Ability of a Clinical Stage LMW-DS Drug to Inhibit Coronavirus Infection of Cells and Suppress Cytokine Secretion from Human Microglia
Received: 19-Jan-2023 / Manuscript No. JIDT-23-87408 / Editor assigned: 24-Jan-2023 / PreQC No. JIDT-23-87408 (PQ) / Reviewed: 06-Feb-2023 / QC No. JIDT-23-87408 / Revised: 13-Feb-2023 / Manuscript No. JIDT-23-87408 (R) / Published Date: 20-Feb-2023 DOI: 10.4172/2332-0877.1000531
Abstract
Most coronaviruses infect animals including bats, birds and mammals, which act as hosts and reservoirs for the viruses, but the viruses can sometimes move host species and infect humans. Coronaviruses were first identified as human pathogens in the 1960s and now there are seven types known to infect humans. Whilst four of these types cause mild-to-moderate respiratory disease, the other three may cause more severe and possibly even fatal disease in vulnerable individuals particularly, with the most recent SARS-CoV-2 pandemic being associated with Severe Acute Respiratory Syndrome (SARS) in many infected people. The aim of the present study was to evaluate the potential of a unique Low Molecular Weight Dextran Sulphate (LMW- DS) clinical stage drug, ILB®, to inhibit infection of human cells by the NL63 coronavirus assessed by immunofluorescence of viral particles, and also to see if the drug directly blocked the interaction of the SARS-CoV-2 viral spike protein with the ACE2 receptor. Furthermore, we evaluated if ILB® could modulate the downstream consequences of viral infection including the reactive cytokine release from human microglia induced by various SARS- CoV-2 variant spike proteins. We demonstrated that ILB® blocked ACE2:spike protein interaction and inhibited coronaviral infection. ILB® also attenuated the Omicron-induced release of pro-inlammatory cytokines, including TNFa, from human microglia, indicating control of post-viral neuroinflammation. In conclusion, given the safety profile of ILB® established in a number of Phase I and Phase II clinical trials, these results highlight the potential of ILB® to treat patients infected with coronaviruses to both limit infectivity and attenuate the progression to severe disease. There is now an opportunity to translate these findings quickly by the clinical investigation of drug efficacy.
Keywords: SARS-CoV-2; LMW dextran sulphate; ACE-2 receptor; Neuroinflammation; Microglia
Introduction
Coronaviruses are a large family of enveloped, positive-stranded RNA pathogenic viruses that cause infectious disease in animals and humans. For example, the highly transmissible SARS-CoV-2 virus causes Coronavirus Disease (COVID-19) which causes a mild to moderate respiratory disease in most humans, but susceptible individuals can become dangerously ill, developing Severe Acute Respiratory Syndrome (SARS) and Acute Respiratory Distress Syndrome (ARDS) that can result in organ damage [1]. The global pandemic of COVID-19 disease that has spread since 2019 has had devastating health, social and economic consequences that remain burdensome [2]. By August 2022 that World Health Organization report 599,825,400 confirmed cases of COVID- 19 and 6,469,458 confirmed deaths representing just over 1% of those infected.
There is now a wealth of information published on the SARS- CoV-2 virus that describe the host receptors, virus transmission, virus structure-function relationships, pathophysiology, co-morbidities, immune response, treatment and the most promising vaccines and anti-viral drugs [3,4]. This explosion of knowledge, and in particular understanding of the mechanisms of SARS-CoV-2 entry into host cells through binding of the viral Spike (S) protein to a key receptor, Angiotensin-Converting Enzyme 2 (ACE2), has led to the development of a wide range of vaccines and other anti-viral drugs that are now controlling disease spread to some extent [5].
However, successive waves of disease derived from emerging and re-emerging viral variants will ensure the persistence of this disease in the global population for the foreseeable future. Simultaneously, persistent and often debilitating, sequelae are being increasingly recognized in convalescent individuals, with fatigue, malaise, dyspnea, defects in memory and concentration and a variety of neuropsychiatric syndromes as the major manifestations, and several organ systems can be involved. Many of these post-viral infection adverse consequences can be linked to neuroinflammation. The socio-economic legacy of this so-called ‘long COVID’ or post-viral syndrome is only now beginning to be realised [6,7] and it is evident it will also present major health and economic challenges.
There is global need for new and improved adjunct anti-viral agents and, in particular, those that can also reduce the symptoms of post-viral syndrome. Here we report the potential of a new class of regenerative medicine, a unique Low Molecular Weight Dextran Sulphate (LMW-DS) called ILB®, to have broad spectrum anti-viral effects on the infection capability and the downstream pathological processes initiated by coronaviruses and, in particular, variants of the SARS-CoV-2 virus.
Materials and Methods
ILB®
ILB® is the sodium salt of LMW-DS containing 16%-19% sulphur with an average MW of 5 kDa (International Publication No. WO 2016/076780, ILB® is in Phase II development to treat the neurodegenerative condition ALS) [8]. ILB® was supplied as the sodium salt dissolved in 0.9% NaCl at 100 mg/ml concentration.
Experimental procedures
Antiviral activity and cytotoxicity of ILB® against human coronavirus NL63: The antiviral activity of eight dilutions of ILB® was explored by pre-incubation with LLC-MK2 cells (LLC-MK2 (rhesus monkey kidney-derived cells) (ATCC CCL-7)) for 1hr before virus addition. Experimental controls were (a) Uninfected untreated cells, (b) Infected untreated cells or (c) Positive control drug (Remdesivir (Selleckchem S8932 PHR1258)). Human coronavirus NL63 (BEI Resources NR-470) plus ILB® were left on the cells for the entire duration of the experiment (48 hrs). The cytotoxicity of the same range of concentrations of ILB® was determined by MTT assay.
LLC-MK2 cells were seeded in 2 × 96-well plates (one for inhibition and one for cytotoxicity) in complete media (DMEM from Gibco, Cat. No. 61965026, supplemented with 10% FBS (Gibco, Cat. no. 10500064) and 1 ×penicillin/streptomycin (Gibco, Cat. no. 15070063) at 4,000 cells/100 µl/well. After seeding, the plates were incubated at RT for 5 minutes for even distribution, and then at 37°C, 5% CO2 until the following day. Virus stocks were diluted into supplemented media (DMEM from Gibco, Cat. no. 61965026 supplemented with 2% FBS (Gibco, Cat. No. 10500064 and 1 × penicillin/streptomycin (Gibco, Cat. No. 15070063), to reach an MOI of 2.0. To calculate MOI, it was estimated that the cell number had doubled to 8,000 cells/well since plating the day before.
Following ILB® dilution and mixing, the cells were preincubated for 1 hr at 37°C in air plus 5% CO2 with ILB® concentrations at 0.005 mg/ ml, 0.014 mg/ml, 0.041 mg/ml, 0.123 mg/ml, 0.370 mg/ml, 1.111 and 10.000 mg/ml or Remdesivir at 0.009 µM, 0.027 µM, 0.082 µM, 0.247 µM, 0.741 µM, 2.222 µM, 6.667 µM and 20.000 µM. After 1 hr, media was removed from the cells and replaced with 50 µl of virus or media (uninfected untreated control) immediately followed by 50 µl of the ILB® dilutions at twice the final concentrations, as they become diluted to the final concentrations by an equal volume of virus or media. Plates were then incubated for 48 hrs at 37°C in air plus 5% CO2, and the ILB® and/or the virus remained with the cultured cells for the entire duration of the experiment. After 48 hrs, the infection plates were washed with PBS, fixed for 30 minutes with 4% formaldehyde, washed again with PBS, and stored in PBS at 4°C until staining.
For the infectivity readout, cells were immunostained for relevant viral particles. Briefly, any residual formaldehyde was quenched with 50 mM ammonium chloride, after which cells were permeabilised (0.1% Triton× 100) and stained with an antibody recognising double-stranded RNA (Caltag Medsystems Ltd, SCI-10010200). The primary antibody was detected with an Alexa-488 conjugate secondary antibody (Life Technologies, A11001), and nuclei were stained with Hoechst. Images were acquired on an CellInsight C × 5 high content platform (Thermo Scientific) using a 4 ×objective, and percentage infection calculated using CellInsight C × 5 software (infected cells/total cells× 100).
For a cytotoxicity test, media was removed from the cells and replaced with 50 µl of supplemented media, followed by 50 µl of the diluted formulations or media. After mixing, the plates were incubated for 48 hrs at 37°C in air plus 5% CO2. Cytotoxicity was detected by MTT assay. Briefly, the MTT reagent (Sigma, M5655) was added to the cells for 2 hrs at 37°C, 5% CO2, after which the media was removed and the precipitate solubilised with a mixture of 1:1 Isopropanol:DMSO for 20 minutes. The supernatant was transferred to a clean plate and signal read at 570 nm.
Normalised percentages of inhibition were calculated using the following formula: Normalized % inhibition

Where appropriate, IC 50 values were generated by iterative curve fitting according to a Logistic equation using KaleidaGragh software (v5.0; Synergy Software).
Percentages of cytotoxicity were calculated using the following formula:
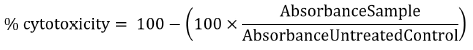
Where appropriate, TC50 values were generated by iterative curve fitting according to a Logistic equation using KaleidaGragh software (v5.0; Synergy Software).
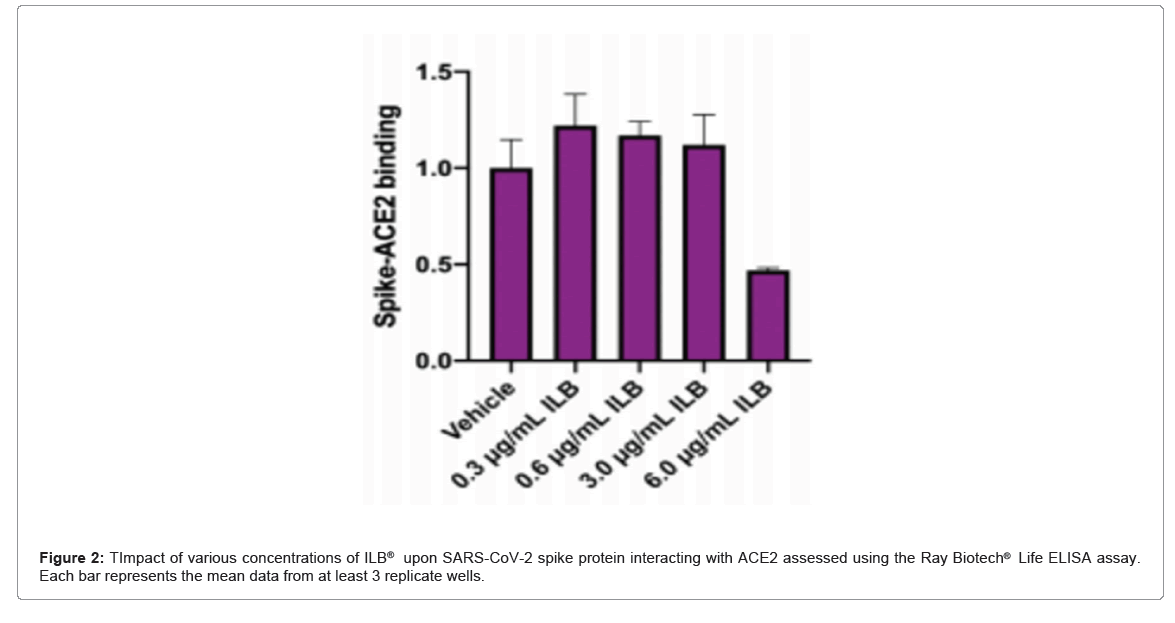
ILB® inhibition of the interaction between SARS-CoV-2 Spike protein and the ACE2 receptor: The Ray Biotech® Life COVID-19 Spike-ACE2 binding assay kit (Catalogue Number: CoV-SACE2) was used exactly as described in the manufacturer’s instructions (Ver 1.6) to assess the ability of a range of concentrations of ILB®, between 0.3 µg/ml and 600 µg/ml, to disrupt the interaction between SARS-CoV-2 Spike protein and ACE2.
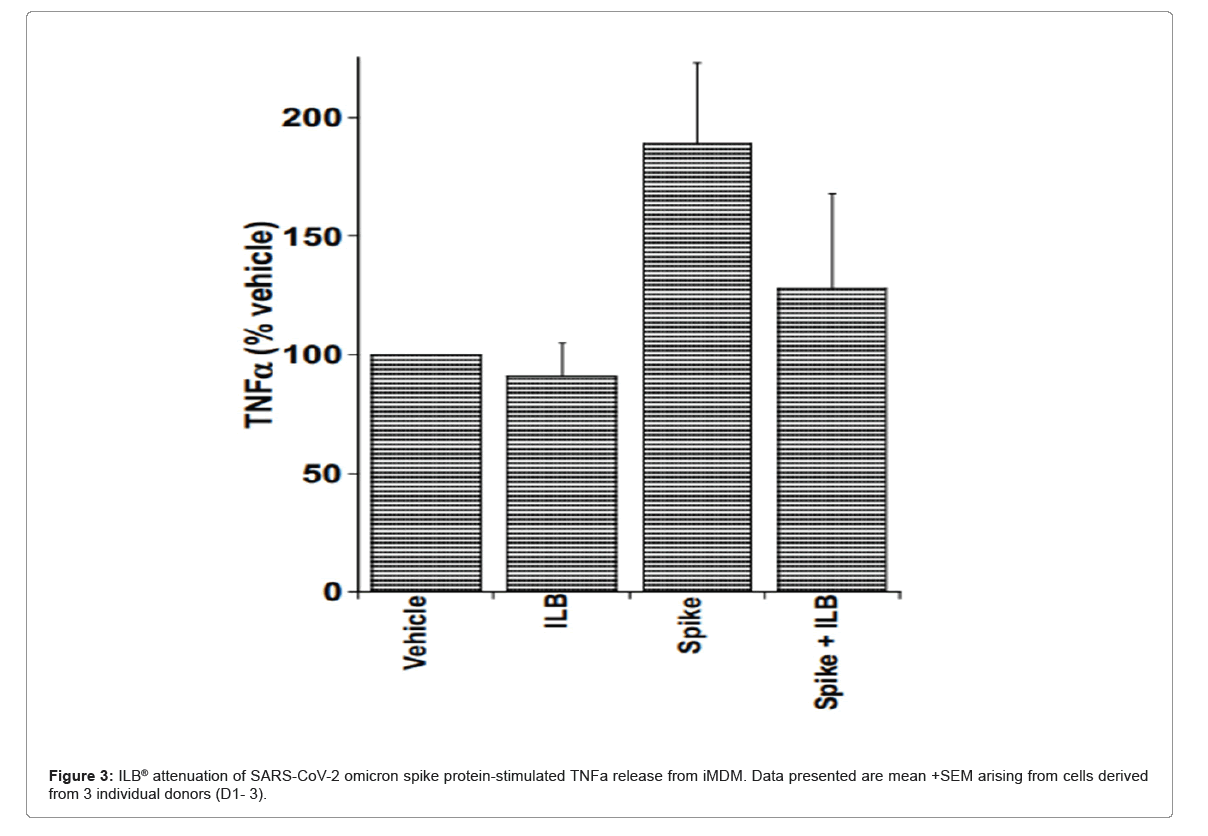
ILB® attenuation of Coronavirus-stimulated cytokine release from human microglia: Peripheral Blood Mononuclear Cells (PBMCs) were isolated from three healthy donors through SepMate density centrifugation (Stem Cell Technologies, 85450) with Ficoll- Paque PLUS (Cytiva 17-1440-03). Monocytes were purified from the PBMC population using the EasySep Human monocyte enrichment kit (STEMCELL Technologies; 19059), plated in 96-well plates and differentiated into microglia (iMDM) with the addition of cytokines; M-CSF, GM-CSF, NGF-β, CCL2 and IL-34 for 5 days at 37°C.
iMDM were pre-incubated in the absence or presence of ILB® (600 mg/mL) for 30 minutes prior to the addition of recombinant SARS-CoV-2 spike protein (‘original [Wuhan]’ (Acro Biosystems SPN C52H9), ‘Delta’ [B.1.617.2] (Acro Biosystems SPN-C52He), ‘Omicron’ [B.1.1.529] (Acro Biosystems SPD-C522e); (1.0 mg/mL or 5.0 mg/mL) in the absence or presence of cross-linker (Abcam; ab18184), and culture for a further 6 hrs or 20 hrs. Positive control stimulation of microglia was also performed by the addition of LPS (100 ng/mL; Invivogen tlrl- b5lps) and, where indicated, BzATP (100 µM) stimulation for the final two hours of the culture period for 6 hrs or 20 hrs. After 6 and 20 hrs, the cell culture supernatants were collected and stored at -20°C for subsequent quantification of the levels of IL-1β, IL-6, MCP-1 and TNFα by Luminex (R and D Systems; LXSAHM-04).
Results
Antiviral activity and cytotoxicity of ILB® against human coronavirus NL63
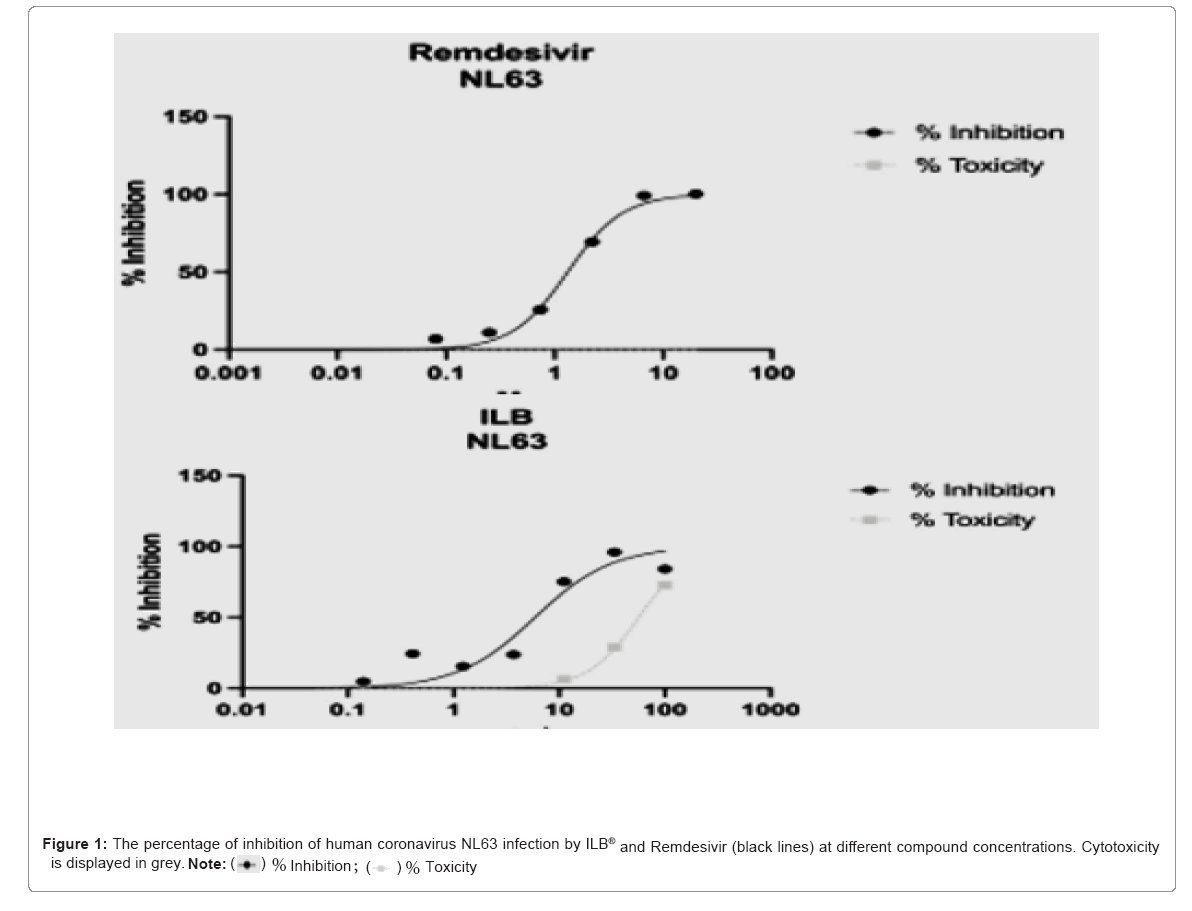
Table 1 displays the EC50, EC90, TC50, TC90 and Selectivity Index (SI50) and SI90 for ILB® and the Remdesivir control. Inhibition of human coronavirus NL63 infection was observed for both the test and control formulations, with an EC50 of 5.90 mg/ml for ILB® and 1.32 μM for Remdesivir. Cytotoxicity was observed only at the highest concentrations of ILB®, with TC50 of 56.5 mg/ml. No significant cytotoxicity was observed at any of the concentrations of Remdesivir tested (Table 1).
| - | EC50 (mg/ml) | TC50 (mg/ml) | SI50 | EC90(mg/ml) | TC90(mg/ml) | SI90 |
|---|---|---|---|---|---|---|
| ILB® | 5.9 | 56.5 | 9.6 | 37.1 | 192.9 | 5.2 |
| - | EC50 (µM) | TC50 (µM) | SI50 | EC90 (µM) | TC90 (µM) | SI90 |
| Remdesivir | 1.32 | ND | ND | - | - | - |
Table 1: EC50, EC90, TC50, TC90, SI50 (=TC50/EC50) and SI90 (=TC90/EC90) values for NL63 against ILB® and Remdesivir. ND: Not determined, due to the inability to extrapolate a curve or the Hill Slope value from the input values.
The graphs in Figure 1 below display the percentage of inhibition of human coronavirus NL63 infection at different ILB® concentrations compared to Remdesivir. Sample values in each plate were normalised to the plate internal controls, where 100% inhibition was derived from the average of the negative control (untreated uninfected) and 0% inhibition was derived from the average of the positive control (untreated infected). The x axes show compound dilutions (mg/ml [ILB®] or μM [Remdesivir]). The curves represent the best fit of the logarithm of compound dilution vsersus the normalised percentage of inhibition (variable slope). Cytotoxicity is displayed in grey, with values normalized to the plate internal control (untreated cells, 100% viability). Percentages of infection and cytotoxicity relative to each ILB® concentration are shown in the Appendix as Supplementary Tables S1 and S2 respectively (Figure 1).
Supplementary results
Percentages of infection IF assay: Table S1 shows the percentages of LLC-MK2 cells infected by human coronavirus NL63 and treated with formulations of ILB® or Remdesivir (assay control) at 48 hrs post- infection. Eight dilutions were tested as indicated in the table. Three technical replicates were performed. Untreated infected and untreated uninfected controls were included (both with media and media without diluent). Wells without cells were also included.
Percentages of cytotoxicity: Table S2 shows the percentages of cytotoxicity of LLC-MK2 cells incubated with formulations of ILB® or Remdesivir (assay control) for 48 hrs. For both compounds, eight dilutions were tested. Three technical replicates were performed. Untreated controls were included (both with media and media without diluent). No cell and Triton X-100-treated controls were also included.
ILB® inhibition of the interaction between SARS-CoV-2 Spike protein and the ACE2 receptor: In each of three independent experiments ILB® reduced the interaction between SARS-CoV-2 spike protein and ACE2 (Figure 2).
ILB® attenuation of SARS-Cov-2 variant spike protein- stimulated cytokine release from human microglia: Figure 3 show that in the absence of LPS, the ‘Omicron’ Spike protein (5.0 mg/mL, in the presence of cross-linker evoked an increase in the levels of TNFα released from microglia and this was reduced by the presence of ILB® (see Donors 1, 2 and 3 at 20 hrs in Figure 3). However, a relatively low concentration of LPS (10 ng/mL) in the presence of the ‘Wuhan’, ‘Delta’ or ‘Omicron’ spike proteins evoked a significant increase in IL-6 and TNFα release that was reduced by the presence of ILB® (see Donor 1 at 20 hrs; Donor 2, at 6 hrs and 20 hrs; Donor 3 at 20 hrs), and an increase in IL-1β that was reduced by the presence of ILB® (see Donor 2 at 20 hrs) (Figure 3).
Discussion
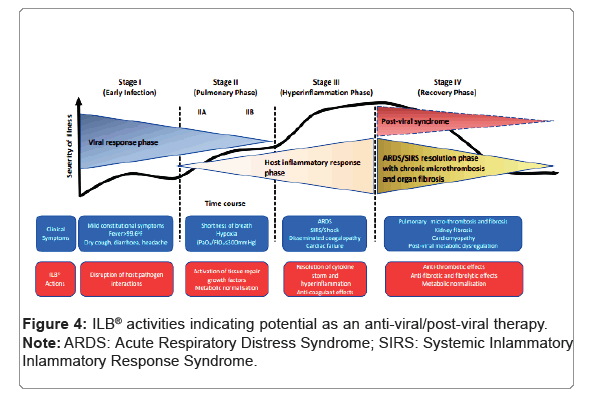
Under the conditions tested, ILB® displayed neutralising activity against infection by human coronavirus NL63 when pre-incubated with the cells for 1 hr prior to infection, with an EC50 of 5.90 mg/ml. Cytotoxicity was observed only at the higher concentrations of ILB®, with a TC50 of 56.5 mg/ml. The ability of ILB® to prevent cell infection is supported by the observation of a direct inhibition of the interaction between SARS-CoV-2 spike protein and ACE2. Taken together, this evidence of anti-viral activity would be predicted to be beneficial to patients by reducing the ability of the SARS-CoV-2 virus to gain entry to cells. The anti-infective activity of ILB®, a clinical grade drug with a proven safety profile in humans [8], points to its substantial potential as a new adjunct anti-viral drug to help counter the uncontrolled growing global burden of Coronavirus disease. Anti-viral drugs can be divided into two major categories: those directed against the host and those directed against the vector. Anti-viral compounds directed against host targets are of interest in the search for broad spectrum anti-viral compounds that may be able to address present or future unknown viral emergences, since they can be directed at pathways that are common to multiple virus types. Of importance, compounds that target the host pathway exploited by viruses may be expected to show a higher barrier to resistance, which is of importance for RNA viruses with a relatively high tendency to mutate, such as Coronaviruses. Highly sulphated Glycosaminoglycans (GAGs) like heparan sulphate are found in and around animal cells and are involved in the infection of many pathogenic enveloped and non-enveloped viruses, including Coronaviruses like SARS-CoV-2 [9-12]. These viruses utilize cell surface glycoconjugates as cellular receptors for attachment, which enable them to take their first step toward establishing infection. The binding of these viruses to cell surface heparan sulphate could be specific but also could be due to nonspecific electrostatic interactions of biological relevance, either possibility suggests the application of heparan sulphate mimetics as anti-viral therapies. Indeed, soluble GAGs, especially heparin mimetics, have been used as competitive inhibitors to block Coronavirus infection [13-15] of relevance, the LMW-DS used in this study, ILB®, acts as a soluble heparin mimetic that can offer a competitive binding site for heparin-binding moieties, thereby preventing receptor interactions [16]. It therefore seems probable that, in this instance, soluble ILB® is acting competitively with cell surface heparan sulphate to bind virus and block cell attachment and internalization via spike protein:ACE2 receptor interactions. Extensive preclinical and clinical studies, reported by us elsewhere [8,16-19] suggest that ILB® may also be a useful tool to counteract the post-viral syndrome that is often associated with Coronavirus infection. Accordingly, ILB® activates natural repair processes to control hyperinflammation and thrombosis, normalise cellular metabolism and remodel damaged tissues [16]. Thus, uniquely, ILB® acts as a broad acting anti-viral drug that targets every step in the progression of viral disease from infection through to cellular pathology. This inference of restoration of cellular and tissue homeostasis in humans is further supported by the studies reported here on ILB® control of cytokine release from human microglia stimulated with the omicron spike protein variant that suggest the ability of ILB® to counteract chronic viral neuroinflammation. In contrast to previous reports [20- 26] by other research groups investigating the ‘Wuhan’ SARS-CoV-2 spike protein with myeloid cells, a clear response to ‘Wuhan’ SARS- CoV-2 spike protein-evoked cytokine response (proposed to act via TLR4 [20-24] was not evident in our studies [20,21] using myeloid induced-monocyte derived microglia (iMDM). LPS contamination of preparation remains a major challenge when studying TLR biology [25] especially for bacteria derived proteins [20] (where trace levels of LPS may remain), and whilst others used mammalian [21-24] or insect cell line-derived spike protein the potential for bacterial contamination remains. Although there are differences in the source of Wuhan spike protein used in previous studies compared to the present study, the SARS-CoV-2 spike proteins used in the present study are generated using mammalian expression systems, and furthermore their direct ability to bind ACE2 has been verified using SPR analysis and also a spike protein binding assay to mammalian cells that was reversible by anti-spike protein neutralising antibodies [27]. Similar validation is evident for the delta and omicron spike proteins used in the present study. It is therefore of interest that the Wuhan and delta spike proteins did not evoke evident cytokine release from the iMDM. In contrast, the omicron spike protein (5.0 mg/mL), in the presence of cross-linker in an attempt to recapitulate the native spike presentation to the surface of the cell, enhancedconsistently TNFα release from the iMDM cells and of potential therapeutic relevance, the enhanced release of TNFα was reduced consistently by ILB®. An ability of ILB® to reduce the release of pro-inflammatory cytokines from virally ‘primed’ microglia has relevance to potential beneficial effects of ILB® to treat numerous post- viral syndromes including long COVID (Figure 4) [28-30].
Conclusion
In conclusion, ILB® displays potential as a host-directed anti-viral drug that inhibits coronavirus infection and may be a useful adjunct anti-viral treatment. In addition, the anti-inflammatory activity of ILB® suggest this drug also has potential to alleviate some of the long term consequences of viral infection in central and peripheral tissues. The clinical stage of development of ILB® offers the opportunity to test the anti-viral and post-viral syndrome activities summarised in clinical trial to determine if the results translate for the benefit of patients infected with Coronaviruses.
Acknowledgements
This work was funded by Tikomed AB.
Disclosures
Patents pertaining to this LMW-DS drug have been filed by Tx Medic AB, a subsidiary of Tikomed AB. LB is coinventor of LMW-DS, and is a founder, shareholder and board member of Tikomed AB. AL, NMB and LB declare consultancy payments from Tikomed AB and/or Axolotl Consulting Ltd for services related to the submitted work. MM is an employee of Virology Research Services Ltd who received payment for services related to some of the submitted work. This study received funding from Tikomed AB. The funder had the following involvement with the study: Approval of the individual study components and the decision to publish the study.
References
- Mistry P, Barmania F, Mellet J, Peta K, Strydom A, et al. (2022) SARS-CoV-2 variants, vaccines, and host immunity. Front Immunol 12:809244.
[Crossref] [ Google Scholar] [ PubMed]
- Koelle K, Martin MA, Antia R, Lopman B, Dean NE (2022) The changing epidemiology of SARS-CoV-2. Science 375:1116-1121.
[Crossref] [ Google Scholar] [ PubMed]
- Varghese PM, Tsolaki AG, Yasmin H, Shastri A, Ferluga J, et al. (2020) Hostpathogen interaction in COVID-19: Pathogenesis, potential therapeutics and vaccination strategies. Immunobiology 225:152008.
[Crossref] [ Google Scholar] [ PubMed]
- Kim S (2022) COVID-19 drug development. J Microbiol Biotechnol 32:1-5.
- Jackson CB, Farzan M, Chen B, Choe H (2022) Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol 23:3-20.
[Crossref] [ Google Scholar] [ PubMed]
- Castanares ZD, Chalon P, Kohn L, Dauvrin M, Detollenaere J, et al. (2022) Pathophysiology and mechanism of long COVID: A comprehensive review. Ann Med 54:1473-1487.
[Crossref] [Google Scholar] [ PubMed]
- Mehandru S, Merad M (2022) Pathological sequelae of long-haul COVID. Nat Immunol 23:194-202.
[Crossref] [Google Scholar] [ PubMed]
- Logan A, Nagy Z, Barnes NM., Belli A, Di Pietro V, et al. (2022) A phase II open label clinical study of the safety, tolerability and efficacy of ILB® for amyotrophic lateral sclerosis. PLoS One 17: e0267183.
[Crossref] [Google Scholar] [PubMed]
- Clausen TM, Sandoval DR, Spliid CB, Pihl J, Perrett HR, et al. (2020) SARS-CoV-2 Infection depends on cellular heparan sulfate and ACE2. Cell 183:1043-1057.e15.
[Crossref] [Google Scholar] [PubMed]
- Yu M, Zhang T, Zhang W, Sun Q, Li H, et al. (2021) Elucidating the interactions between heparin/heparan sulfate and SARS-CoV-2-related proteins an important strategy for developing novel therapeutics for the COVID-19 Pandemic. Front Mol Biosci 7:628551.
[Crossref] [Google Scholar] [PubMed]
- Liu L, Chopra P, Li X, Bouwman KM, Tompkins SM, et al. (2021) Heparan sulfate proteoglycans as attachment factor for SARS-CoV-2. ACS Cent Sci 7:1009-1018.
- Kearns FL, Sandoval DR, Casalino L, Clausen TM, Rosenfeld MA, et al. (2022) Spike-heparan sulfate interactions in SARS-CoV-2 infection. Curr Opin Struct Biol 76:102439.
[Crossref] [Google Scholar] [PubMed]
- Mangiafico M, Caff A, Costanzo L. (2022) The role of heparin in COVID-19: An update after two years of pandemics. J Clin Med 11:3099.
[Crossref] [Google Scholar] [PubMed]
- Guimond SE, Mycroft West CJ, Gandhi NS, Tree JA, Le TT, et al. (2022) Synthetic heparan sulfate mimetic pixatimod (PG545) potently inhibits SARSCoV-2 by disrupting the spike-ACE2 interaction. ACS Cent Sci. 2022 8:527-545.
[Crossref] [Google Scholar] [PubMed]
- Hu Y, Jo H, DeGrado WF, Wang J. (2022) Brilacidin a COVID-19 drug candidate, demonstrates broadspectrum antiviral activity against human coronaviruses OC43, 229E, and NL63 through targeting both the virus and the host cell. J Med Virol 94:2188-2200.
[Crossref] [Google Scholar] [PubMed]
- Logan A, Belli A, Di Pietro V, Tavazzi B, Lazzarino G, et al. (2022) The mechanism of action of a novel neuroprotective low molecular weight dextran sulphate: New platform therapy for neurodegenerative diseases like amyotrophic lateral sclerosis. Front Pharmacol 13:983853.
[Crossref] [Google Scholar] [PubMed]
- Hill LJ, Botfield HF, Begum G, Qureshi O, Vigneswara V, et al. (2021) ILB® resolves inflammatory scarring and promotes functional tissue repair. NPJ Regen Med 6:3.
[Crossref] [Google Scholar] [PubMed]
- Lazzarino G, Mangione R, Belli A, Di Pietro V, Nagy Z, et al. (2021). ILB® attenuates oxidative/nitrosative stress and mitochondrial dysfunction in patients with amyotrophic lateral sclerosis. J Pers Med11:794.
- Lazzarino G, Di Pietro V, Rinaudo M, Nagy Z, Barnes NM, et al. (2022). ILB®, a low molecular weight dextran sulphate, restores glutamate homeostasis, amino acid metabolism and neurocognitive functions in a rat model of severe traumatic brain injury. Int J Mol Sci 23:8460.
[Crossref] [Google Scholar] [PubMed]
- Shirato K, Kizaki T (2021) SARS-CoV-2 spike protein S1 subunit induces pro-inflammatory responses via toll-like receptor 4 signaling in murine and human macrophages. Heliyon 7:e06187.
[Crossref] [Google Scholar] [PubMed]
- Zhao Y, Kuang M, Li J, Zhu L, Jia Z, et al. (2021) SARS-CoV-2 spike protein interacts with and activates TLR41. Cell Res 31:818-820.
[Crossref] [Google Scholar] [PubMed]
- Frank MG, Nguyen KH, Ball JB, Hopkins S, Kelley T, et al. (2022) SARSCoV-2 spike S1 subunit induces neuroinflammatory, microglial and behavioral sickness responses: Evidence of PAMP-like properties. Brain Behav Immun 100:267-277.
[Crossref] [Google Scholar] [PubMed]
- Barhoumi T, Alghanem B, Shaibah H, Mansour FA, Alamri HS, et al.( 2021) SARS-CoV-2 coronavirus spike protein-Induced apoptosis, inflammatory, and oxidative stress responses in THP-1-like-macrophages: Potential role of angiotensin-converting enzyme inhibitor (perindopril). Front Immunol. 12:728896.
- Olajide OA, Iwuanyanwu VU, Adegbola OD, Al-Hindawi AA (2022) SARS-CoV-2 spike glycoprotein S1 induces neuroinflammation in BV-2 microglia. Mol Neurobiol 59:445-458.
[Crossref] [Google Scholar] [PubMed]
- Mancek Keber M, Jerala R (2015) Postulates for validating TLR4 agonists. Eur J Immunol 45:356-70.
[Crossref] [Google Scholar] [PubMed]
- Shahanshah K, Mahnoush S, Christopher L, John WS, Rashmin CS, et al. (2021) SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway. ELife 10:e68563.
[Crossref] [Google Scholar] [PubMed]
- Harrison N, Richardson L, Pallini C, Morano I, Jinks E, et al. (2022) A cell-based, spike protein binding assay highlights differences in antibody neutralising capacity for SARS-CoV-2 variants. Biorxiv 7-19.
- Awogbindin IO, Ben-Azu B, Olusola BA, Akinluyi ET, Adeniyi PA, et al. (2021) Microglial implications in SARS-CoV-2 infection and COVID-19: Lessons from viral RNA neurotropism and possible relevance to parkinson's disease. Front Cell Neurosci 15:670298.
[Crossref] [Google Scholar] [PubMed]
- Bouayed J, Bohn T (2021) The link between microglia and the severity of COVID-19: The "two-hit" hypothesis. J Med Virol 93:4111-4113.
[Crossref] [Google Scholar] [PubMed]
- Mondelli V, Pariante CM (2021) What can neuroimmunology teach us about the symptoms of long COVID? Oxf Open Immunol 1-5.
[Crossref] [Google Scholar] [PubMed]
Citation: Logan A, Mazzon M, Cowley J, Harrison N, Morano IN, et al. (2023) Ability of a Clinical Stage LMW-DS Drug to Inhibit Coronavirus Infection of Cells and Suppress Cytokine Secretion from Human Microglia. J Infect Dis Ther 11: 531. DOI: 10.4172/2332-0877.1000531
Copyright: © 2023 Logan A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1686
- [From(publication date): 0-2023 - Nov 21, 2024]
- Breakdown by view type
- HTML page views: 1516
- PDF downloads: 170