A Systematic Review on Evolving the Bowel Cancer Screening Programme in England: Targeted Screening for Individuals with First Degree Family History of Bowel Cancer
Received: 20-May-2022 / Manuscript No. jcmhe-22-64367 / Editor assigned: 23-May-2022 / PreQC No. jcmhe-22-64367 (PQ) / Reviewed: 06-Jun-2022 / QC No. jcmhe-22-64367 / Revised: 13-Jun-2022 / Manuscript No. jcmhe-22-64367 (R) / Published Date: 20-Jun-2022 DOI: 10.4172/2168-9717.1000758
Abstract
First degree relatives of bowel cancer cases have an approximately twofold risk of the disease compared with those without a family history, and the more affected first degree relatives an individual has, the higher the absolute and relative risk for developing bowel cancer. At present, widespread screening of average risk individuals for bowel cancer occurs in England; however, the Bowel Cancer Screening Programme does not currently provide a framework for the identification of a family history of bowel cancer. The aim is to determine the cost effectiveness and benefits of offering targeted screening to individuals with a first degree family history of bowel cancer. The primary outcomes examined the prevalence of developing bowel cancer and estimated the cost effectiveness to inform screening policy decisions. The secondary outcome evaluated the possible screening barriers in the adoption of bowel screening of first degree relatives. Data presented suggests that screening first degree relatives of patients with bowel cancer is likely to be cost effective to the NHS, and that compared with the average risk group using colonoscopy, advanced neoplasia was significantly more prevalent in those with first degree relatives with bowel cancer. Targeted screening would mean less long term health costs however, given the limited resources on NHS cost analysis data, cost savings associated with targeted screening are largely unknown; although data presented from European studies strongly suggests that it may be optimistic. Opportunities for this review include its recommendation to modify the screening criteria to extended to first degree relatives of bowel cancer patients and for the National Screening Committee to implement this protocol nationwide.
Keywords: Bowel cancer; First degree relatives; Family history; Colonoscopy; Systematic review
Introduction
Bowel cancer is the second most common cause of cancer related mortality in men and women in the United Kingdom [1]. The earliest phases of bowel cancer start with a generalised disorder of cell replication and with the appearance of masses of enlarged polyps showing proliferative abnormalities. The bowel is part of the digestive system and is divided into two parts: the small bowel and the large bowel. Bowel cancer, also known as colorectal cancer (CRC), starts when cells in the bowel lining become damaged and then grow exponentially, forming a cluster of cells called a tumour [2]. Most bowel cancer cases will develop from these precancerous polyps; however, this progression from cancerous polyps to early invasive cancer can take years. The phase between early and late stage cancerous cells is estimated to be five to ten years [3]. Therefore, early diagnosis is crucial because patients diagnosed with bowel cancer at the earliest stage have a better than 90% chance of surviving for five years, while for those diagnosed at the latest stage, it drops down significantly to just 6% [4].
Approximately 10% of all bowel cancer cases are thought to be caused by a change in a known gene that can be passed down through a family. More specifically, individuals have a much higher chance of developing bowel cancer with a genetic condition called Lynch syndrome, which causes approximately 3% of all bowel cancer cases. Therefore, if one or more relatives have had a known genetic condition linked to bowel cancer, such as Lynch syndrome, it may also indicate increased risk, and they are more likely to be diagnosed at a younger age [1]. Cairns et al. classified the risk of developing bowel cancer based on family history without identification of any hereditary conditions into two groups: a high moderate risk group, where three or more relatives (in a first degree association: parent, brother, sister or child) have been affected over the age of 50 years or more than two relatives affected before the age of 60; and a low moderate risk group, where one first degree relative under the age of 50 or two first degree relatives over the age of 60 have been affected [5]. The more affected first degree relatives (FDRs) an individual has, the higher his or her absolute and relative risk for developing bowel cancer [6].
At present, only widespread screening of average risk individuals for bowel cancer occurs in the United Kingdom; however, the Bowel Cancer Screening Programme does not currently provide a framework for the identification of a family history of bowel cancer. Given the prevalence of bowel cancer in the UK, examining other factors that focus on potentially higher risk, yet still asymptomatic individuals with a first degree family history of bowel cancer (where first degree relation is defined as one’s parent, sibling or child related by blood) and maybe a reasonable subgroup of the population for early standardised screening.
To set the stage, first, this article will present a literature review on the history of the practices currently in place for bowel cancer screening. Then, we observed the data on the prevalence of adenomas in first degree relatives, especially below the age of 50, as it will be useful for estimating the yield of screening before the age of 50. Finally, we refined estimates of cost effectiveness for individuals in this age group.
In writing this review, the goal is to determine whether offering colonoscopy as the primary screening, through the Bowel Cancer Screening Programme, to individuals with a first degree family history of bowel cancer would be more cost effective and beneficial prior to the national screening eligibility age. In addition, individuals were distinguished according to the number of affected first degree relatives. The objectives of this review are to systematically assess the literature on the effectiveness of colonoscopy screening in individuals with an increased risk of a bowel cancer family history. Therefore, it is necessary to demonstrate the prevalence of adenomas and bowel cancer in first degree relatives, as well as its potential for cost effectiveness and uptake rates to attain national screening optimisation and achieve most of the screening value.
Background
In England, screening programmes for cancer are only recommended for cervical, breast and bowel cancer. The National Bowel Cancer Screening Programme (BCSP) began operating in 2006 to discover bowel cancer disease in its early stages and treat it adequately before it poses a threat to the individual [7].
The programme is coordinated by five regional screening hubs, and in each region, individuals who are registered with a general practitioner are eligible for screening from age 55 years, and then at age 60 years, biennially, thereafter up to and including age 74 years. At present, the Bowel Cancer Screening System (BCSS) is used as a source of demographic information, excluding prior subject medical history information, to identify eligible men and women residing in England who are within the screening age range and registered with a GP practice. With the experience of working in one of the screening programme hubs, several requests are received with enquiries to self-refer to the programme from individuals who have not been invited to take part in screening because they do not meet the current eligibility age for screening; however, they call attention to having a family history of bowel cancer.
Consequently, they are advised to either wait to be eligible for screening or to contact their GP if they are experiencing symptoms of bowel cancer. This unavailable service and circumstance prompted an interest in pursuing an investigation around screening for increased risk individuals with a family history of bowel cancer and identifying an opportunity for further research in providing a service that BCSP is currently lacking.
Literature Review
A literature search was undertaken using the BPP Online Library to find journal articles for this study. This critical review of the literature on bowel cancer is an important initial first step to understanding what other research has already been done in this area. Numerous healthcare databases, multiple search engines, and various journal searches were used. Material from abstracts was reviewed if the full text articles were unavailable, and keyword searches were used according to relevance. This review aims to provide a perspective on how the topic area of interest has developed and to identify the current state of research to recognise its key issues. It is confined to a discussion of bowel cancer screening in countries in the European Union (E.U.) and the United States (U.S.A.).
Screening for bowel cancer
The World Health Organisation defines screening as the process of using simple tests to identify a healthy population who may be at increased risk of a condition but may appear asymptomatic [8]. The WHO states that the success of a screening programme for a population depends upon specific fundamental principles. First, the target disease should be a common form of cancer with high morbidity and mortality. Second, the screening tests should be acceptable, safe, accurate and relatively inexpensive. Sensitivity is defined as the effectiveness of a test in detecting disease in those who have that disease. Specificity defines the extent to which a test gives negative results in those who are free of disease. Positive predictive value is defined as the extent to which subjects have the disease in those who provided a positive test result. Unlike screening programmes for breast and prostate cancers, bowel cancer screening has reduced mortality from colon cancer and detected early bowel cancer; it has also decreased the incidence of bowel cancer through the detection and removal of precancerous lesions. Studies have shown that screening for bowel cancer provided 152 to 313 life years gained per 1000. 40 year old individuals [9].
Bowel cancer screening recommendations
At any time, approximately nine million adults in England are eligible for bowel cancer screening [1]. Faecal immunochemistry test screening (FIT) is a newer version of the guaiac based faecal occult blood test (gFOBT) and is offered through BCSP. FIT carefully measures the colonic blood in a stool sample, since upper gastrointestinal globin is degraded readily by digestive proteolytic enzymes. It is an antibody to human globin that does not cross react with dietary meats [10]. The sensitivity of the test can be adjusted, which enables it to detect a trace amount of blood and thus identify more cancers earlier.
The programme invites individuals for screening from age 60 years and biennially thereafter up to and including age 74 years. Individuals are asked to collect a stool sample and to return the completed kit to the regional hub for processing. If the FIT yields an abnormal result, they are referred to their local screening centre for diagnostic investigations. In some areas of England, BCSP invites people aged 55 for a one off flexible sigmoidoscopy that examines inside the rectum and the lower sigmoid colon, where the majority of noncancerous growths called, polyps and other bowel cancers start. During the procedure, biopsies can be taken for examination in the laboratory, and if the samples yield an abnormal result, they are referred to a specialist cancer service [1]. In support of a petition signed by half a million people, in early 2019, England and Wales announced that they were lowering the bowel cancer screening age from 60 to 50 [1]. This was following a recommendation from the UK National Screening Committee (UK NSC), which is responsible for the review processes and setting recommendations for the bowel cancer screening programme in all four nations of the UK.
Implementation of the NSC recommendations varies across the UK, and currently, there are discussions on the future of combining FIT and flexible sigmoidoscopy in the screening programme that is under review. According to the report by the Sheffield School of Health and Related Research [11], which pointed out that cost effectiveness depends upon the proportion of those who are invited to partake, there is currently less than 50% for endoscopic screening but is projected to be close to 67% for FIT screening. In addition, the report highlighted the uncertainty of the available endoscopy resources required to provide the screening options, where England is reported to have only found resources to reach 43% of GP practices.
Currently, the following two options have been outlined by the NSC UK consultation for future consideration: (A) combine flexible sigmoidoscopy screening at trial uptake and quality standards to 58-60 year olds with a lower sensitivity FIT; or (B) offer FIT to 50-74 year olds at thresholds below 93 ug/g and decommission, not start, flexible sigmoidoscopy screening (or for most GP surgeries) as a primary test [1].
Bowel cancer screening interventions
Current screening options for bowel cancer include faecal occult blood testing; sigmoidoscopy and colonoscopy are all effective in preventing bowel cancer mortality versus no screening [12]. The evidence for and limitations of these various screening modalities are discussed. The overall goal of bowel cancer screening is to reduce cancer specific mortality in average risk individuals, which is supported by Lieberman for decreasing the burden of bowel cancer for early stage cancer and precancerous adenomas [13]. The argument for favoring fecal occult blood testing to endoscopic screening is mainly due to the exclusive focus of randomized control trials of the latter, and as a result, this can be misleading in the context of bowel cancer screening. There is still some controversy regarding the optimal screening intervention for bowel cancer with the current screening options.
The sensitivity of faecal immunochemical testing to detect blood in the stool can be adjusted to be more or less accurate. Allison et al. concluded that the measures of accuracy in FIT screening vary greatly between tests within a specific field of expertise and according to how the test is applied with various confusing cut off levels, which can make its application in real life complex [14]. The implication to adjust the sensitivity threshold determines the number of individuals referred for colonoscopy screening. Therefore, the lower the threshold is, the more sensitive the test is to faecal blood, which will require more colonoscopy referrals and increase the demand for endoscopy resources on the NHS. The BCSP has implemented FIT to be used suboptimally at a higher threshold (low level of sensitivity), which ultimately means that a vast number of cancers that could be prevented will go undetected [13].
Dating back to the 1990’s, an observational study by Winawer et al. cited in Zhang et al. recognised the major protective effect of lower gastrointestinal endoscopy against bowel cancer through the detection and removal of precancerous adenomas [12,15]. Supported by authors Anderson, et al. who aimed to identify factors that may predict finding isolated adenomatous lesions on screening colonoscopy, found that the sensitivity of colonoscopy for bowel cancer detection in the entire colon was 58%-75% [16]. A case–control study of subjects aged 70–85 years within the linked Surveillance, Epidemiology, and End Results (SEER) Medicare database reported that both screenings with flexible sigmoidoscopy and colonoscopy were associated with reductions in overall bowel cancer incidence, although the magnitude of the reduction was greater for colonoscopy [17]. More recently, data examining the incidence of bowel cancer in both sexes also recognised a 53% drop in both incidence and mortality related to colonoscopic removal of adenomatous polyps [10].
Contrasting findings are raised from an observational study looking at the benefit of FS screening in reducing the mortality and incidence of proximal bowel cancer in which the study implied that the benefit of FS is not uniform for bowel adenomas that arise in different areas of the bowel lining, endorsing colonoscopy screening as the better option, similarly reported by Mack et al. describing that colonoscopy is the gold standard of screening [18,19]. However, a case–control study Baxter et al. compared bowel cancer mortality after screening with colonoscopy and other screening interventions and found that it was the most invasive and costly screening intervention for bowel cancer [20]. Consequently, flexible sigmoidoscopy screening may emerge with a higher attendance rate than colonoscopy screening. The UK National Screening Committee has recommended bowel cancer screening in all average risk individuals above the age of fifty, supporting screening via faecal occult blood testing or flexible sigmoidoscopy. However, it does not currently provide a framework for screening age or invention in the high moderate risk population, explicitly first degree relatives of bowel cancer relatives.
Risk among first degree relatives of bowel cancer patients
A prospective study of bowel cancer risk among first degree relatives of patients with bowel cancer determined an age adjusted relative risk of bowel cancer in this group compared to the general population [19]. The relative risk increased in participants with two or more first degree relatives affected by bowel cancer. Similarly, a systematic review of case– control and cohort estimates that first degree relatives of bowel cancer patients have a twofold increased risk of developing bowel carcinoma [21]. Risk increased further with the increasing number of first degree relatives affected and in relatives of patients diagnosed with bowel cancer when less than 45 years of age. However, in individuals with less pronounced family histories, surveillance has been recommended from age 40 or ten years before the age of diagnosis of the youngest relative. The categorisation of high moderate risk group inclusion criteria consists of a familial combination where affected relatives are first degree relatives of each other with at least one being a first degree relative of the patient. If both parents are affected, these count as being a first degree relative and three affected first degree relatives of any age (for example, a parent and a blood related aunt/uncle and/or grandparent), at least one of whom is a first degree relative of the patient, or two siblings/one parent or two siblings/one offspring combinations, or both parents and one sibling. However, there should be no affected relative two siblings, no more than two children or child plus sibling. The high moderate risk groups are appropriately increased to warrant low intensity surveillance comprising 5 yearly colonoscopy between age 50 and age 75 years. As a recommendation for screening, polyps should be snared, and adenoma surveillance should be pursued thereafter if a benign neoplasm is confirmed.
Recommendations for screening first degree bowel cancer relatives
Screening participation across the UK is less than 60% of the population; therefore, an alternate strategy is to focus on potential higher risk groups [1]. These groups include those with a first degree family history of bowel cancer, which is at an increased level of risk but not as strong as those with a defined genetic syndrome [22]. The authors of Guidelines for Colorectal Cancer and the Surveillance in Moderate and High Risk Groups, Cairns et al. recommend that some months before surveillance is due, clinical validation of all individuals considered at increased risk of bowel cancer is necessary to ensure it is still appropriate [5]. There are no UK implementation strategies to introduce bowel cancer screening specifically among first degree relatives of individuals with bowel cancer.
However, European recommendations (Quirke, Lambert and Vieth) and other groups, including the American Society of Colon and the American Cancer Society (U.S. Preventive Services Task Force) do recommend more exhaustive bowel screening in this population starting at age 40 years or ten years prior to the age at diagnosis of their first degree relative [23,24].
The NHS Cancer Reform Strategy has guidance notes on bowel surveillance for individuals with a family history indicating a moderate risk, to ensure that surveillance colonoscopy is restricted to those who are most likely to benefit has assumed greater importance. Recommendations on referrals based on family history and at risk individuals are currently only coordinated through centres with a specialist interest, such as regional genetics services or medical gastroenterology centres. It acknowledges the need for high quality risk assessment and counselling for those at high risk from bowel cancer as a consequence of their family history, but currently, there is variability in service delivery according to local circumstances and availability of resources. NHS bowel screening pilot studies undertaken in England and Scotland have shown that the detection rate of adenomas is six to nine per 1000 examinations [5].
The BCSP has adopted this guideline with some modifications; however, there is still the need to quickly track interventions and improve patient access to screening by distinguishing those with a family history and presenting a moderate high risk of bowel cancer.
Screening participation and barriers to bowel cancer screening
Rawl et al. identified two distinct characteristics as barriers to screening: (A) the doctor's knowledge of the subject's risk of bowel cancer and (B) the subject's perception of risk and fears about diagnosis and screening [25]. In addition, published studies have shown that a major reason for nonattendance to flexible sigmoidoscopy screening has been a lack of belief that it is worth the personal effort to attend and its effectiveness in reducing bowel cancer mortality [26]. Among most of the cited reasons for nonparticipation, such as conflicts with work, inconvenience and being too busy, not having any current health problems or symptoms of bowel cancer was the most widely reported reason for nonadherence to screening appointments [27]. Given this evidence, one could infer that with an increased perception of personal risk for disease, individuals are likely to be more adherent to participating. A review by Kerrison et al. of several large randomised controlled trials (RCTs) showed that a single flexible sigmoidoscopy (FS) screen between the ages of 55 and 64 can significantly reduce the incidence and mortality of the disease among people who complete the diagnostic test [28]. In brief, improving bowel cancer screening participation involves not only having interventions with high sensitivity and specificity but also being user friendly, less disruptive (ideally one off) and considerate to the level of risk to secure high uptake, as proposed by Hoff [26].
NHS cancer reform strategy: Cost effectiveness
According to guideline data by Cairns et al. the NHS tariff permits an estimate of costs for bowel cancer follow up, using £80 for a review outpatient visit, £170 for a CT scan of chest abdomen and pelvis and £476 for a colonoscopy [5]. Over 5 years, assuming a relatively modest follow up regime, a six month outpatient visit with a CT scan at one year and a colonoscopy once in the five years of follow up would cost £1100 per patient over 5 years. Considering the population size and prevalence of those affected by bowel cancer, such a follow up programme would probably cost approximately £250 000 per annum. This figure highlights the expense of clinical practice with uncertain benefits. The National Strategy for Cancer aims to help the reformed NHS deliver cancer services that are amongst the best in the world. It is accompanied by a commitment to a £200 million cancer transformation fund and sets out a proposed new 5 year Cancer Strategy for England to deliver its recommendations [29].
In England, the current cancer spending has increased by 27% since 2015, and cancer is now the third largest disease programme, costing the NHS approximately £4.35 billion a year [29]. Lansdorp-Vogelaar et al. describe screening cost effectiveness analysis as a tool that correctly compares the health and economic consequences of different interventions [27]. It can assist in identifying the screening modality for adoption in population screening that will yield the greatest health benefits, given any resource constraints. An important characteristic of the effectiveness of any screening programme is quality; therefore, for any test to be effective, it must first be completed effectively. Research finds that only 50% adherence to follow up endoscopy after a positive faecal blood test undergoes follow up in US clinical practice [30]. Furthermore, ScHARR reports that cost effectiveness depends upon the proportion of those individuals who are invited to partake and a study by Vijan et al. on cost effectiveness estimation, best modelled adherence and the costs of screening as two potentially important variables [11,31].
The cost effectiveness analysis does not determine which intervention is optimal but rather which intervention will provide the greatest health and cost benefits; therefore, if there is good evidence that can indeed estimate an increase in screening participation amongst those who would have otherwise remained unscreened, then it would present an appropriate measure for consideration.
Optimal screening
The UK Bowel Cancer Screening Programme does not currently provide a framework for the identification of bowel cancer family history. Data collected up to more than 30 years demonstrate that a high incidence of advanced adenomas is increased in individuals with a family history of bowel compared to the general population, which may be detected at an early stage [32]. Walshe et al. highlight the importance of considering high risk strategies as part of population screening initiatives [33]. In their study, they found that the ‘low risk’ individuals (55.7%) were referred back to primary care without the need for colonoscopy, emphasising the potential benefits of differentiating real from perceived risk, enabling more appropriate allocation of screening resources. In Europe, screening guidelines for bowel cancer recommend colonoscopy for high risk patients and FIT for the standard risk group [23].
Mesher et al., suggested that a family history of bowel cancer may reduce the reliability of a negative FIT test, specifically by decreasing the negative predictive value; therefore, it is not possible to establish how biannual FIT (with full colonoscopy for those with a positive test) would compare to those at increased risk of bowel cancer [32]. In France, individuals with an increased risk for bowel cancer are identified using a standardised form joined to the invitation letter sent to all eligible individuals for screening. Sportes et al. show that those identified as high risk are then excluded from the screening programme using faecal tests and directly referred for colonoscopy [34]. This study also reports a high compliance rate of colonoscopy screening for the at increased risk population, perhaps as a result of the generalisation of bowel cancer screening throughout France, enabling better patient information about the disease.
Summary
In conclusion, considerable evidence in the literature describes an association between bowel cancer family history and higher advanced adenoma yield at screening, and the presented pooled study data advocate for the prospective integration of modified screening procedures into population screening programmes. Kerrison et al. identified that most of the screening programs in Europe, including the UK, do not recognise the presence of a family history of bowel cancer and screen all eligible participants as average risk with no previous measures that distinguish a separation of screening pathways for high to moderate risk subjects of bowel cancer [28]. The intention is to replace these current screening processes, and based on the French experience using a standardised form, access to improved healthcare can be expected. The current lack in screening refinement is again summarised by the Chief Executive of Bowel Cancer UK as a response to the interim review, commenting that the NHS “is too frequently providing the worst service to the people at highest risk and this is surely in contradiction to aims of diagnosing people earlier so we can save more lives” [1].
Methods
Philosophy
Historically, Stigler cited in Shiffrin and Stigler documented that the practice of actual combining of data from independent studies appeared as early as 1805 [35]. A systematic review is based on a clearly stated research question and aims to identify relevant studies, appraise their quality and evaluate the evidence using explicit methodology [36]. It is because of this explicit and organised approach that systematic reviews have become an increasingly important form of scientific communication and as a proportion of reports in large scientific search databases such as PubMed, where they have increased over 14000% since 1987, over 500% since 2000, and over 200% since 2010 [37]. The conduct and reporting of this review conform with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols on reporting standards and guidelines for authors to use when developing their review manuscripts for publication [38].
Aims
This study was designed to assess the current bowel cancer screening status, knowledgebase, beliefs and barriers with regard to screening among first degree relatives of patients diagnosed with bowel cancer aged 40 years or older. The main study objectives were as follows:
• Examine the prevalence of developing bowel cancer in first-degree relatives of patients who are being screened for bowel cancer.
• To evaluate current compliance with and stage of adoption of bowel screening of first-degree relatives.
• Estimate cost effectiveness and analysis for public policy decisions for the screening of high-risk bowel cancer individuals.
Approach
The decision to conduct a systematic review and meta-analysis for this protocol was due to the relatively short timeframe allocated for its completion. In addition to the nature of the research question, a long duration of pertinent outcomes is required and the extensive time allowance necessary for the application for an NHS Ethics Community Approval.
Study designs
A study design refers to the method used to evaluate the investigation in question. In this review protocol, the focus was on randomised control trials (RCTs) and observational cohort studies; all considered high quality methodologies [28]. Observational cohort studies allow the incidence of disease in exposure groups to be calculated while being efficient for diseases with a long latency period between disease exposure and development, such as bowel cancer, where the phase between early and late stage cancerous cells is estimated to be five to ten years [3]. This type of study design is easier and relatively cheaper to perform than randomised trials; however, it can be subject to selection bias, which tends to overestimate the effect of screening and the overall cost effectiveness [39]. RCTs have a powerful advantage because they provide the best opportunity to control for confounding biases, and they can offer a decade or more follow-up period of bowel cancer incidence and mortality [28].
Search strategy and inclusion criteria
Three sets of standardised search strategies were developed for the overall study, one for the cost effectiveness data, the second for the prevalence of bowel cancer in first-degree relatives and the third for screening participation behaviour. All studies, including journal articles and theses that will be included, must meet the following criteria: (1) published randomised control trials (RCTs), observational epidemiological, and cohort studies assessing any form of diagnostic surveillance aimed at early detection of bowel cancer in first-degree relatives; (2) studies with more than ten years of follow-up of bowel cancer mortality; (3) studies of adults at higher risk of bowel cancer due to family history, or previous adenomatous polyps; (4) reported data on the cost effectiveness on a specified screening intervention in first-degree relatives; (5) authors assessing the effects of colonoscopy versus no screening on bowel cancer incidence or mortality; and data from other systematic reviews studies or meta-analyses were excluded (Table 1).
Table 1: Key Words and Medical Subject Headings (MeSH) Used to Identify Articles in the PubMed, Science Direct and ProQuest Databases (from 2000- 2018)
| PubMed | Science Direct | ProQuest |
|---|---|---|
| [Colorectal cancer OR bowel cancer OR screening OR colonoscopy] | [Mortality OR recommendation OR participation OR perception] | [Costs OR cost effectiveness] |
| AND | AND | AND |
| [First-degree relatives OR family history] | [Screening OR colonoscopy] | [First-degree relatives OR family history] |
| AND | AND | AND |
| [Risk] | [First-degree relatives OR family history] | [Risk] |
Literature search
To capture as many relevant citations as possible, this systematic review was conducted in the medical and scientific databases PubMed, Science Direct and ProQuest; articles published between January 1, 2000, and the most recent publications were searched to identify primary studies of first-degree bowel cancer screening participation, mortality and cost effectiveness. To answer the research question within the allocated short timeframe, a combination of search phrases, always in conjunction with the keywords “first-degree relatives” or “family history”, were chosen to capture the concepts involved, including “colorectal cancer” or “screening” or “mortality” or “colonoscopy” or “recommendation” or “perception” or “participation” or “risk” or “costs” or “cost-effectiveness” (Table 1). The choices of articles were subject to the former inclusion judgement and were only reviewed in full if study abstracts met these criteria to maximise efficient research. Additionally, we manually searched the reference lists of the included studies to identify additional papers and yield material with more relevancy.
Data collection and risk of bias assessment
Data on the first author, year of publication, study design, programme delivery and context (namely, country), sample size, outcome, analysis, and screening participants are all factors to be examined and extracted from eligible papers.
The primary outcomes will measure bowel cancer mortality rates of a 10 year or more follow-up period, sensitivity of colonoscopy screening, and the incidence of bowel cancer in the screening groups.
Secondary outcomes will assess screening participation adherence and overall cost effectiveness. Descriptive statistics will be used to report the characteristics of both outcome assessments. The studies were categorised as RCTs or observational cohort studies as previously mentioned. To assess the risk of bias independently for RCTs, the evaluation was performed according to randomisation sufficiency, allocation discrepancy, selective outcome reporting and blinding of participants, and study assessors of PRISMA standards [38]. Observational cohort studies were evaluated using the selection of participants, comparability of cohorts, exposure and outcome assessment and follow-up adequacy. Each risk bias outcome was assessed as high, medium or low (Figure 1 and Table 2).
Table 2: Summary of the characteristics of the reviewed studies published between 2002 and 2018. Examining pathological prevalence, screening behaviour and the cost effectiveness and analysis of screening first-degree relatives of bowel cancer patients.
| Author/Year/ Country | Title | Study Design/Sample Setting | Description of Search Strategy/Arm | Results/Conclusions OR Outcome Variables | Risk of bias |
|---|---|---|---|---|---|
| Bauer et al., 2018, Germany | Invitation to Screening Colonoscopy in the Population at Familial Risk for Colorectal Cancer (CRC) | The cluster-randomised, multicentre study, first-degree relatives of patients with CRC across Germany nationwide | Screening participation behaviour | Increase in the participation of their first-degree relatives in screening colonoscopy. The frequency of neoplasia that was found underscores the need to screen relatives even before they reach the usual age threshold for screening. | Low |
| Dillon et al., 2018, Australia | Family history-based colorectal cancer screening in Australia: A modelling study of the costs, benefits, and harms of different participation scenarios | Microsimulation Screening Analysis (MISCAN) model, 2480 participants in the Australasian Colorectal Cancer Family Registry (ACCFR), classified into 3 risk categories | Cost-effectiveness data and screening participation | For those at moderately increased risk, higher adherence to recommended screening was also highly cost effective. Investing in public health strategies to increase adherence to appropriate CRC screening will save lives and deliver high value for money. | Low |
| Dove-Edwin et al., 2005, UK | Prevention of colorectal cancer by colonoscopic surveillance in individuals with a family history of colorectal cancer: 16 year, prospective, follow-up study | Prospective, observational study, 16-year follow up. 1678 individuals, classed into four groups | Prevalence and relative risk of developing bowel cancer | Colonoscopic surveillance reduces the risk of colorectal cancer in people with a strong family history. The study confirms that individuals with a lesser family history may not require surveillance under age 45. | Medium |
| Imperiale et al., 2002, USA | Results of screening colonoscopy among persons 40 to 49 years of age | Retrospective, cross-sectional analysis. 906 consecutive persons, aged 40 to 49 years, employer-based screening- colonoscopy programme | Prevalence and relative risk of developing bowel cancer | Colonoscopic detection of colorectal cancer is uncommon in asymptomatic persons 40 to 49 years of age. The low yield of screening colonoscopy in this age group is consistent with current recommendations about the age at which to begin screening in persons at average risk. | High |
| Ladabaum, Ferrandez., &Lanas, 2010, Spain | Cost-effectiveness of colorectal cancer screening in high-risk Spanish patients: use of a validated model to inform public policy | Decision analytic Markov model, Model tailored to reflected CRC epidemiology and costs in persons with first-degree relatives in Spain, superimposing colonoscopy every 5 or 10 years from ages 40-80 years | Cost-effectiveness data | Colonoscopic screening of first-degree relatives of persons with CRC may be cost saving in public systems like that of Spain. Decision analytic modelling tailored to regional considerations can inform public policy decisions. | High |
| Lindberg et al., 2017, Denmark | Outcome of 24 years of national surveillance in different hereditary colorectal cancer subgroups leading to more individualised surveillance | Prospective, observational study, 13444 surveillance sessions, including 8768 incidence sessions and 24- year follow-up | Prevalence and relative risk of developing bowel cancer | Individuals from families with a strong history of CRC could be offered 5-year surveillance colonoscopies (unless findings at the preceding surveillance session indicate shorter interval) and individuals from moderate-risk families could be handled with the population-based screening programme for CRC after an initial surveillance colonoscopy. | High |
| Manne et al., 2002 USA | Correlates of colorectal cancer screening compliance and stage of adoption among siblings of individuals with early-onset colorectal cancer | Cross-sectional study design. 1,183 patients which 504 siblings were assessed for CRC screening practices, perceived risk of CRC, perceived severity of CRC, the preventability of CRC, cancer-related distress, and sibling relationship closeness | Screening participation behaviour | CRC screening acceptance was relatively high among siblings of individuals diagnosed with CRC prior to age 56. Physician and family recommendation were also strong correlates. | High |
| Marc et al., 2009, Canada | Colorectal cancer screening among first-degree relatives of colorectal cancer patients: benefits and barriers | Cross-sectional study, questionnaire. A survey was sent to 747 first-degree relatives, aged 40 or older | Screening participation behaviour | Five constructs influencing CRC screening included: salience and coherence, perceived susceptibility, response efficacy, social influence, and cancer worries and individuals with a first-degree family history of CRC should be specifically addressed with a consistent guideline within a population-based screening programme | Low |
| Naber et al., 2018, USA | Cost-Effectiveness of Age- Specific Screening Intervals for People With Family Histories of Colorectal Cancer | Microsimulation Screening Analysis (MISCAN) model, Estimate costs and effects of colonoscopy screening strategies varying in age range and interval. | Cost-effectiveness data | In individuals with a family history of CRC, it is cost effective to gradually increase the screening interval if several subsequent screening colonoscopies have negative results and no new cases of CRC are found in family members. | Medium |
| Quintero et al., 2016, Spain | Risk of Advanced Neoplasia in First-Degree Relatives with Colorectal Cancer: A Large Multicenter Cross-Sectional Study | Cross-sectional analysis prospectively collected cross- sectional data. 8,498 individuals, 3,015 were defined as a familial-risk group, 3,038 as average-risk group | Prevalence and relative risk of developing bowel cancer | Individuals having two FDR with colorectal cancer showed an increased risk of advanced neoplasia compared to those with average-risk for colorectal cancer. The data suggest that screening colonoscopy guidelines should be revised in the familial-risk population. | Medium |
Results
Study characteristics
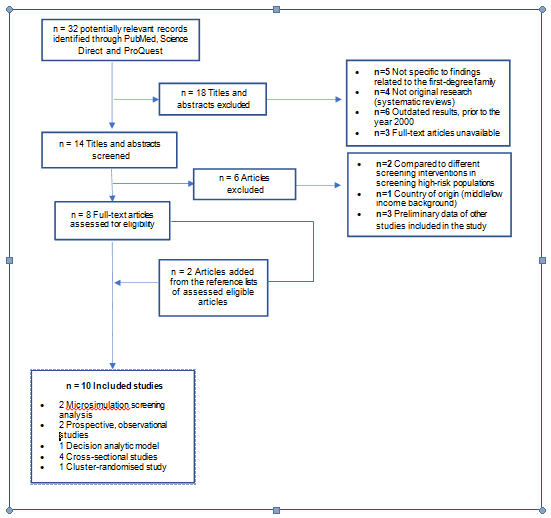
The search retrieved 32 articles addressing bowel cancer in first-degree relatives. After abstract review, 14 papers were considered eligible. On full paper review, eight were then excluded, as shown in Figure 1. A further two were added from the reference lists of the remaining papers, bringing the total number of articles included in this review to 10. The basic characteristics of the included studies are presented in Table 2 (a more detailed overview is presented in the Appendix). The majority of the articles reported took place in Europe, specifically, Germany (n=1, 10%), Denmark (n=1, 10%), Spain (n=2, 20%) and the United Kingdom (n=1, 10%) [40-43]. One study was set in Australia (n=1, 10%), three studies in North America (30%): one in Canada and two in the United States, and the studies that examined screening use within opportunistic programmes (n=4, 40%) employed cross-sectional designs (n=4, 40%). All studies had sample sizes of over 1000 participants [9,15,44]. The North American studies differed from the European studies and Australian study in their population definitions. The North American studies differed from the European studies in their population definitions. Despite this difference, all the studies included in the review were similar with regard to targeted age demographics, methods for first-degree bowel cancer screening data, and urban setting.
Methodological quality of studies
Study quality was mostly considered moderate in all studies reviewed using PRISMA as the assessment tool to evaluate the methodological quality of the included studies [38]. The level of compliance is subject testing varied between studies but was consistent within a given study, which provided confidence in the internal validity; however, the risk of bias was high in 30% of articles. All studies reported on the allocation of participants, and 50% used a randomised cross-sectional study design.
Prevalence and relative risk of developing bowel cancer
Among the studies that assessed the clinical prevalence of bowel cancer in first-degree relatives (FDRs), the results from average-risk patients who underwent colonoscopic screening, Imperiale et al. (2002) showed that 78.9% had no detected lesions, 10.0% had hyperplastic polyps, 8.7% had tubular adenomas, and 3.5% had advanced neoplasms, none of which were cancerous (95% confidence interval). Compared with the average-risk group using colonoscopy, advanced neoplasia was significantly more prevalent in individuals having at least two FDRs with bowel cancer that had a 95% confidence interval [CI] for patients aged less than 60 years [41,45]. Lesions were found in those patients aged less than 50 years (P=0.02); however, only one advanced adenoma was detected in a participant below the age of 50 years (P=0.03). In one of the studies, adjusted proportion with advanced neoplasia was highest in group 4 (Amsterdam criteria positive) and lowest in individuals from group 1 (1 FDR affected, <45 yrs). When adjusted for age and sex, adenomas were seen on follow-up colonoscopies after the initial examination in 26% of group 4, 25% of group 3 (>3 FDRs affected, two generations), 21% of group 2 (at 2 FDRs affected), and 13% of group 1, showing an increased relative risk for developing bowel cancer to those individuals with at least 2 FDRs. Age 41 was the average screening age in all the studies showing significant adenoma prevalence.
Estimated cost effectiveness
As with all public health interventions, cancer screening programmes must measure their value and the health outcomes achieved per currency spent for the whole population. Adjusted average cost effectiveness ratios based on the work of Vijan et al. showed an increase adjusted to the year 2000 in currency [31]. The cost of time to receive screening and followup services added approximately 20% more to the cost effectiveness of 5-year endoscopy screening in the USA. Similarly, Naber et al. show that replacing 10-year screening with 5-year screening would be more cost saving and in terms of cost effectiveness per QALY gained in people with 1, 2 and 3 affected FDRs, respectively [9]. The relatively high cost of bowel cancer care compared to screening in Spain accounts for the result that screening was cost saving overall, where screening costs accounted for 52% of total costs with colonoscopy in high-risk patients every 10 years and 66% of costs with colonoscopy every 5 years, while cancer care costs decreased substantially to 44% and 30% of total costs for the two screening strategies, respectively [42]. Overall, for people with at least 1 affected first-degree relative or at high risk of bowel cancer, screening beginning at an age of 40 years is most cost-effective.
Screening behavior
The relative adherence and compliance of bowel cancer first-degree family members compared to the general population was significantly greater in all reviewed studies, indicating their willingness and the likelihood of participating in screening if they were offered bowel cancer screening. The results of Bauer et al. demonstrated that the colonoscopy uptake rates were 99/125 (79%) in the intervention group (first-degree relatives) and 97/136 (71%) in the control group [40]. This indicates that the active invitation of first-degree relatives resulted in a higher rate of colonoscopy than the conventional opportunistic screening. Dillon et al. reported that those at moderately increased risk reported higher adherence to recommended screening, which was also highly cost-effective [44]. 37% of those in the highest risk categories screened according to guidelines compared to 18% at average risk reported to underscreen. The pooled relative compliance was 95% CI. Using the scales developed by Rawl and Mack surveyed a specific group of first-degree relatives of bowel cancer patients through the Ontario Familial Colon Cancer Registry, containing a potential 772 first-degree relatives of bowel cancer patients [19,25]. Approximately 64% of these relatives had been screened reported ncouragement from a physician was a strong correlate of actual screening behaviour and up to 34% felt to be of high or intermediate risk. Manne et al. reported that physician and family recommendations were also strongly correlated with bowel cancer screening [15].
Discussion
Colonoscopic surveillance is effective in preventing bowel cancer in individuals from families with a first degree family history of bowel cancer. However, colonoscopic surveillance in families at moderate risk does not seem to be indicated before the age of 40, and this is true even for firstdegree relatives of young patients. The results in this review are viewed in the context of previous studies addressing the cost effectiveness of bowel cancer screening. However, the majority of the cost effectiveness of screening has usually been studied in theoretical average-risk populations [5,27,31]. These multiple studies examining various strategies, including FIT, flexible sigmoidoscopy, colonoscopy and CT-colonography, suggest that bowel cancer screening is cost effective. This systematic review demonstrates how a validated microsimulation or decision-analytic model can be adapted to reflect a specific region to inform public screening policy decisions.
Two types of cost effectiveness ratios are often reported in the literature: A cost effectiveness ratio comparing each intervention strategy with the standard of care, often a “no intervention” scenario, and an incremental cost effectiveness ratio comparing each strategy with the next most effective alternative, which may or may not be a “no intervention” scenario [46]. Here, cost effectiveness was measured as the net cost of the screening services divided by the number of quality-adjusted life years (QALY) saved. Net costs are the value of resources used in providing preventative services plus any follow-up services. The results of the QALY data presented suggest that screening first-degree relatives of patients with bowel cancer is likely to be highly cost effective and perhaps even cost saving in the NHS.
Limitation of the studies reviewed
A systematic review is an increasingly important form of scientific communication that can evaluate the evidence from numerous studies using an explicit methodology [36]. However, an important drawback of this form of communication is that it can also be difficult to combine and compare the findings of different studies because authors often conduct their investigations differently, especially in regard to the sample population, the study design and, consequently, the study outcomes. The studies included were predominantly cross-sectional study designs, which are a type of observational study over a limited period analysing data from a population [28]. The limitation of having many studies use this design is that the risk of bias is relatively high; therefore, it is difficult to determine the quality of the results. Given a greater time allocation for research, a wider database search would overcome this limitation in the future.
Most medical reviews are known to contain only published and readily available studies because medical researchers tend to publish studies that show a significant effect and are less likely to submit negative results [47]. In the UK study, expected outcomes were estimated using concurrent population rates and published estimates of the relative risk concerning family history [32]. Therefore, there may have been further unreported cases of cancer due to a lack of a robust control group and, as a consequence, may affect the validity of outcomes.
Limitations of the review
Targeted screening would mean less endoscopy demand; however, the likelihood of cost reductions associated with targeted screening is still largely unknown given the limited availability of NHS data to analyse costs. However, the presented data from the USA, Europe and Australia studies strongly suggest that it may be rather optimistic. Another important limitation of targeted screening is the risk of making it complex and not understandable for both eligible subjects and to the flow of screening processes.
Consequently, it could decrease the participation rate and thereby limit the expected benefit of targeting. However, on the other hand, since the low perceived risk of bowel cancer is considered a barrier to screening itself, targeting first-degree relatives who are at higher risk of bowel cancer could result in higher participation rates [5]. Nevertheless, there are several areas of uncertainty that need to be cleared before a decision on customisation is made and to which screening algorithm to apply. One possibility is to intensify screening by coordinating the screening of first-degree relatives before the national eligible screening age (55 years), directly through the Bowel Cancer Screening Programme system, and eventually transition to join average-risk population screening; or the other possibility is to separate the patient pathway of screening for firstdegree relatives (high-risk group) to offer more sensitive or more frequent testing throughout the individual’s screening pathway.
Importance of the study
The World Health Organisation’s (WHO) definition of screening generally makes bowel cancer a suitable disease for screening, as it has a high incidence with significant morbidity and mortality and a long asymptomatic preclinical phase and is relatively curable if detected in its early stages. Given the evidence shown in this study, targeting early screening for first-degree relatives is justified as a rational recommendation for the National Screening Committee to implement the project at a national level. The challenging aspect of bowel cancer screening is presented in the availability of acceptable and relatively inexpensive screening processes; therefore, the contribution this study could make to the field is twofold. First, the main findings from the study are that the prevalence and risk of bowel cancer in first-degree relatives is significantly higher than the average-risk population, resulting in higher adherence and compliance rates among the screening target group; authors Vijan et al. have linked increased cost effectiveness to increased screening adherence [31]. Second, focusing on early bowel cancer screening based on bowel cancer family history will also help reduce future wait time from GP referrals, as approximately half of hospitals are already in breach of wait time targets for urgent diagnostic tests [1].
Conclusion
Early screening of bowel cancer provides the best opportunity to detect precancerous cells in the lining because the disease pathology progression from cancerous adenomas to invasive cancer can take years; hence, it is treatable, and cost-effective interventions can be put in place that fit within the National Health Service budget. The evidence synthesised from the studies in this review strongly suggests that customising bowel cancer screening to target higher-risk groups will optimise the use of available resources, especially in the NHS, with a limited financial budget. Currently, endoscopic capacity in the UK does not meet the needs for bowel cancer screening other than faecal occult blood testing; therefore, the possible outcomes of targeting first-degree relatives of bowel cancer patients instead of entire populations at relatively low average risk of bowel cancer is to optimise the use of available resources to reduce costs and unfavourable effects of screening activity, such as missed endoscopic appointments.
Conflict of Interest
The author has no competing interests to declare that are relevant to the content of this article.
Ethical Considerations
Ethics considerations and since this is a systematic review of work done by other researchers, ethical approval is not needed.
Ethics considerations and since this is a systematic review of work done by other researchers, ethical approval is not needed.
References
- Rosen P, Young GP, Levin B, Spann S (2005) Colorectal cancer in clinical practice: prevention, early detection and management. 2nd Ed. CRC Press.
- Jayatilleke N, Pashayan N, Powles J (2012) Burden of disease due to cancer in England and Wales. J Public Health 34(2): 287–295.n[Crossref] [Google Scholar] [PubMed]n
- Cairns S, Scholefield J, Steele R, Dunlop M, Thomas H, et al. (2010) Guidelines for colorectal cancer screening and surveillance in moderate and high-risk groups. Gut 59: 666-689.n[Crossref] [Google Scholar] n
- Butterworth A, Higgins J, Pharoah P (2006) Relative and absolute risk of colorectal cancer for individuals with a family history: A meta-analysis. Eur J Cancer 42(2): 216-227.n[Crossref] [Google Scholar] [PubMed]n
- Logan R, Patnick J, Nickerson C (2012) Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut 61: 1439-1446.n[Crossref] [Google Scholar] [PubMed]n
- World Health Organisation (2019) Screening and early detection of cancer.
- Naber SK, Kuntz KM, Henrikson NB, Williams MS, Calonge N, et al. (2018). Cost-effectiveness of age-specific screening intervals for people with family histories of colorectal cancer. Gastroenterology 154(1): 105–116.n[Crossref] [Google Scholar] [PubMed]n
- Issa I, Noureddine M (2017) Colorectal cancer screening: An updated review of the available options. World J Gastroenterol 23(28):.5086-5096.n[Crossref] [Google Scholar] [PubMed] n
- Sheffield School of Health and Related Research (ScHARR) (2018) Summary report to the department of health.
- Zhang J, Cheng Z, Ma Y, He C, Lu Y, et al. (2017) Effectiveness of screening modalities in colorectal cancer: a network meta-analysis. Clin Colorectal Cancer 16: 252-263.n[Crossref] [Google Scholar] [PubMed]n
- Lieberman D (2009) Clinical practice: Screening for colorectal cancer. N Engl J Med 361: 1179–1187.n[Crossref] [Google Scholar] [PubMed]n
- Allison J, Fraser C, Halloran S, Young, G (2014) Population screening for colorectal cancer means getting FIT: the past, present, and future of colorectal cancer screening using the faecal immunochemical test for haemoglobin. Gut Liver 8(2): 117–130.n[Crossref] [Google Scholar] [PubMed]n
- Manne S, Markowitz A, Winawer S, Meropol N, Haller D, et al. (2002) Correlates of colorectal cancer screening compliance and stage of adoption among siblings of individuals with early-onset colorectal cancer. Health Psychol 21(1): 3-15.n[Google Scholar] [PubMed] n
- Anderson J, Al pern Z, Messina C, Lane B, Hubbard P, et al. (2004). Predictors of proximal neoplasia in patients without distal adenomatous pathology. Am J Gastroenterol 99:.472-477.n[Crossref] [Google Scholar] [PubMed]n
- Ko C, Doria RV, Barrett M, Kamineni A, Enewold L et al. (2019) Screening colonoscopy and flexible sigmoidoscopy for reduction of colorectal cancer incidence: A case–control study. PloS One 14(12): e0226027.n[Crossref] [Google Scholar] [PubMed]n
- Demers AA, Kliewer E, Singh H, Mahmud S, Nugent Z, et al. (2010) The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology 139: 1128-1137.n[Crossref] [Google Scholar] [PubMed]n
- Mack L, Cook L, Temple W, Carlson L, Hilsden R, et al. (2009) Colorectal cancer screening among first-degree relatives of colorectal cancer patients: Benefits and barriers. Ann Surg Oncol 16(8): 2092-3100.n[Crossref] [Google Scholar] [PubMed] n
- Baxter N, Warren J, Barrett M, Stukel T, Doria RV (2012) The association between colonoscopy and colorectal cancer mortality in a U.S. cohort according to the site of cancer and colonoscopist speciality. J Clin Oncol 30: 2664-2669.n[Crossref] [Google Scholar] [PubMed]n
- Johns L, Houlston R (2001) A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol 96(10): 2992-3003.n[Crossref] [Google Scholar] [PubMed]n
- Kwak E, Churg D (2007) Hereditary colorectal cancer syndromes: an overview. Clin Colorectal Cancer 6(5): 340-344.n[Crossref] [Google Scholar] [PubMed]n
- Quirke P, Risio M, Lambert R, Karsa L, Vieth M (2011) Quality assurance in pathology in colorectal cancer screening and diagnosis-European recommendations. Virchows Arch 458(1): 1–19.n[Crossref] [Google Scholar] [PubMed]n
- U.S.Preventive Services Task Force (2002) Screening for colorectal cancer: recommendation and rationale. Ann Intern Med 133:. 573-584.n[Crossref] [Google Scholar] [PubMed]n
- Rawl S, Champion V, Menon U, Loehrer P, Vance G, et al. (2001) Validation of scales to measure benefits and barriers of colorectal cancer screening. J Psycho Oncol 19: 47–63.n[Crossref] [Google Scholar] n
- Hoff G (2010) Colorectal cancer screening in an expanding panorama of screening programmes. Best Pract Res Clin Gastroenterol 24: 521-527.n[Crossref] [Google Scholar] [PubMed]n
- Lansdorp VI, Knudsen A, Brenner H (2010) Cost-effectiveness of colorectal cancer screening–an overview. Best Pract Res Clin Gastroenterol 24(4):.439-449.n[Crossref] [Google Scholar] [PubMed]n
- Kerrison R, Wagner VC, Green T, Gibbins M, Macleod U, et al. (2019) Rapid review of factors associated with flexible sigmoidoscopy screening use. Prev Med 120:.8-18.n[Crossref] [Google Scholar] [PubMed]n
- Department of Health and Social Care (2011) The national cancer strategy.
- Miglioretti D, Rutter C, Bradford S, Zauber A, Kessler L, et al. (2008) Improvement in the diagnostic evaluation of a positive faecal occult blood test in an integrated health care organisation. Med Care 46(9): S91-S96.n[Crossref] [Google Scholar] [PubMed]n
- Vijan S, Hwang E, Hofer T, Hayward R (2001) Which colon cancer screening test? a comparison of costs, effectiveness, and compliance. Am J Med 111:.593–601.n[Crossref] [Google Scholar] [PubMed]n
- Mesher D, Dove EI, Sasieni P, Vasen H, Bernstein I, et al. (2014) A pooled analysis of the outcome for prospective colonoscopic surveillance for familial colorectal cancer. Int J Cancer 134: .939–947.n[Crossref] [Google Scholar] [PubMed].n
- Walshe M, Moran R, Boyle M, Cretu I, Galvin Z, et al. (2017) High-risk family colorectal cancer screening service in Ireland: Critical review of clinical outcomes. Cancer Epidemiol 50: 30-38.n[Crossref] [Google Scholar] [PubMed] n
- Sportes A, Catajar N, Charles S, Bejou B, Mary F, et al. ( 2018) Invitation letter with a standardised form is a reliable tool to exclude increased risk patients from organised faecal immunological testing-based colorectal cancer screening program. Dig Liver Dis 50(12): 1339-1342.n[Crossref] [Google Scholar] [PubMed]n
- Shiffrin R, Borner K, Stigler S (2018) Scientific progress despite irreproducibility: A seeming paradox. PNAS 115 (11): 2632–2639.n[Crossref] [Google Scholar] n
- Khan KS, Kunz R, Kleijnen J, Antes G (2003) Five steps to conducting a systematic review. J R Soc Med 96(3): 118–121.n[Crossref] [Google Scholar] [PubMed] n
- Johnson B, Hennessy E (2019) Systematic reviews and meta-analyses in the health sciences: Best practice methods for research syntheses. Soc Sci Med 233: 237-251.n[Crossref] [Google Scholar] [PubMed]n
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, et al. (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. BMJ 4(1): g7647.n[Crossref] [Google Scholar] [PubMed]n
- Bauer A, Riemann J, Seufferlein T, Reinshagen M, Hollerbach S, et al. (2018) Invitation to screening colonoscopy in the population at familial risk for colorectal cancer. Dtsch Arztebl Int 115(43): 715–722.n[Crossref] [Google Scholar] [PubMed]n
- Lindberg L, Ladelund S, Frederiksen B, Smith HL, Bernstein I (2017) Outcome of 24 years national surveillance in different hereditary colorectal cancer subgroups leading to more individualised surveillance. J Med Genet 54(5): 297-304.n[Crossref] [Google Scholar] [PubMed] n
- Ladabaum U, Ferrandez A, Lanas A (2010) Cost-effectiveness of colorectal cancer screening in high-risk Spanish patients: use of a validated model to inform public policy. Cancer Epidemiol Biomarkers Prev 19(11): 2765–2776.n[Crossref] [Google Scholar] [PubMed]n
- Dove EI, Sasieni P, Adams J, Thomas H (2005) Prevention of colorectal cancer by colonoscopic surveillance in individuals with a family history of colorectal cancer: 16 year, prospective, follow-up study. BMJ 331(7524): 1047.n[Crossref] [Google Scholar] [PubMed]n
- Dillon M, Flander L, Buchanan DD, Macrae FA, Emery JD, et al. ( 2018) Family history-based colorectal cancer screening in Australia: A modelling study of the costs, benefits, and harms of different participation scenarios. PLoS Med 15(8): e1002630.n[Crossref] [Google Scholar] [PubMed]n
- Quintero E, Carrillo M, Leoz M, Cubiella J, Gargallo C, et al. (2016) Risk of advanced neoplasia in first-degree relatives with colorectal cancer: a large multicenter cross-sectional study. PLoS Med 13(5).n[Crossref] [Google Scholar] [PubMed]n
- Maciosek M, Solberg L, Coffield A, Edwards N, Goodman M (2006) Colorectal cancer screening: Health impact and cost-effectiveness. Am J Prev Med 31(1): 80- 89.n[Crossref] [Google Scholar] [PubMed]n
- Higgins J, Green S (2011) Cochrane handbook for systematic reviews of interventions.
Citation: Chigonda RL (2022) A Systematic Review on Evolving the Bowel Cancer Screening Programme in England: Targeted Screening for Individuals with First Degree Family History of Bowel Cancer. J Comm Med Health Educ 12: 758. DOI: 10.4172/2168-9717.1000758
Copyright: © 2022 Chigonda RL. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1447
- [From(publication date): 0-2022 - Mar 14, 2025]
- Breakdown by view type
- HTML page views: 1202
- PDF downloads: 245