Research Article Open Access
A Tailored Self-Assembling Monolayer for Monitoring Biomarkers in Cell Culture Media Using the liSPR System
| Anja Henseleit*, Natalie Haustein, Carolin Pohl, Thomas Bley and Elke Boschke | |
| Technische Universität Dresden, Institute of Food Technology and Bioprocess Engineering, 01062 Dresden, Germany | |
| Corresponding Author : | Anja Henseleit Technische Universität Dresden Institute of Food Technology and Bioprocess Engineering 01062 Dresden, Germany Tel: +49 0351 46334272 Fax: +49 0351 46337761 E-mail: anja.henseleit@tu-dresden.de |
| Received: November 13, 2015; Accepted: November 28, 2015; Published: December 9, 2015 | |
| Citation: Henseleit A, Haustein N, Pohl C, Bley T, Boschke E (2015) A Tailored Self-Assembling Monolayer for Monitoring Biomarkers in Cell Culture Media Using theliSPR System. J Biochip Tissue 5:112. doi:10.4172/2153-0777.1000112 | |
| Copyright: © 2015 Henseleit A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Journal of Bioengineering and Bioelectronics
Abstract
Surface plasmon resonance (SPR) spectroscopy is a promising chip-based biosensor technology that can quantify various biomarkers without molecular labels or time-consuming sample preparation. The liSPR spectrometer is an inexpensive, convenient, portable, and robust SPR system. A drawback is that the chips’ sensing surfaces are fragile as they consist of a thin layer of gold sputtered directly on a polymer slide, without the Cr or Ti adhesive layers typically used in other SPR chips. Furthermore, interfering substances, particularly non-target proteins, may adsorb to the gold due to its hydrophobicity. This can lead to non-specific sensor signals or even inactivation of the ligands and hence false SPR signals. Thus, to improve the system’s reliability the bare gold surface must be modified.
Here, we present a tailored sensor surface for measuring human serum albumin (HSA) levels of human hepatocytes cultivated in vitro in media containing huge numbers of proteins capable of adsorbing to a bare gold surface. The key feature is a mixed self-assembled monolayer (SAM) comprising two types of alkanethiols: one for covalently attaching ligand molecules – here antibodies – to the chip and one for suppressing non-specific adsorption of interfering sample components. In addition to describing the new sensor surface we present data on its levels of immobilized antibody, non-specific adsorption of cell culture components, and HSA binding capacities. Calibration curves (obtained using HSA-spiked media samples) are also presented showing that the rapid, convenient sensor system provides similar sensitivity and reliability to standard ELISA methodology.
| Keywords |
| Surface plasmon resonance (SPR); Human serum albumin (HSA); Self-assembled monolayer (SAM) |
| Introduction |
| Surface plasmon resonance (SPR) spectroscopy exploits the excitation of surface plasmons by light energy. In the typically used Kretschmann configuration [1], light passes through a prism and is then totally reflected at a liquid-metal interface [2,3]. |
| Under certain conditions (when the incident angle, phase, wavelength and intensity of the light are appropriate) the electrons in the metal resonate and a surface plasmon wave is generated within an evanescent field that propagates along the metal-liquid interface [4]. Consequently the intensity of the reflected light declines, and the reduction in intensity can be detected by a charge-coupled device (CCD) camera. |
| The excitation is highly influenced by changes in the refractive index close to the metal interface, which may be caused by molecular association and dissociation events on a sensor’s surface. In anglemodulated SPR systems the shift of the incident light angle, where the reflected light intensity dip occurs, is used to detect the target [5]. For this purpose, a target-specific ligand has to be attached to the sensor surface and the sample solution has to be injected by an integrated microfluidic system. |
| The first commercial optical SPR-based was the Biacore, initially produced by Pharmacia AB in the early 1990’s and subsequently GE Healthcare [6]. Since then increasing numbers and types of platforms have been released, ranging from low-cost benchtop to high-throughput, high-sensitivity systems. The platform specifically addressed here is the robust, inexpensive, angle-modulated bench-top liSPR system supplied by capitalis technology GmbH [7]. The disposable sensor chips of the liSPR system are polymer slides with a 50 nm layer of gold. The optical elements are integrated at the bottom of the sensor chip, which makes the design compact [5]. However, only chips with bare gold surfaces are currently offered by capitalis technology, in contrast (for instance) to the pre-functionalized carboxylated sensors available for the Biacore system. Furthermore, there is a lack of reproducible functionalization protocols for the fragile gold layer of theliSPR chips. |
| The absence of pre-functionalization is disadvantageous for probing samples containing non-target proteins, because they tend to adsorb to gold randomly. This can lead to both non-specific sensor signals and denaturation of antibodies used as ligands. Thus, for the specific identification and determination of a target, surface functionalization, for instance by creation of a self-assembled monolayer (SAM), is needed. |
| SAMs that are widely used to provide gold surfaces with tailored functionalities consist of alkanethiols [3,8]: chains of saturated hydrocarbons, with a thiol group at one end and an anchor group, which can be used for ligand immobilization, at the other. They spontaneously form a functional SAM in the presence of a gold sensor surface via chemisorption of the thiol groups on the gold sensor surface, and van-der-Waals forces between the chains [9]. Thus, alkanethiols of appropriate lengths form densely packed hydrophilic layers tilted at about 30° to the surface [10]. |
| To characterize the utility of the resulting SAM for measuring concentrations of target proteins in cell culture media using theliSPR system, in cooperation with Dr. Marx and colleagues (Institute of Biotechnology, TU Berlin), we have investigated its interactions with human serum albumin (HSA), a HSA-specific antibody, and nontarget proteins. |
| HSA is a heart-shaped, 66.5 kDa protein with three homologous domains [11]. In humans it is continuously synthesized by the hepatocytes [12] and is the most abundant blood plasma protein [13]. Thus, HSA production is often used to characterize the viability of in vitro cultivated hepatocytes [14-16]. Using of 3-dimensional culture systems, the typical network of the liver can be mimicked and hepatocytes cultures can be kept vital for several weeks [17]. Furthermore, hepatocytes can be co-cultivated with other tissues in separate chambers connected by a circulating microfluidic in so-called multi-organ chips or multi-compartmental bioreactors that simulate, potentially, the entire human body. This allows analysis of complex interactions between different cells and metabolic roles of various substances, as illustrated by the co-cultivation of liver and skin cells [16]. To monitor the hepatocytes’ viability the cited authors measured HSA levels during 28-day cultivation periods, and found much lower concentrations (3.3 - 3.8 nM) than in pure hepatocyte cultures (88 - 170 nM). In these and other previous experiments they measured HSA using a commercial ELISA (enzyme linked immunosorbent assay), which is reliable but inappropriate for rapid, high-throughput monitoring. Thus, a specific aim of this study was to develop a SPR assay for monitoring HSA levels in cell culture media from this multiorgan- chip. |
| To combine opportunities for stable attachment of HSA-specific antibodies and repellence of non-target proteins, we used a mixed SAM comprising two alkanethiols: an anchoring thiol consisting of a 11-carbon alkane chain and six EG groups terminated with a carboxyl group for covalent immobilization of the antibody; and a proteinresistant blocking thiol of the same alkane chain length but terminated only with three EG groups. The longer EG spacer of the anchor-thiol is intended to enhance the accessibility of the functional groups [18]. We varied ratios of these two alkanethiols to identify optimal ligand densities and protein-resistance for measuring the target protein in crude cell culture media. For this purpose we compared antibody immobilization levels, protein-resistance, and HSA binding capacities at each of the tested alkanethiol ratios. We also obtained comparative measurements using a standard ELISA technique to assess theliSPR system’s utility for measuring HSA concentrations of in vitro hepatocyte cultures. |
| Materials and Methods |
| Materials |
| Sensor chips were obtained from capitalis technology GmbH (Germany). HSA-specific antibodies and a Human Albumin ELISA Quantitation Set were purchased from Biomol GmbH (Germany). As a reference, Bromodeoxyuridine (BrdU)-specific antibodies were obtained from BioLegend Inc. (USA). MaxiSorp immunoplates were obtained from Nunc GmbH & Co. KG (Germany). TBST (Tris buffered saline with Tween® 20, pH 8.0) running buffer and HSA were purchased from Sigma-Aldrich Chemie GmbH (Germany). The blocking-thiol (HS-C11-EG3) and anchoring-thiol (HS-C11-EG6- OCH2-COOH) were obtained from ProChimia Surfaces Sp. z o.o. (Poland). An amine coupling kit was purchase from GE Healthcare Europe GmbH (Germany), and UV/Ozone ProCleaner from NanoAndMore GmbH (Germany). All other chemicals were analytical grade and obtained from VWR International GmbH (Germany). |
| Preparation of the gold surface and the SAMs |
| Gold surfaces of the sensor chips were treated with UV/Ozone ProCleaner for 30 min and rinsed with pure ethanol. They were then incubated overnight at 30°C with mixtures of the alkanethiols prepared freshly from 10 mM stock solutions in degassed absolute ethanol, at ratios expressed as percentages of the anchor-thiol (e.g. a 70 % solution had a 70:30 anchoring to blocking thiol ratio). Following this incubation they were thoroughly rinsed sequentially with ddH2O, absolute ethanol, ddH2O, 100 mM HCl, 50 mM NaOH, 0.5% (v/v) SDS, and ddH2O, and finally dried under a nitrogen stream. |
| SPR measurements |
| The experiments were carried out using aliSPR system (capitalis technology GmbH, Germany) at 30 °C with a flow rate of 5 μL/s. The samples were pumped 100 times backwards and forwards over the sensor surface to enhance contact time without wasting sample solution. The sensing surface of each chip, as supplied, is a 50 nm thick, 12×3 mm gold layer [5,7]. The surface is illuminated by three LEDs, each monitoring a selected 9×0.8 mm area. A CCD camera with a spatial resolution of 1280 rows and angular intensity distribution of 960 columns detects the reflected light. In our experiments levels of protein bound to the surface were measured in pixels, each roughly corresponding to 41 resonance units (RU) or 41 pg/mm2 [19]. The raw data were evaluated using Microsoft Excel. |
| Examination of protein-resistance |
| SAMs with each ratio of the alkanethiols were prepared to test their protein-resistance by incubating them with undiluted HepaRG medium (William’s Medium E, 10 % fetal calf serum, 100 units per mL penicillin, 100 mg/mL streptomycin, 5 mg/mL human insulin, 2 mM L-glutamine, and 50 μM hydrocortisone hemisuccinate), which is used to cultivate hepatocytes in the 3-D system mentioned above [16]. The details of the medium and the cultivation are described elsewhere [16]. They were then rinsed with ddH2O for 3 min and the degree of nonspecific adsorption was determined by measuring their SPR responses, using ddH2O as running medium. |
| Antibody immobilization |
| The antibodies were immobilized using an amine coupling kit following the manufacturer’s instructions, with 10 mM sodium acetate (pH 4.5) as both running liquid and diluent. Initially, the carboxylated surfaces were activated for 10 min by injecting 1-ethyl-3-(3-(dimethyl amino)-propyl)carbodiimide/N-hydroxysuccinimide (EDC/NHS). Then they were incubated with a 1.4 μM solution of the antibodies for 10 min, followed by ethanolamine-HCl for 10 min to block the remaining active groups. Finally, after flushing with sodium acetate their immobilization levels were determined from the difference between SPR signals before activation and after the blocking [20]. |
| Specific HSA binding capacity |
| To assess the specificity of the HSA detection a reference surface was prepared too, offline, by immobilizing HSA-specific as well as BrdUspecific antibodies by inverse micro-contact printing in the 17-channel connection plate [5], using 5 μL of the antibody solutions and the amine coupling kit according to the manufacturer’s instructions. The carboxylated surface was activated for 10 min by incubation with EDC/NHS, then with 1.4 μM of the antibodies dissolved in 10 mM sodium acetate (pH 4.5) for 1 h. After blocking with ethanolamine- HCl for 30 min HSA solutions at selected concentrations in HepaRG medium were injected onto the antibody-modified surface for 10 min. Dissociation was monitored by replacing the sample solution with TBST for about 3 min. The degree of binding was determined by measuring the SPR signal shortly after injection stopped. Since this resulted in incomplete dissociation, HSA was finally removed from the antibodies by injecting 100 mM glycine-HCl (pH 2.2) for 72 s to regenerate the antibody surface. |
| ELISA measurements |
| For comparative measurements HSA was measured using the Human Albumin ELISA Quantitation Set according to the manufacturer’s instructions. Briefly, 69 nM solutions of HSA-specific antibody were incubated for 1 h at 25°C on MaxiSorp immunoplates. After the washing and blocking steps, HSA samples dissolved in HepaRG medium were added to and incubated in the antibodymodified wells for 1 h at 25°C. HSA was detected by adding 0.3 nM secondary horseradish peroxidase (HRP) labeled HSA-specific antibody and the substrate 3,3',5,5'-tetramethyl benzidine. After 15 minutes, the reaction was stopped by adding 0.18 M H2SO4 and the resulting absorbance at 450 nm was measured using a Genios Multifunction Fluorescence, Absorbance and Luminescence Microplate Reader (Tecan Group Ltd., Switzerland). To avoid erroneous signals due to contaminants (e.g. dust) in the wells, a reference measurement at 580 nm was subtracted from all signals. |
| Results and discussion |
| Examination of the protein-resistance |
| Although functionalization of gold surfaces using alkanethiols has been described in several articles [18,21], such protocols for theliSPR system are still lacking. A complicating factor is that the gold layer of liSPR chips is sputtered directly on the polymer slides without the Cr or Ti adhesion layer typically used in other SPR systems. Hence, common functionalization protocols, e.g. Technical Bulletin AL-266 from Sigma-Aldrich, Inc. based on sonication or long exposure to acidic ethanol (pH 2) would lead to detachment of the gold from theliSPR chips. Thus, we have established a specific functionalization protocol for theliSPR system, involving incubation with alkanethiols in pure ethanol overnight followed by extensive rinsing steps instead of sonication to remove the unbound alkanethiols. |
| A surface functionalization procedure should both stably immobilize the antibodies and provide resistance to non-specific binding by potentially interfering sample components. Non-specific adsorption induces high background signals, thereby reducing sensitivity and potentially generating false-positive outcomes. Thus, we introduced the blocking-thiol into the SAM to enhance the proteinresistance of the sensing layer. Proteins may adsorb non-specifically to the sensor, by replacing water molecules in the interphase between the surface and bulk solution at levels depending (inter alia) on their molecular size [22]. Thus, for measuring targets in real samples, ethylene glycol (EG) groups are often integrated into the alkanethiols because of their extraordinary hydrophilicity, and thus repellence of hydrophobic substances [23,24]. To attach antibodies the SAM has to be functionalized with carboxyl (COOH) anchor groups, to which target-specific antibodies can be covalently immobilized via their primary amine groups using an amine coupling procedure. |
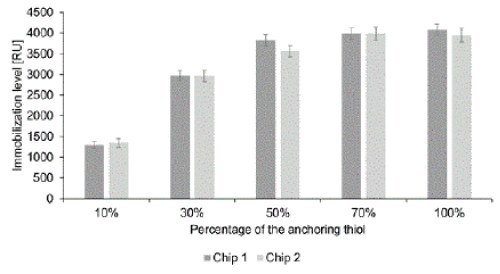
| To check that the functionalization of theliSPR gold surface worked as expected, we first measured the non-specific adsorptivity of the SAMs with each tested ratio of thiols, without immobilized antibodies, by exposing them to HepaRG medium for 10 min, followed by a 3-min rinsing. Thus, only strongly bound molecules that were not removed by such rinsing contributed to the measured signals. Rinsing is essential in SPR to avoid deviations in the bulk refractive index interfering with the sensor signal. However, we used ddH2O as eluent to avoid possible interference from buffer components, such as salts or the surfactant Tween® 20. The results are shown in Figure 1. |
| The degree of non-specific binding was highest with the bare gold surface. In addition, in trials with the SAMs it increased with increases in concentration of the anchoring thiol, as expected since the carboxyl group induces some fouling through electrostatic interactions [25]. The SAMs with the highest and lowest anchoring thiol contents (100 and 10%), adsorbed 48 and 95% less protein than the bare gold surface, respectively, showing that the functionalization worked as expected and created a hydrophilic surface. |
| To obtain information about the homogeneity of the SAMs we determined standard deviations of amounts of proteins nonspecifically bound in the selected 9×0.8 mm sensing area. The results show that the homogeneity was very similar for the pure anchoring thiol SAM and gold surface, but decreased with increases in the concentration of the blocking thiol (the standard deviation for the 10% anchoring thiol SAM was six times higher than the standard deviation for the gold surface). Such deviations could be due to phase separation of the two alkanethiols; a known problem of mixed SAMs [21]. |
| Immobilization of the antibodies |
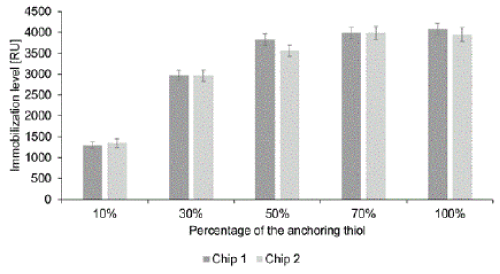
| Another requirement for the mixed SAM is stable immobilization of an appropriate antibody (via covalent bonding to the displayed COOH groups of the anchoring thiols) to enable continuous specific monitoring of HSA levels during cultivations. To compare the antibody immobilization capacity of the SAMs with each alkanethiol ratio we determined amounts of the HSA-specific antibody immobilized from a 1.4 μM solution (Figure 2). |
| The immobilization level of the antibody increased with increases in the anchoring thiol content up to 50%, at which the surface seems to be saturated. Previous analysts have even detected lower antibody immobilization levels when using a SAM consisting solely of anchoring thiol than when using one with 10% anchoring thiol [21], probably because a SAM with a single chain length hinders access to the activated carboxyl groups. Accordingly, in our experiments the different alkanethiol chain lengths of the mixed SAM appeared to enhance accessibility of the anchor group. The observed immobilization levels were also within the range recorded for dense antibody monolayers, giving 1300 - 6500 RU signals [26]. It should be noted that high antibody concentrations are desirable for concentration measurements [27], but too dense packing can hinder access to the antigen binding sites [28]. |
| Specific HSA binding capacity |
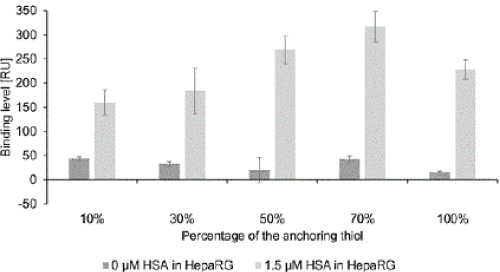
| To identify the optimal composition of the SAM, the target binding capacity must also be considered. Therefore, the specific binding of HSA was determined by injecting HepaRG medium spiked with 1.5 μM HSA (Figure 3). To ensure that positive results were not falsepositives due to interfering sample components we also measured HSA binding to a reference surface with immobilized bromodeoxyuridine (BrdU)-specific antibody. BrdU is a synthetic analog of thymidine used for labeling nucleic acids. Thus, it has no relation to HSA or any other sample components. |
| The binding signals for HSA increased with increases in the anchoring thiol content up to 70%. Surprisingly, they were weaker with the 100% anchoring thiol SAM, possibly because if the concentration of anchoring groups is too high the antibodies may bind via multiple primary amines, thereby limiting their flexibility and reducing their activity [29]. The results obtained using the reference antibody confirmed the specificity of our system for HSA. |
| In addition to the HSA-specific binding capacity, we investigated the degree of non-specific binding of substances in the HepaRG medium to the immobilized HSA-specific antibodies. In contrast to the results obtained without immobilized antibodies (Figure 1), no obvious trend in non-specific binding was detected with increasing concentration of the carboxyl groups. Thus the antibody molecules presumably cover the carboxyl groups, which has an additional blocking effect, as carboxyl groups induces some fouling through electrostatic interactions. |
| The SAM with the 70% thiol ratio provided the strongest HSA binding signals, high homogeneity (a standard deviation of just 7% in measurements using four channels per chip) and reproducibility of 99% (from the two independent measurements). Thus, to test the potential of the proposed assay for measuring HSA concentrations in cell culture media we obtained a concentration series using this SAM, and samples spiked with HSA at concentrations ranging from 0.8 to 385 nM. |
| liSPR vs. ELISA sensitivity |
| HSA concentrations in the multi-organ chips mentioned above have been previously determined by a sandwich ELISA, in which HSAcontaining samples are incubated in wells with previously immobilized HSA-specific antibodies and bound HSA is then recognized by an enzyme-labeled secondary HSA-specific antibody [16]. Since the antibody used is polyclonal, the same antibodies can be used for either the immobilization or recognition step. |
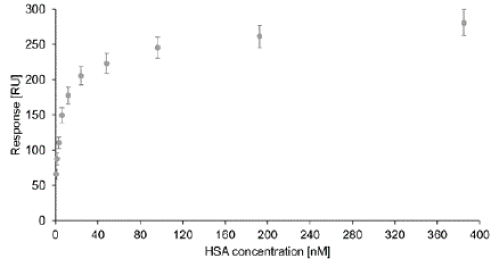
| As shown in Figure 4, a typical saturation curve was recorded, with a limit of detection (LOD) of the proposed assay of 59 RU (mean signal from a pure HepaRG media injection plus three times the standard deviation of measurements from six channels). Thus, by using the 70% anchor thiol SAM and the proposed SPR assay it is possible to detect as little as 0.8 nM HSA specifically in cell culture media containing up to 10% fetal calf serum. |
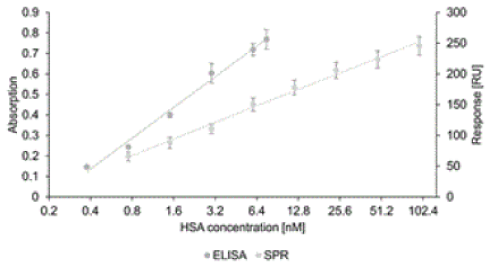
| This assay was used to generate a calibration curve, similar to the SPR-generated curve, using samples of HSA spiked in HepaRG medium at concentrations ranging from 0.1 to 7.5 nM. In both cases, to determine unknown HSA concentrations in cell culture media the log-linear ranges below the saturation levels of the curves should be used, as illustrated in Figure 5. |
| The regression equation and correlation coefficient obtained from the ELISA were Δabsorption = 0.2167 × ln(HSA) + 0.3329 and 0.9912, respectively, for HSA concentrations ranging from 0.4 to 7.5 nM. The regression equation and correlation coefficient obtained using the SPR assay were ΔRU = 38.65 × ln[HSA] + 75.539 and 0.9923, respectively, for concentrations ranging from 0.8 to 96.2 nM HSA. |
| Minimal HSA concentrations in media when hepatocytes purely and together with skin cells cultivated in the multi-organ-chip system mentioned above reportedly range from ca. 3.3 to 170 nM [16]. This means, cell culture samples containing HSA concentrations above 96.2 nM (e.g. from pure hepatocyte cultures or advanced cultures) have to be diluted at least 1 to 30 for the SPR assay in a defined manner. This is necessary due to the saturation of binding signals above 96.2 nM. |
| However, unlike the SPR assay the ELISA relies on enzyme-based signal enhancement, the additional recognition steps increase costs and operation times, and of course a labeled counterpart of the target is needed. Furthermore, ELISAs consume time and samples, while in marked contrast theliSPR instrument affords high-sensitivity with minimal sample requirements and real-time detection. |
| Conclusion |
| Previously published methods for functionalizing SPR chips cannot be applied toliSPR chips as they have no adhesive layer to stabilize the gold on the polymer slides. |
| Thus, we have established a new, milder functionalization protocol. It involves formation of a SAM composed of two alkanethiols with different functionalities designed to provide resistance to non-specific protein binding and opportunities for stably attaching ligands. |
| A specific aim was to obtain a for quantifying HSA in cell culture media using theliSPR system. Thus, the ratio of the alkanethiols was optimized in terms of immobilization levels of HSAspecific antibodies, non-specific adsorption of interfering substances and HSA binding capacities. A SAM containing 70% of the alkanethiol capped with carboxyl groups yielded the best results and was investigated further in comparative trials with a well-established ELISA. Robust ELISA and SPR calibration curves were obtained, using samples of HepaRG medium spiked with HSA, providing log-linear ranges of 0.4 - 7.5 nM HSA and 0.8 - 96.2 nM HSA, respectively. The results show that both assays are capable of monitoring HSA levels of in vitro hepatocyte cultures. However, the presented SPR assay avoids the need for any additional molecular label and provides opportunities for high-throughput multiplexing [7]. In further work we aim to develop the system to enable simultaneous detection of multiple biomarkers in single samples of cell culture media, using sensing spots with different target-specific ligands. |
| The presented functionalization and immobilization protocols could also be easily adapted for other applications, with appropriate adjustments of alkanethiol ratios in the SAMs if necessary. |
| Acknowledgements |
| The authors thank Dr. Yildiz (Department of Chemistry, Izmir Institute of Technology, Turkey) as well as Prof. Dr. Homola and M. Bocková (Institute of Photonics and Electronics, Academy of Sciences, Czech Republic) for useful discussions. We also thank E.-M. Materne (Institute of Biotechnology, TU Berlin) for providing the HepaRG medium of the multi-organ chip. This work was financially supported by the European Union and the Free State of Saxony (SAB project UNILOC). |
| References |
|
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 10440
- [From(publication date):
December-2015 - Jul 01, 2025] - Breakdown by view type
- HTML page views : 9587
- PDF downloads : 853
