 induces BACE1 expression. Neurobiol Dis 44: 116-124.
- Huttunen HJ, Kovacs DM (2008) ACAT as a drug target for Alzheimer's disease. Neurodegener Dis 5: 212-214.
- Puglielli L, Konopka G, Pack-Chung E, Ingano LA, Berezovska O, et al. (2001) Acyl-coenzyme A: Cholesterol acyltransferase modulates the generation of the amyloid beta-peptide. Nature Cell Biol 3: 905-912.
- Hutter-Paier B, Huttunen HJ, Puglielli L, Eckman CB, Kim DY, et al. (2004) The ACAT inhibitor CP-113,818 markedly reduces amyloid pathology in a mouse model of Alzheimer’s disease. Neuron 44: 227-238.
- Bryleva EY, Rogers MA, Chang CC, Buen F, Harris BT, et al. (2010) ACAT1 gene ablation increases 24(S)-hydroxycholesterol content in the brain and ameliorates amyloid pathology in mice with AD. Proc Natl Acad Sci U S A 107: 3081-3086.
- Arosio B, D'Addario C, Gussago C, Casati M, Tedone E, et al. (2014) Peripheral blood mononuclear cells as a laboratory to study dementia in the elderly. Biomed Res Int 2014: 169203.
- Anchisi L, Dessì S, Pani A, Mandas A (2013) Cholesterol homeostasis: A key to prevent or slow down neurodegeneration. Front Physiol 3: 486.
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140: 918-934.
- Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308: 1314-1318.
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J (2009) Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci 29: 3974-3980.
- Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG (2009) Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener 4: 47.
- Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, et al. (2004) Inflammatory proteins in plasma and the risk of dementia: The Rotterdam study. Arch Neurol 61: 668-672.
- Bettcher BM, Kramer JH (2013) Inflammation and clinical presentation in neurodegenerative disease: A volatile relationship. Neurocase 19: 182-200.
- Gamba P, Leonarduzzi G, Tamagno E, Guglielmotto M, Testa G, et al. (2011) Interaction between 24-hydroxycholesterol, oxidative stress and amyloid-bin amplifying neuronal damage in Alzheimer’s disease: Three partners in crime. Aging Cell 10: 403-417.
- Gamba P, Guglielmotto M, Testa G, Monteleone D, Zerbinati C, et al. (2014) Up-regulation of b-amyloidogenesis in neuron-like human cells by both 24- and 27-hydroxycholesterol: Protective effect of N-acetyl-cysteine. Aging Cell 13: 561-572.
- Curcio CA, Presley JB, Millican CL, Medeiros NE (2005) Basal deposits and drusen in eyes with age related maculopathy: Evidence for solid lipid particles. Exp Eye Res 80: 761-775.
- Curcio CA, Presley JB, Malek G, Medeiros NE, Avery DV, et al. (2005) Esterified and unesterified cholesterol in drusen and basal deposits of eyes with age-related maculopathy. Exp Eye Res 81: 731-741.
- Peiretti E, Mandas A, Abete C, Vinci M, Piludu S, et al. (2014) Age-related macular degeneration and cognitive impairment show similarities in changes of neutral lipids in peripheral blood mononuclear cells. Exp Eye Res 124: 11-16.
- Khan TK, Alkon DL (2006) An internally controlled peripheral biomarker for Alzheimer’s disease: Erk1 and Erk2 responses to the inflammatory signal bradykinin. Proc Natl Acad Sci U S A 103: 13203-13207.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 2777
- [From(publication date):
June-2017 - Dec 23, 2024] - Breakdown by view type
- HTML page views : 2134
- PDF downloads : 643