Research Article Open Access
Abdominal Stereotactic Body Radiotherapy: Local Control and Correlation to Biologically Equivalent Dose
| Simon Kirste1, Hans Trautsch1, Marc-Benjamin Messmer1, Rolf Wiehle1, Hans-Christian Rischke1, Felix Momm2, Anca-Ligia Grosu1 and Thomas B. Brunner1* | |
| 1Department of Radiation Oncology, University Hospitals Freiburg, Freiburg, Germany | |
| 2Department of Radiation Oncology, Ortenau Hospital Offenburg, Offenburg, Germany | |
| *Corresponding Author : | Thomas B Brunner Department of Radiation Oncology University Hospitals Freiburg, Freiburg, Germany Tel: +49-761-270- 94070 E-mail: thomas.brunner@uniklinik-freiburg.de |
| Received: February 11, 2016; Accepted: March 07, 2016; Published: March 10, 2016 | |
| Citation: Kirste S, Trautsch H, Messmer MB, Wiehle R,Rischke HC, et al. (2016) Abdominal Stereotactic Body Radiotherapy: Local Control and Correlation to Biologically Equivalent Dose. OMICS J Radiol 5:216. doi:10.4172/2167-7964.1000216 | |
| Copyright: © 2016 Brunner TB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Radiology
Abstract
Aim: We conducted a retrospective study to report on the outcome and toxicity of stereotactic body radiotherapy (SBRT) and to analyze the correlation of local control (LC) and biological equivalent dose (BED) of patients treated with SBRT for upper abdominal lesions.
Methods: Patients who completed stereotactic radiotherapy treatment between 05/2007 and 03/2012 were enrolled into the analysis. Different fractionation regimens depending on the dose to organs at risk (OAR) were given. The primary endpoint was LC determined on imaging studies. Toxicities were classified according to CTCAE v4. Physical dose was converted into BED (α / β = 10 Gy) and a tumor control probability model (TCP) was used for dose-response relationship. For outcome analyses the Kaplan-Meier method and log rank tests were used.
Results: Forty-two patients were treated with SBRT. Thirty-three patients with 39 lesions were evaluable for further analysis with a median follow-up of 15.2 months. Lesions were located in liver (n = 28), hepatic hilum (n = 8), nodal (n = 2), adrenal (n = 2) and pancreatic head (n = 1). Local failure was observed in 7 lesions at a mean time of 7.3 months (range, 1.5 - 17.9) resulting in a LC rate of 83% at one year and 63% at two years. No significant difference for intra- vs extrahepatic lesions was observed. Lesions treated with a BED ≥ 59.5 Gy had a better LC vs. lesions treated with a BED < 59.5 Gy (88% vs. 68%, p = 0.027). Median overall survival was 14.5 months (2 - 32.5). Observed toxicities were mild (grade 2 in 4 patients, grade 3 in 1 patient).
Conclusion: Upper abdominal SBRT is well tolerated and results in excellent local control if BED is ≥ 59.5 Gy. We therefore propose to aim for doses at or above this BED.
| Keywords |
| Stereotactic radiotherapy; Abdominal; Biological equivalent dose; Liver; Toxicity |
| Introduction |
| Stereotactic radiation therapy is a high precision radiation technique that uses very high doses of radiation to a single target volume in order to achieve a tumor ablative effect. It is characterized by a sharp dose gradient thereby sparing organs at risk close to the target volume [1]. After implementation as a treatment for cranial lesions [2] stereotactic radiotherapy has become increasingly used over the last decade as extra cranial stereotactic radiotherapy, also called stereotactic body radiotherapy (SBRT). First it was applied in the lung for metastatic lesions or primary lung tumors [3,4], but its use for lesions in the upper abdominal region soon became another indication [5]. One reason for the slower adoption of SBRT in the abdominal region compared to lung was the inherent problem of organ movement in the abdomen and the proximity of various organs at risk, such as liver, small bowel, stomach and kidneys. With conventional radiation therapy techniques it was not possible to deliver tumor ablative doses to lesions in the upper abdomen. With the introduction of SBRT in combination with modern radiation techniques, intensity modulated radiotherapy (IMRT) and image-guided radiotherapy (IGRT); it was feasible to precisely deliver high ablative tumor doses to the target volume to eradicate tumor cells [6]. The abdominal region that is most frequently treated is the liver for primary liver tumors or for secondary intrahepatic lesions. Lymph nodes, adrenal metastases or pancreatic tumors are other areas that can be treated with SBRT. A number of studies have shown that the dose can be safely escalated without doselimiting toxicity. Encouraging local control and low toxicity rates could be achieved [7-10] for primary liver tumors as well as hepatic metastasis. |
| Improvements in systemic therapy have resulted in better survival of patients with metastatic disease and a new treatment paradigm, the oligometastatic state, was defined [11,12]. For patients with a limited number of metastases the use of aggressive local treatments can result in prolonged disease-free survival and a possibility of cure [13]. For that reason aggressive local therapies have an increasingly important role. So far surgery is the primary treatment modality when opting for an aggressive treatment approach resulting in a 5-year survival of approximately one third of patients after complete resection of liver metastasis [14,15]. However a significant number of these tumors are unresectable or patients are not suitable for surgery due to comorbidities or low performance status and represent a cohort of potential candidates for SBRT. |
| In the present study we aim to report the outcome and toxicity of SBRT in a single center after introduction of SBRT and to identify predictive parameters for local control. Since we have used different fractionation regimens for lesions with and without proximity to critical organs at risk, we were especially interested in the dose-response relationship of this cohort. |
| Methods |
| Patient’s eligibility |
| Patients undergoing SBRT in the upper abdomen for oligometastatic disease from May 2007 to March 2012 were included in the analysis. Patients with any primary tumor were eligible. SBRT was defined as radiation therapy using hypofractionation, a stereotactic patient setup and image guidance. Histological confirmation of disease was obtained in all patients except for patients with Klatskin tumors in which diagnosis was based on typical imaging and elevated CA19- 9 values. SBRT was performed in patients who were not eligible for other standard local treatments, e.g. resection, chemoembolization or radiofrequency ablation. No further sites of tumor activity beyond those treated by SBRT were allowed. All patients were discussed at the institutional tumor board and informed consent was obtained from every patient before the start of treatment. The study was approved by the institutional ethic comity and review board. |
| Stereotactic body radiotherapy treatment planning and treatment delivery |
| Staging was performed by contrast enhanced (IV and oral) computed tomography (CT), magnetic resonance imaging (MRI) and / or positron emission tomography (PET / CT) to exclude distant metastasis or uncontrolled extra-hepatic disease. |
| Patient immobilization was guaranteed by a customized, external vacuum mattress. Breathing-related tumor motion was minimized by abdominal compression. Planning CT slice thickness was 2 mm. Depending on image quality; CT images were fused with MRI and / or PET / CT images in treatment position to facilitate gross tumor volume (GTV) delineation. 4-D-information was used to define internal target volumes (ITVs) in 27 patients. Finally, ITVs were expanded by 4 mm to create planning target volumes (PTVs). Dose was prescribed to the 60% or 95% isodose line that surrounded the PTV depending on the proximity to organs at risk (OARs). Organs at risk were typically the stomach, small bowel and kidney. Depending on dose to OARs, 3 × 12.5 Gy, 5 × 7 Gy, 7 × 5 Gy, 6 × 5 Gy and 12 × 4 Gy were given every other day. Image-guidance (IGRT) was performed by cone beam CT before each fraction. SBRT was administered by a linear accelerator with energies of 6 or 18 MV using intensity-modulated radiotherapy, dynamic arcs or multiple coplanar static beams. Patients with stomach or duodenum close to the PTV were treated with proton pump inhibitors during and after SBRT. |
| Study end points and statistical analysis |
| The primary endpoint was infield local control (LC) defined as absence of either growth or increase in metabolic activity of the treated lesion. LC and survival rates were calculated from the date of the first fraction of SBRT. Follow-up imaging and toxicity evaluation were performed at 3 months intervals. Treatment response was assessed on CT, MRI, and / or PET / CT scanning. In the case of local relapse, follow-up images were registered to the radiation treatment plan. All images were re-evaluated for local control by an experienced radiologist, nuclear medicine physician and radiation oncologist. Re-occurrence or progressive disease within the PTV was counted as local failure. Progression outside the irradiated volume was counted as distant progression. |
| Secondary endpoints were toxicity, progression free survival (PFS) and overall survival (OS). Survival curves were generated by the Kaplan-Meier method using SPSS software version 22.0 (IBM, USA). Differences among survival curves were compared using the log-rank test. p values and 95% confidence intervals were two-sided, and a p < 0.05 was considered as statistically significant. Acute and late toxicities were scored according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. To compare different fractionation schedules, irradiation doses were converted into biological effective dose (BED) using the linear-quadratic model and a α / β-ratio of 10 Gy for tumor with the following formula [16]: |
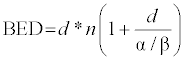 |
| where d refers to dose / fraction and n to fraction number. |
| We will also report the equivalent dose in 2 Gray fractions (EQD2) to be able to compare the results to a fraction size of 2 Gy that is usually used in clinical practice. |
 |
| To analyze the relationship between BED and tumor control rates a tumor control probability (TCP) model was implemented [17]. |
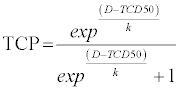 |
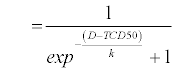 |
| D refers to dose analysed, TCD50 describes the dose that achieves 50% local control and coefficient k refers to the slope of the curve. For this model patients with a follow-up time shorter than 6 months were excluded. |
| Results |
| Patient and treatment characteristics |
| From May 2007 to March 2012, 42 patients underwent treatment for 48 lesions using upper abdominal SBRT. Thirty-three patients with 39 lesions were eligible for analysis. Reasons for exclusion were conventional fractionated percutaneous therapy prior to SBRT (n = 5), a prescribed BED < 28 Gy to the tumor (n = 3) and termination of therapy after the first fraction because of patient choice (n = 1). The patient characteristics and treatment details are summarized in Table 1. |
| The median age for the whole population at the time of SBRT was 70 years (range, 24-87) with a median performance status (Karnofsky) of 90% (40-100%). The most common site of SBRT was the liver (26 / 39). Other sites were the bile ducts (n = 8), lymph nodes (n = 2), adrenal gland (n = 2) and pancreas (n = 1). The most common primary tumor was colorectal cancer (12 / 33) and cholangiocarcinoma (11 / 33). 58% (19 / 33) of the patients had metastasis in other organs that were treated and controlled at the start of SBRT. 76% (25 / 33) of patients received systemic therapy before the start of radiotherapy. |
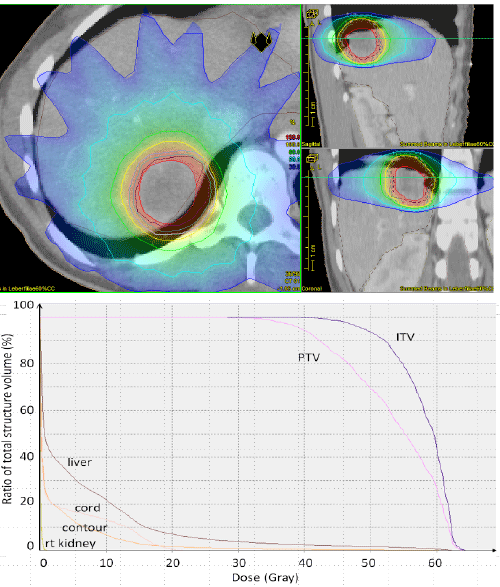
| The maximum tumor diameter ranged from 1.4 cm to 10 cm with a mean of 3.5 cm. The tumor volume delineated on the planning CT (GTV) had a wide range with a mean of 29.6 cm3 (range, 1.3 cm3 - 325.5 cm3) resulting in a mean PTV volume of 93 cm3 (range, 7.7 cm3 - 712.1 cm3). In terms of dose the median prescribed physical dose to the PTV was 35 Gy (range, 21 Gy - 48 Gy) in 5 fractions (range 3 - 12) corresponding to a median BED for all lesions of 59.5 Gy (range, 35.7 Gy - 84.4 Gy). Dose was prescribed to the 60% isodose and 95% isodose line in 22 / 39 and 17 / 39 cases. The most common fractionation regimen was 5 fractions of 7 Gy (21 / 39). To compare the differences in dose resulting from different fractionation regimens and dose prescription at different isolines, the PTV encompassing dose and the PTV encompassing BED were calculated. The median physical dose encompassing the PTV was 31.7 Gy (range, 16.8 Gy - 46.4 Gy) and the median BED encompassing the PTV was 51.7 Gy (range, 22.4 Gy - 86.4 Gy). Figure 1 shows a typical treatment plan for an intrahepatic lesion. |
| Clinical outcomes |
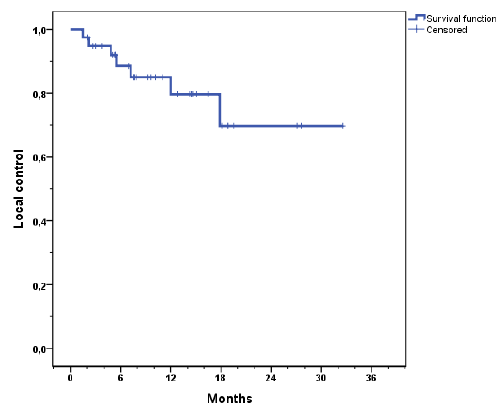
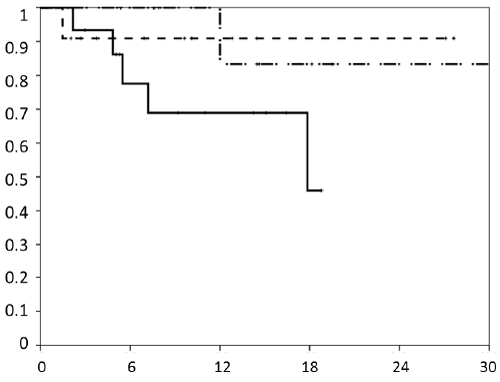
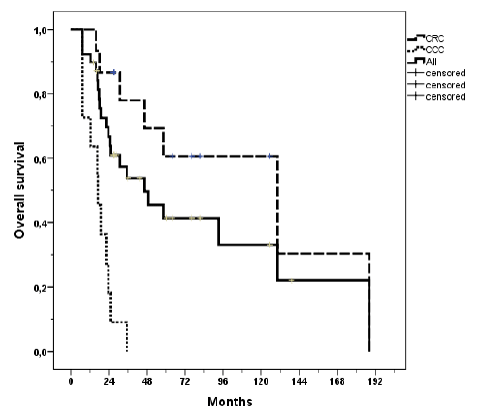
| The median follow-up was 11.4 months and 15.2 months for surviving patients. Actuarial local control rates at 1 and 2 years for the whole cohort were 83% and 63% respectively and 79% and 63% for liver metastasis only (Figure 2a). The mean recurrence free time was 25.8 months. Local progression was observed in 7 (18%) lesions with a mean time to relapse of 7.3 months (range, 1.5 - 17.9). Five failures were in-field recurrences and two failures have to be regarded as marginal misses occurring at the field border. Actuarial local control for all lesions stratified by BED is shown in Figure 2b. Local control was better with higher BED (p = 0.027). The median time to local recurrences was 28.3 months when the BED was ≥ 59.5 Gy and 11 months when the BED was ËÂ? 59.5 Gy. Local control by histology is shown in Figure 2c. |
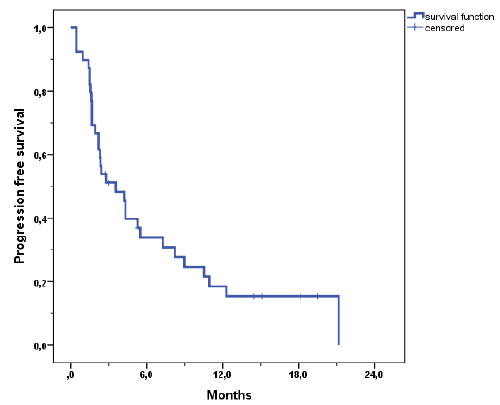
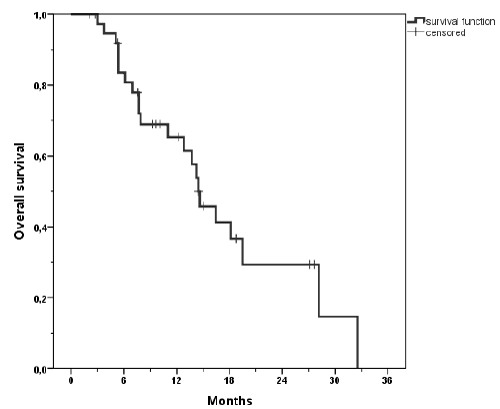
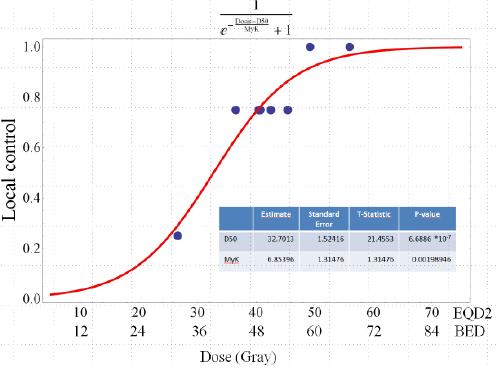
| Progression free survival was poor with a mean of 6.6 months (Figure 3). The median survival was 14.5 months (range, 2 - 32.5). 1-year actuarial overall survival was 66% and 2 year actuarial overall survival was 26% (Figure 4a). Patients with colorectal carcinoma had a median overall survival of 130 months from initial diagnosis. When calculating the tumor control probability we found that a BED of 60 Gy resulted in a 90% control probability at one year (Figure 5). |
| Toxicity |
| SBRT was well tolerated in all patients without developing high grade acute toxicity. The most common acute toxicity was nausea grade 2 (n = 3) and upper abdominal pain grade 2 (n = 1). One patient developed cholangitis one week after completion of radiotherapy which resolved completely. Another patient died three months after irradiation for an adrenal metastasis from colon cancer because of liver failure. He had pre-existing liver disease (cirrhosis Child-Pugh stage B with 9 points) and thrombocytopenia after multiple chemotherapy cycles. The mean liver dose was 5 Gy with a total liver volume of 1565 mL. Therefore we classified this as a non-treatment related death. Concerning late toxicity there was one patient with a single 3 cm × 2 cm × 2 cm intrahepatic metastasis from breast cancer close to the hilum in segment VI / VIII who developed a bile duct stenosis at the hepatic bifurcation diagnosed by MRI 2 years after completion of radiotherapy (Grade 2). This was treated by endoscopic papillotomy and balloon dilatation followed by a temporary bile duct endoprothesis. The hilum of the liver received a maximum dose of 55.6 Gy (median of 46.4 Gy in 3 fractions 0.5 ml received 50 Gy). At follow-up imaging of the liver complete response was observed. Further follow-up showed partial resolution of cholestatic parameters in temporal relationship with termination of fulvestrant medication. This estrogen receptor antagonist is cleared by the hepatobiliary route and the observed complication was categorized to be partly treatment related and partly drug related. At latest follow-up the patient was alive and free-from disease. |
| Discussion |
| The aim of this study was to evaluate our experience using SBRT for lesions in the upper abdominal area. The results of the present study demonstrate that SBRT is safe and effective for the treatment of metastases to upper abdominal organs, predominantly the liver and the liver hilum. This cohort of patients represents the first experience with SBRT in our center. LC at 1 and 2 years were 83% and 63%. Grade 3 toxicity occurred in only one patient (3%). We observed 7 local failures in 39 treated lesions with a mean time to relapse of 7.3 months. Progression occurred inside the PTV in five lesions and on the field border in two cases that could have potentially been avoided by increasing the treatment margin (Table 2). |
| These results compare favorable to other studies. There are 5 prospective phase I / II trials investigating the role of SBRT in the upper abdomen including hepatic and extra- hepatic lesions [18-22]. Trials using a BED of 75-93.6 Gy reported a similar LC rate of 67% - 82%. The best LC rate could be achieved in a trial by Rusthoven et al. with LC rates of 92% and 91% at one and two years. An explanation for the superior LC rate may be the fractionation regimen of 3 Gy × 20 Gy resulting in a BED of 180 Gy. |
| The largest retrospective studies were published by Dewas et al. and Fumagalli et al. reporting on a series of 78 and 113 hepatic lesions treated with SBRT in a comparable time period showing dose as the major prognostic factor for local control as well as tumor diameter and volume [23,24]. Nonetheless the influence of tumor size remains controversial in the literature with other studies showing no influence of tumor size in lesions treated with high single doses [25]. In our study correlation of tumor size to local control did not show a significant result. The reason for this may be the quite heterogeneous population. Local control rates were comparable in all studies, however recent studies with a higher total dose report better local control rates of up to 96% at one and two years without greater toxicities [26]. With the ongoing improvement of radiation techniques and better understanding of dose constraints for organs at risk the search for the optimal dosetoxicity relation continues. |
| Important factors that influence the outcome of SBRT and pose challenges for comparisons of different studies are patient and treatment heterogeneity in terms of patient selection, different tumor histologies, hepatic versus extra-hepatic disease, different fractionation regimens and total dose resulting in different BED and effects of systemic therapies. These factors have to be considered carefully when comparing study results in SBRT. Despite of this, one of the most intriguing findings of the study was the statistically significant relationship between BED and local control. The median prescribed dose to the PTV was a BED of 59.5 Gy in our study. This compares significantly lower to a pooled analysis on hepatic metastases from colorectal carcinoma (CRC) jointly analyzed by Stanford, University of Colorado and Princess Margaret hospital [27]. Stratifying local control by a dose below 75 Gy BED versus ≥ 75 Gy BED, local control at one year was 48% and 84%, respectively. In our analysis all lesions were treated with a BED of < 75 Gy. Stratifying our cohort at the median BED of 59.5 Gy, a statistically significant difference for local control could be observed for the two groups. Local control rate at one year for BED ≥ 59.5 Gy was 88% compared to 68% for BED < 59.5 Gy. We explain the similar local control rate by the fact that we had a mixture of different primaries, including only 12 patients with CRC, whereas Chang et al. exclusively reported on liver metastases from CRC. Local control of SBRT for metastasis from colorectal cancer has been found to have worse in-field control rate, previously. Takeda et al. compared CRC with metastases from other primary cancers and also found CRC to be less well controlled compared to metastases from NSCLC and head and neck primaries [28]. This is further underpinned by a report by Kress et al. where the same median BED was used as in our study [29]. In their series 1 year local control rate was 72%. We hypothesize that the lower control rate is due to the fact that this group was only analysing patients with CRC, again. Local control rate for CRC only at one year in our cohort was 68%. Of a total of 7 local failures 5 were metastasis from CRC. Of note, the follow-up time for patients alive was almost identical in our trial compared to the pooled analysis on colorectal metastases to the liver by Chang et al. [27]. |
| Another explanation for the comparable local control rates in patients treated with a comparably low BED could be the fact that more than half of the lesions in our study cohort were treated with a prescription dose that was prescribed to the 60% isodose by that creating a steep dose gradient resulting in much higher doses in the center of the lesion. One could hypothesize that the maximum dose being higher than the actual prescription dose might be responsible for the observed good local control rates with a rather low BED compared to contemporary SBRT series. |
| Despite of good local control rates the overall survival was low in our study, 26% at 2 years. This can be explained by the fact that many patients with poor prognostic factors (extrahepatic disease, multiple chemotherapy regimens) have been included. Seventy-six percent of the patients received chemotherapy prior to SBRT reflecting advanced disease stages negatively influencing the outcome results. In this patient cohort no strict rules concerning additional systemic therapies were applied. Most of the patients had multiple systemic therapies before or after stereotactic radiotherapy. All patients except one had controlled primary tumor or other controlled metastasis. |
| Treatment was very well tolerated in this trial with a low rate of acute and late toxicities. The only relevant late toxicity observed, was a grade 3 bile duct stricture requiring stenting two years after SBRT in a patient with a centrally located liver metastasis from breast cancer. As discontinuation of fulvestrant led to resolution of toxicity, we postulate a combined cause between radiation and drug induced cholestasis in our patient. Grade three bile duct obstruction was also reported in two patients in the series from Stanford using single fraction SBRT (1 Gy × 25 Gy) in 77 patients with pancreatic cancer and in two patients in a report from the Mayo Clinic [30,31]. This group indicates the doses given to the porta hepatis being Dmax of 55 Gy and 51.6 Gy as well as D5cc 54.0 and 47.8 Gy in 3 Gray fractions in the absence of constraints for the porta hepatis. This corresponds to BED (α / β 10 Gy) values of 155.8, 140.4, 151.2 and 124.0 Gy. We have changed practice for tumours in the porta hepatis since the occurrence of this complication to hypofractionated treatment with 12 Gy × 4 Gy corresponding to a BED (α / β 10 Gy) of 67.2 Gy and did not observe this type of complication again since the new fractionation regimen has been introduced [32]. |
| The current study has the characteristic limitations inherent to a retrospective analysis, concerning inclusion criteria, potential underestimation of toxicity and treatment inhomogeneity. To account for fractionation heterogeneity we calculated the BED. Furthermore we are aware that small sample size and heterogeneity of the patient cohort, such as different localizations of the lesions, as well as different primary tumors are other aspects to keep in mind when analyzing the results. Systemic therapies may have also contributed to local control of treated sites as most of the patients had multiple systemic therapies before or after stereotactic radiotherapy. |
| Conclusion |
| In conclusion this trial demonstrates that local control after SBRT correlates with BED. Lower doses than reported in the literature recently have been sufficient to achieve acceptable local control rates in patients with upper abdominal lesions treated with SBRT. A BED of at least 59.5 Gy should be used for SBRT. This cautious approach to abdominal SBRT was generally very well tolerated and will therefore be evaluated in a prospective randomized trial. |
References
- Lax I, Blomgren H, Näslund I, Svanström R (1994) Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. ActaOncol 33: 677-683.
- Leksell L (1951) The stereotaxic method and radiosurgery of the brain. ActaChirScand 102: 316-319.
- Guckenberger M, Allgäuer M, Appold S, Dieckmann K, Ernst I, et al. (2013) Safety and efficacy of stereotactic body radiotherapy for stage 1 non-small-cell lung cancer in routine clinical practice: a patterns-of-care and outcome analysis. J ThoracOncol 8: 1050-1058.
- Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, et al. (2010) Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 303: 1070-1076.
- Herfarth KK, Debus J, Lohr F, Bahner ML, Rhein B, et al. (2001) Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J ClinOncol 19: 164-170.
- Sterzing F, Brunner TB, Ernst I, Baus WW, Greve B, et al. (2014) Stereotactic body radiotherapy for liver tumors : Principles and practical guidelines of the DEGRO Working Group on Stereotactic Radiotherapy. StrahlentherOnkol 190: 872-881.
- Goodman KA, Wiegner EA, Maturen KE, Zhang Z, Mo Q, et al. (2010) Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J RadiatOncolBiolPhys 78: 486-493.
- Rusthoven KE, Kavanagh BD, Cardenes H, Stieber VW, Burri SH, et al. (2009) Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J ClinOncol 27: 1572-1578.
- Wulf J, Guckenberger M, Haedinger U, Oppitz U, Mueller G, et al. (2006) Stereotactic radiotherapy of primary liver cancer and hepatic metastases. ActaOncol 45: 838-847.
- Méndez Romero A, Wunderink W, Hussain SM, De Pooter JA, Heijmen BJ, et al. (2006) Stereotactic body radiation therapy for primary and metastatic liver tumors: A single institution phase i-ii study. ActaOncol 45: 831-837.
- Hellman S, Weichselbaum RR (1995) Oligometastases. J ClinOncol 13: 8-10.
- Badakhshi H, Grün A, Stromberger C, Budach V, Boehmer D (2013) Oligometastases: the new paradigm and options for radiotherapy. A critical review. StrahlentherOnkol 189: 357-362.
- Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: Analysis of 1001 consecutive cases. Ann Surg 230: 309-318.
- Aloia TA, Vauthey JN, Loyer EM, Ribero D, Pawlik TM, et al. (2006) Solitary colorectal liver metastasis: Resection determines outcome. Arch Surg 141:460-466.
- Hughes KS, Rosenstein RB, Songhorabodi S, Adson MA, Ilstrup DM, et al. (1988) Resection of the liver for colorectal carcinoma metastases. A multi-institutional study of long-term survivors. Dis Colon Rectum 31: 1-4.
- Fowler JF1 (1989) The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol 62: 679-694.
- Okunieff P, Morgan D, Niemierko A, Suit HD (1995) Radiation dose-response of human tumors. Int J RadiatOncolBiolPhys 32: 1227-1237.
- Hoyer M, Roed H, Traberg Hansen A, Ohlhuis L, Petersen J, et al. (2006) Phase II study on stereotactic body radiotherapy of colorectal metastases. ActaOncol 45: 823-830.
- Lee MT, Kim JJ, Dinniwell R, Brierley J, Lockwood G, et al. (2009) Phase I study of individualized stereotactic body radiotherapy of liver metastases. J ClinOncol 27: 1585-1591.
- Méndez Romero A, Wunderink W, Hussain SM, De Pooter JA, Heijmen BJ, et al. (2006) Stereotactic body radiation therapy for primary and metastatic liver tumors: A single institution phase i-ii study. ActaOncol 45: 831-837.
- Tse RV, Hawkins M, Lockwood G, Kim JJ, Cummings B, et al. (2008) Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J ClinOncol 26: 657-664.
- Katz AW, Carey-Sampson M, Muhs AG, Milano MT, Schell MC, et al. (2007) Hypofractionated stereotactic body radiation therapy (SBRT) for limited hepatic metastases. Int J Rad OncBiolPhys 67: 793-798.
- Dewas S, Bibault JE, Mirabel X, Fumagalli I, Kramar A, et al. (2012) Prognostic factors affecting local control of hepatic tumors treated by Stereotactic Body Radiation Therapy. RadiatOncol 7: 166.
- Fumagalli I, Bibault JE, Dewas S, Kramar A, Mirabel X, et al. (2012) A single-institution study of stereotactic body radiotherapy for patients with unresectable visceral pulmonary or hepatic oligometastases. RadiatOncol 7: 164.
- Herfarth KK, Debus J, Lohr F, Bahner ML, Rhein B, et al. (2001) Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J ClinOncol 19: 164-170.
- Goodman BD, Mannina EM, Althouse SK, Maluccio MA, Cárdenes HR5 (2016) Long-term safety and efficacy of stereotactic body radiation therapy for hepatic oligometastases. PractRadiatOncol 6: 86-95.
- Chang DT, Swaminath A, Kozak M, Weintraub J, Koong AC, et al. (2011) Stereotactic body radiotherapy for colorectal liver metastases: a pooled analysis. Cancer 117: 4060-4069.
- Takeda A, Sanuki N, Kunieda E1 (2014) Role of stereotactic body radiotherapy for oligometastasis from colorectal cancer. World J Gastroenterol 20: 4220-4229.
- Kress MS, Collins BT, Collins SP, Dritschilo A, Gagnon G, et al. (2012) Stereotactic body radiation therapy for liver metastases from colorectal cancer: analysis of safety, feasibility, and early outcomes. Front Oncol 2: 8.
- Barney BM, Olivier KR, Miller RC, Haddock MG (2012) Clinical outcomes and toxicity using stereotactic body radiotherapy (SBRT) for advanced cholangiocarcinoma. RadiatOncol 7: 67.
- Yoon H, Oh D, Park HC, Kang SW, Han Y, et al. (2013) Predictive factors for gastroduodenal toxicity based on endoscopy following radiotherapy in patients with hepatocellular carcinoma. StrahlentherOnkol 189: 541-546.
- Momm F, Schubert E, Henne K, Hodapp N, Frommhold H, et al. (2010) Stereotactic fractionated radiotherapy for Klatskin tumours. RadiotherOncol 95: 99-102.
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2a | Figure 2b | Figure 2c |
 |
 |
 |
 |
| Figure 3 | Figure 4a | Figure 4b | Figure 5 |
Relevant Topics
- Abdominal Radiology
- AI in Radiology
- Breast Imaging
- Cardiovascular Radiology
- Chest Radiology
- Clinical Radiology
- CT Imaging
- Diagnostic Radiology
- Emergency Radiology
- Fluoroscopy Radiology
- General Radiology
- Genitourinary Radiology
- Interventional Radiology Techniques
- Mammography
- Minimal Invasive surgery
- Musculoskeletal Radiology
- Neuroradiology
- Neuroradiology Advances
- Oral and Maxillofacial Radiology
- Radiography
- Radiology Imaging
- Surgical Radiology
- Tele Radiology
- Therapeutic Radiology
Recommended Journals
Article Tools
Article Usage
- Total views: 11089
- [From(publication date):
April-2016 - Jul 13, 2025] - Breakdown by view type
- HTML page views : 10164
- PDF downloads : 925
