Short Communication Open Access
ABCB1 Gene Polymorphisms Associated with Risk of Parkinson's Disease and Their Functional Relevance
Ramakrishnan V* and Akram Husain RS
Genetics Laboratory, Faculty of Allied Health Sciences, Chettinad Academy of Research and Education, Kelambakkam, Chennai, India
- *Corresponding Author:
- Ramakrishnan V
Faculty of Allied Health Sciences,
Chettinad Academy of Research and Education,
Chettinad Health City, Kelambakkam- 603 103
Tamil Nadu, India
Tel: 0039-40-3994622
Tel: 04447419041 E-mail: rkgenes@gmail.com
Received Date: August 18, 2016; Accepted Date: September 27, 2016; Published Date: October 04, 2016
Citation: Ramakrishnan V, Akram Husain RS (2016) ABCB1 Gene Polymorphisms Associated with Risk of Parkinson’s Disease and Their Functional Relevance. J Alzheimers Dis Parkinsonism 6:265. doi: 10.4172/2161-0460.1000265
Copyright: © 2016 Ramakrishnan V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Parkinson’s disease (PD) is the most common human neurodegenerative disease characterized by resting tremor, bradykinesia, muscle rigidity, impaired postural reflexes, psychiatric symptoms and aggregation of α-synuclein protein. This disease occurs in autosomal recessive pattern, affecting 3% of population above 65 years [1]. The environmental and genetic factors have been documented to play vital role in the pathogenesis of Parkinson’s disease [2]. Numerous genes have been found to be associated with PD risk in both sporadic, familial patients through Genome-wide Association Studies (GWAS) by next generation sequencing and genotyping methods such as PINK1, SLC45A3, SNCA, ACMSD, PARKIN, ATP13A2, GSTs, VPS35, HLA, GBA, RIT2, LRRK2, MTHFR and ABCB1 [3].
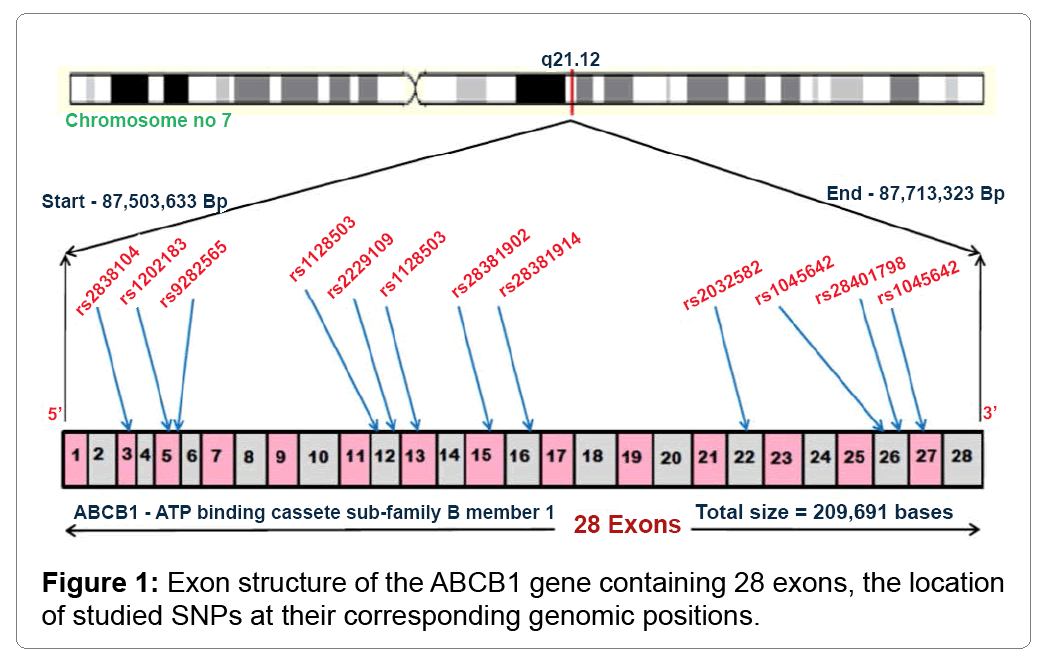
Pesticide exposure is known environmental risk factor for Parkinson’s disease. A relationship between pesticides and PD was first described in the year 1983 by the use of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) [4]. The exposure to pesticide and other environmental substances alone is not sufficient to cause PD, other factors, such as genetic variants and polymorphisms in genes are necessary in PD development [5]. One of such gene is ATP-binding cassette; sub-family B (MDR/TAP), member 1 (ABCB1) gene encodes the P-glycoprotein (P-gp), a transmembrane protein (170kDa) belonging to conserved super family of ATP-binding cassette (ABC) transporters. The P-gp is an essential component of the Blood Brain Barrier (BBB) responsible for active efflux of molecules from brain endothelium. This glycoprotein influences the bioavailability and therapeutic response of various drugs such as anti-epileptic’s, anti-depressants, anti-psychotics and glucocorticoids [6]. ABCB1 gene plays crucial role in regulating, protecting the intra-cerebral concentrations caused by neuro-toxicants [7]. Till date, more than hundred single nucleotide polymorphisms (SNPs) have been identified in ABCB1 gene, which are localized in the promoter, intronic and exonic regions (Figure 1) [8].
Even though there are several polymorphisms, only some of them are functionally evaluated and found to be associated with PD development. Likewise ABCB1 gene polymorphisms were widely studied in PD patients of Asian and Caucasian ethnic background.
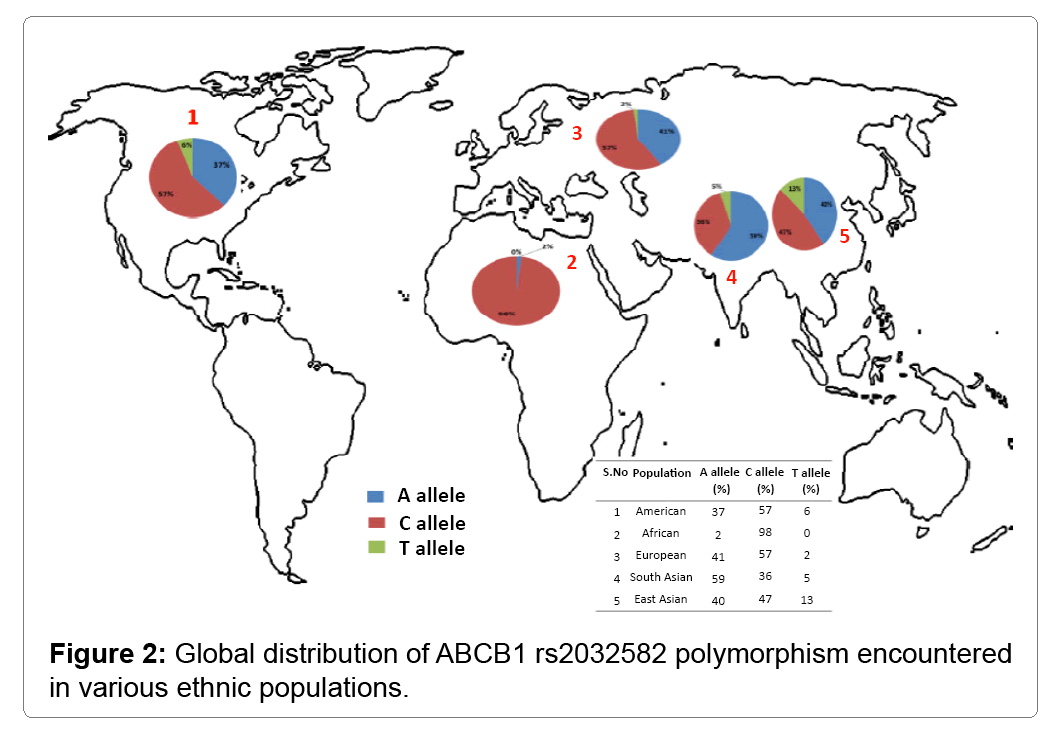
In addition to PD, ABCB1 SNPs have been studied in the other neurological disorders including epilepsy, bipolar disorder and Alzheimer’s disease [9]. This review focuses on the functionally relevant genetic polymorphisms observed in ABCB1 gene and 24 synonymous variants have been identified in the exonic region. The synonymous rs1128503 (C1236T) polymorphism located in 12th exon, C>T refers to the glycine residue placed in the external surface of N-terminal domain which shows to have effect on protein folding and influence on some drug inhibitors [10]. The rs1045642 c.3435C/T (Ile1140Ile) SNP located in 27th exon often referred as silent polymorphism, C>T correlates to isoleucine residue observed in the internal regions of C-terminal domain which cause differential expression of P-gp in neuronal tissues, plasma drug concentration and decrease in mRNA stability [11]. The nonsynonymous triallelic variant c.2677G/T/A (Ala893Ser/Thr) rs2032582 found in 22nd exon has shown the effects on protein function and found to be associated with alteration of digoxin efflux in vitro studies [12]. Figure 2 explains the global distribution of rs2032582 polymorphism which has been studied under the 1000 Genomes Project [13]. To date, there are several publications that document the presence of these three SNPs with their genetic predisposition, progression and response to treatment [14]. The mechanism behind synonymous genetic variants predisposes to specific diseases are unknown; it may be possible effect of altered mRNA stability or due to protein conformation. Additionally, disease-associated SNP may not be causal itself, it might be in the linkage disequilibrium with another causal variant present in the same region or in closely located gene. The distribution of associated SNPs may vary from one geographical location to another, strengthening the importance of screening in unrelated geographic populations. It is also recommended to evaluate a wider range of ABCB1 polymorphisms that can be correlated with several neurological disorders.
References
- Brown RC, Lockwood AH, Sonawane BR (2005) Neurodegenerative diseases: An overview of environmental risk factors. Environmental Health Perspectives113: 1250-1256.
- BekrisLM, Mata IF, Zabetian CP (2010)Thegenetics of Parkinson disease. Journal of Geriatric Psychiatry and Neurology 23: 228-242.
- Lu Y, Tan L, Shen N, Peng J, Wang C, et al. (2016) Possible association of CCDC62 rs12817488 polymorphism and Parkinson’s disease risk in Chinese population: A meta-analysis. Scientific Reports 6: 23991.
- Langston JW, Ballard P, Tetrud JW, Irwin I (1983) Chronic Parkinsonism in humans due to a product of meperidine- analog synthesis. Science 219: 979-980.
- Brown TP, Rumsby PC, Capleton AC, Rushton L, Levy LS (2006) Pesticides and Parkinson's disease-is there a link? Environmental Health Perspectives114: 156-164.
- Loscher W, Potschka H (2005) Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2: 86-98.
- Lee G, Bendayan R (2004) Functional expression and localization of P-glycoprotein in the central nervous system: Relevance to the pathogenesis and treatment of neurological disorders. Pharm Res 21: 1313-1330.
- Yan-Hong LI, Yong-Hua WA, Yan LI, Ling YA (2006) MDR1 gene polymorphisms and clinical relevance. ActaGeneticaSinica 33: 93-104.
- Tan EK, Chan DK, Ng PW, Woo J, Teo YY, et al. (2005) Effect of MDR1 haplotype on risk of Parkinson disease. Archives of Neurology 62: 460-464.
- Zschiedrich K, König IR, Brüggemann N, Kock N, Kasten M, et al. (2009) MDR1 variants and risk of Parkinson disease. Journal of Neurology 256: 115-120.
- Zhou Z, Chen Q, Zuo D, Wang H, Hua Y, et al. (2015) ABCB1 (rs1128503) Polymorphism and response to chemotherapy in patients with malignant tumors-evidences from a meta-analysis. Int J ClinExp Med 8: 265-272.
- Brambila-Tapia AJ (2 013) MDR1 (ABCB1) Polymorphisms: Functional effects and clinical implications. Rev Invest Clin 65: 445-454.
- 1000 Genomes Project Consortium (2015) A global reference for human genetic variation. Nature 526: 68-74.
- Maraganore DM, De Andrade M, Lesnick TG, Strain KJ, Farrer MJ, et al. (2005) High-resolution whole-genome association study of Parkinson disease. The American Journal of Human Genetics 77: 685-693.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 11886
- [From(publication date):
October-2016 - Jul 03, 2025] - Breakdown by view type
- HTML page views : 10974
- PDF downloads : 912