Research Article Open Access
A Survey of Lawsuits Filed for the Complaint of Tardive Dyskinesia Following Treatment with Metoclopramide
| Eli D. Ehrenpreis1*, Asha Krishnan BS2, Aimee Alexoff BS2, Dylan Smith BS2 and Saran Wilensky BA3 | |
| 1Clinical Associate Professor of Medicine, University of Chicago, Department of Gastroenterology, NorthShore University HealthSystem; Medical Director, Center for the Study of Complex Diseases, Research Institute, NorthShore University HealthSystem, 1001 University Place, Evanston, IL, 60201, USA | |
| 2Data Analyst, Center for the Study of Complex Diseases, Research Institute, NorthShore University HealthSystem, 1001 University Place, Evanston, IL, 60201, USA | |
| 3Student, Columbia Law School, 435 West 116th Street, New York, NY, 10025, USA | |
| Corresponding Author : |
Eli D. Ehrenpreis |
| Received December 19, 2014; Accepted January 20, 2015; Published January 23, 2015 | |
| Citation: Ehrenpreis ED, Krishnan A, Alexoff A, Smith D, Wilensky S (2015) A Survey of Lawsuits Filed for the Complaint of Tardive Dyskinesia Following Treatment with Metoclopramide. Clin Pharmacol Biopharm 4:131. doi: | |
| Copyright: © 2015 Ehrenpreis ED, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Background: Tardive dyskinesia (TD) is a severe neurologic adverse reaction occurring with long-term use of metoclopramide. Aim: To examine all reported legal cases involving metoclopramide-induced TD prior to June 16, 2014 in an effort to inform physicians and practitioners of the current legal risks associated with the prescription of metoclopramide. Methods: A search of the Westlaw database for cases that contained the terms metoclopramide and tardive dyskinesia was performed. This dataset was then queried for cases brought against physicians or practitioners. Full length reports were obtained and information was pulled regarding plaintiff demographics, medical specialty of the defendant, plaintiff’s TD symptoms, lawsuit claims, and case outcome. Results: There were ninety-six cases in which patients filed a claim that met our search criteria. Eighty-five cases (88.5%) were brought against the brand name and/or generic medicine manufacturers as failure to warn claims. There were eleven cases brought against physicians or practitioners. Eight of eleven lawsuits (72%), of cases were either dismissed, settled or were in the process of settlement. There has also been an increase in the number of malpractice cases filed related to metoclopramide induced TD since the FDA issued its black box warning in February, 2009. Conclusions: There have been a small number of cases directed at physicians related to the prescription of metoclopramide and the development of TD, most of which were either dismissed or settled.
| Keywords |
| Metoclopramide; Tardive dyskinesia; Medico legal; Failure to warn; Malpractice; Motility |
| Introduction |
| Metoclopramide is a dopamine receptor antagonist and antiemetic that is used to treat Gastroesophageal Reflux Disease (GERD) and gastroparesis. Metoclopramide is also a treatment for chemotherapy and post-operative-induced nausea and vomiting [1,2]. Although this medicine has been available on the United States market since 1979, CNS side effects consisting of depression, nightmares and extrapyramidal symptoms have been commonly reported. An important and potentially permanent neurologic adverse reaction, occurring with long-term use of metoclopramide, is the movement disorder: Tardive Dyskinesia (TD) [1]. Long-term use, advanced age, and female gender are risk factors for TD [1,3]. Studies have suggested that incidence of metoclopramide-induced TD is as high as 34.5% [1,4]. In February 2009, the Food and Drug Administration (FDA) issued a Black Box warning for TD occurring after long term usage of metoclopramide [5]. |
| Although the clinical risk of TD associated with long-term metoclopramide usage has been reviewed, there have been few studies examining legal occurrences related to this topic [1,4,6]. Our group previously identified the legal claims related to metoclopramide and TD using a Google Scholar search on December 15th, 2010. This study found that there were an increasing amount of cases brought against manufacturers of metoclopramide following the issuance of the Black Box warning by the FDA [6]. |
| Several studies have looked at potential liability and physician risk relative to medical malpractice claims [7,8]. For example, a study by Localio et al. assessed physicians’ risk and found that healthcare providers were generally successful in cases brought against them for substandard care related to any injury experienced by a patient [7] That study suggested that 18% of all malpractice lawsuits resulted in the defendant verdict and 35% of cases were dropped or dismissed, while the majority of cases (39%) resulted in plaintiff settlement [9]. No prior studies have determined the specific risk to physicians who are named in lawsuits related to the prescription of metoclopramide. |
| We examined all reported legal cases involving metoclopramideinduced TD in an effort to inform physicians and practitioners of the current legal risks associated with the prescription of metoclopramide. The focus of this survey is to review all lawsuits filed against physicians prescribing metoclopramide. |
| Methods |
| Study design |
| Westlaw (West Publishing Co., Eagan, Minnesota) is an online legal reporting service that was used to create the dataset for this study. It is comprised of over 40,000 databases that include case law, legislation, administrative materials, newspaper and magazine articles, public records, law journals, law reviews, treatises, and legal forms. This database includes state and federal reports from all 50 states. Westlaw gathers cases including both issued decisions and complaints and then categorized them by subject area, jurisdiction, and keywords [10]. |
| Sampling criteria |
| The dataset was created by searching the Westlaw database for all cases containing the terms “metoclopramide” or “Reglan” and “tardive dyskinesia” (search: “adv: (metoclopramide Reglan) and (tardive dyskinesia).” The search was not bounded by year. The earliest case was filed in April, 2000 and the most recent case in May, 2014. Cases that were currently pending before judges across the country were not included in the search. |
| All appeals were excluded from the total number of cases to avoid analysis of multiple case filings for the same lawsuit. The remaining cases were queried for court orders and filings of lawsuits brought against pharmaceutical companies, physicians or practitioners. |
| Data analysis |
| Full-text filings of these court cases were obtained from the database, reviewed and analyzed individually. Exact procedures for filing a case varies by jurisdiction, but most commonly a written complaint is brought to the court and defendants are served. Information for the study was pulled from the cases when available. These cases were used to collect demographic information on patients and medical indication for prescription. Information on the medical specialty of the defendant and the amount of time they had been practicing before being sued was also obtained. When reported, details of the disorder were assessed. These included specific symptoms associated with the complaint of TD and their duration. Legal data regarding the type of lawsuit and the outcome of the cases was noted. Information regarding practices of named practitioners was obtained using a Google search. The search was performed between June 2, 2014 and June 16, 2014. |
| Failure to warn was defined as “a defect that occurs when a manufacturer does not place a warning on the packaging of products that could cause injury if the danger is unknown” [11]. Medical malpractice was defined as professional negligence of a healthcare provider who fails to provide adequate treatment, which causes injury or death to the patient, with most cases involving medical error [12]. |
| Results |
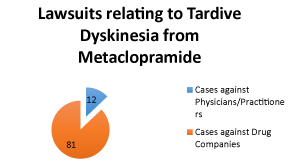
| There were ninety-six cases in which patients filed a claim that met our search criteria. All cases under the search of, “metoclopramide” or “Reglan” and “tardive dyskinesia” (search: “adv: (metoclopramide Reglan) and (tardive dyskinesia)” were included in our analysis. However, within that dataset any lawsuits currently pending were excluded. Eighty-five cases (88.5%) were brought against the brand name and/or generic medicine manufacturers as failure to warn claims. Eleven cases (11.5%) were brought against physicians or practitioners (Figure 2). Cases were filed as failure to warn (5 cases, 45%) or medical malpractice (6 cases, 55%). This evidence is pivotal in examining physician’s liability while prescribing metoclopramide. From this study, physicians should conclude that it is necessary to inform patients about the risk of TD after prolonged usage of metoclopramide. Additionally, prescribers should carefully monitor patients for symptoms and prescribe with caution. |
| Plaintiffs demographics and medical specialty of defendant physicians |
| There was a female predominance in the dataset, with eight female plaintiffs (73%). Ages were available for two plaintiffs, who were 77 and 80 years old. The duration of therapy ranged from 0.7 years to 10 years, with an average of 4.0 years. Gastroesophageal reflux (GERD) and nausea were the most common reasons for prescription, in six and four cases respectively. Three patients received metoclopramide for vomiting/morning sickness, two for gastroparesis, and one patient for loss of appetite. Three defendant physicians were gastroenterologists, three were family medicine practitioners, two physicians practiced internal medicine and one was a general surgeon. One defendant was a pharmacist. The average amount of time in practice for these healthcare providers prior to the lawsuit was 29 years (Table 1). |
| Reported symptoms of tardive dyskinesia |
| Reported symptoms were available in six of the eleven patients (Table 2). These were consistent with the general definition of TD, except for one case blaming the medicine for developmental disabilities in a child born to a mother who took the medicine while pregnant. |
| Lawsuit claims and case outcome |
| Outcomes were available in eight of eleven lawsuits, and these cases were either dismissed, settled or were in the process of settlement (Table 3). An additional case was in the process of settlement. |
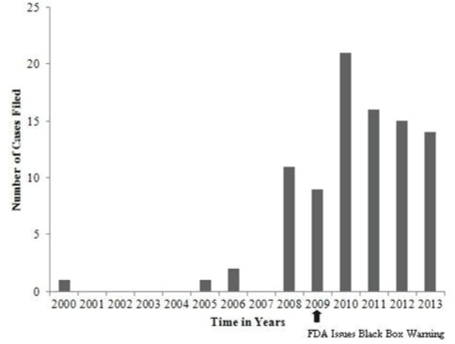
| Malpractice cases filed for metoclopramide-induced td over time |
| As can be seen in (Figure 1), there was an increase in the number of malpractice cases filed related to metoclopramide induced TD since the FDA issued its black box warning in February, 2009. The largest number of these cases was filed in 2010. |
| Discussion |
| Although a variety of neurologic and other adverse medication reactions have been associated with short and long-term use of metoclopramide, the development of TD following chronic treatment with metoclopramide prompted the FDA to place a Black Box warning to its medication label in February, 2009 [6]. TD is characterized by involuntary repetitive movements of the extremities, tongue protrusion, puckering, pursing of the lips, lip smacking, grimacing, rapid eye movements and/or impaired movement of the fingers [6]. TD is a known side effect of medications that are dopaminergic system blockers including phenothiazines such as thorazine and prochlorperazine. Development of TD appears to be directly related to the duration of use of metoclopramide and its cumulative dose. Highest risk for TD is seen in the el derly, especially older women [1], and people who have received prolonged treatment with the medicine [6]. There is no known treatment for tardive dyskinesia and the condition is generally irreversible. |
| Consistent with the aforementioned risks for TD, data collected in the current review indicates that the majority of lawsuits had female plaintiffs. The Black Box warning suggests that metoclopramide treatment should not exceed three months. Plaintiffs in the current review were all found to all exceed this interval of treatment, with the duration of reported therapy ranging from 0.7-10 years. Neurologic symptoms were described in six cases (54.5%) and were typical of TD. |
| In our study the majority of lawsuits were categorized as either failure to warn (45%) or malpractice (55%) [12]. In failure to warn cases, the physician reportedly does not provide sufficient information on risks associated with the drug use and, in medical malpractice cases; the practitioner allegedly falls below the accepted standard of practice in the medical community and causes injury or death to the patient. Review of clinical findings suggests that cases consisted of patients with typical risk factors for TD including female gender, chronic use and advanced age. |
| The Westlaw database has been previously used to study medical malpractice litigation in cases related to penile prostheses [12] and otolaryngology [13]. Both studies found that in the majority of the cases (57.5% and 58% respectively), the claims against the physician or practitioner was dismissed. Another study that focused on the medico legal risks associated with image-guidance in endoscopic sinus surgery found fewer cases resulted in the defendant verdict (36.7%) than our study [14] In our analysis, eight of eleven lawsuits (72%), of cases were either dismissed, settled or were in the process of settlement. Similar results were found in a retrospective study by Localio et al. [7]. Their group used a team of doctors, nurses, and medical records administrators to search New York State medical records to assess the frequency of malpractice claims among patients that have been subjected to medical negligence. These records were reviewed individually for adverse events or negligence and compared to data on malpractice claims. It found that physicians and practitioners were rarely found liable [7]. |
| Black Box warnings represent the most severe caution that the FDA places for a prescription medication. These warnings are used to draw attention to potentially fatal, life-threatening, or disabling ADRs, and often suggest use restriction of the identified medications. These warnings usually arise after the identification of a specific toxicity from post-marketing surveillance [6]. |
| This study found an increase in the number of lawsuits filed following issuance of the Black Box warning for metoclopramide prescription by the FDA. Our group and others [14-18] have previously described a variety of unintended consequences following Black Box warnings for medications. These include decreased prescriptions of needed medications such as anti-depressant medication to elderly patients and an increased number of medication-related lawsuits. A variety of specialists, including gastroenterologists, were named in the lawsuits. Many of these physicians had been practicing medicine for an extended period of time. |
| This study was limited by the amount of data available in the Westlaw database. This is particularly true regarding clinical information about plaintiffs. All cases in this database have been reported by lawyers and are therefore susceptible to reporter bias. |
| To the present, there have been a relatively small number of cases that have been filed against healthcare providers related to prescription of metoclopramide. In addition, relatively favorable outcomes are reported in these cases. Nonetheless, several suggestions are made to practitioners who are prescribing metoclopramide to their patients. First, it should be documented that any patient who needs to stay on the medication for more than several weeks has been warned about the potential risk for TD. Documentation of cumulative duration of medication therapy with updates at each patient visit is advised. In our opinion, patients receiving the medication for a cumulative time period of greater than three months should also have a formal, signed consent that includes an explanation of the risk of TD in their chart [19-23]. |
| In summary, there have been a small number of cases directed at physicians related to the prescription of metoclopramide and the development of TD. Generally the claims were either dismissed or settled. However, the number of cases filed by patients who developed TD after receiving metoclopramide has increased since the issuance of the Black Box warning by the FDA. Caution and careful documentation is advised when prescribing this drug for chronic use. The use of a consent form with explanation of the risk of TD is advised in patients requiring a cumulative exposure to metoclopramide greater than three months in duration. |
| Acknowledgements |
| Eli D. Ehrenpreis is acting as guarantor of the article. Aimee Alexoff and Asha Krishnan were involved in initial study design, review of database material, data analysis, and manuscript preparation. Dylan Smith participated in data analysis, creation of study database, and manuscript preparation. Sarah Wilensky role included data acquisition and review of database material. Eli D. Ehrenpreis was responsible for study development, study design, and manuscript preparation. All authors have reviewed and approved the final version of the manuscript. |
References
- Rao AS, Camilleri M(2010) metoclopramide and tardive dyskinesia. Aliment PharmacolTher31:11-19.
- Sewell DD, Kodsi AB, Caligiuri MP, Jeste DV (1994) Metoclopramide and tardive dyskinesia. Biol Psychiatry 36:630-632.
- Yang YX, Leonard CE, Freeman C, Hennessy S (2011)The effect of a physician-targeted intervention on metoclopramide prescribing practice.TherClin Risk Manag7:359-365.
- Kenney C, Hunter C, Davidson A, Jankovic J (2008). Metoclopramide, an increasingly recognized cause of tardive dyskinesia. J ClinPharmacol 48:379-384.
- US Food and Drug Administration (2009). FDA Requires Boxed Warning and Risk Mitigation Strategy for Metoclopramide-ContainingDrugs.
- Ehrenpreis ED, Deepak P, Sifuentes H, Devi R, Du H, Leikin JB (2013). The metoclopramide black box warning for tardive dyskinesia: effect on clinical practice, adverse event reporting, and prescription drug lawsuits. Am J Gastroenterol108:866-872.
- Localio AR, Lawthers AG, Brennan TA, Laird NM, Hebert LE (1991) Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med.325:245-251.
- Dorsey ER, Rabbani A, Gallagher SAConti RM, Alexander GC.(2010). Impact of FDA black box advisory on antipsychotic medication use. Arch Intern Med 170:96-103.
- Read S, Hill HF (2005) Dermatology's malpractice experience: clinical settings for risk managementJ Am AcadDermatol53:134-137.
- "Westlaw├?┬«." Gale Encyclopedia of American Law.Ed. Donna Batten.3rd ed. Vol. 10. Detroit: Gale, 2010. 370-372. Gale Virtual Reference Library.
- Cheeseman, Henry R.,Goldman, Thomas F. The Paralegal Professional. New Jersey: Prentice Hall; 2010.
- Louisell, David W, Harold Williams, Charles Kramer (2004) Medical malpractice. N Engl J Med 350:283-92.
- Bateman DN, Gokal R, Dodd TR, Blain PG (1981)The pharmacokinetics of single doses of metoclopramide in renal failure. Eur J ClinPharmacol19:437-441
- Tenback DE, van Harten PN (2011). Epidemiology and risk factors for (tardive) dyskinesia.Int Rev Neurobiol98:211-30.
- Ceilley R, Eisenthal A(2009) The Unintended Effects of a Boxed Warning.J ClinAesthet
- .Olfson M, Marcus SC, Druss BG (2008) Effects of Food and Drug Administration warnings on antidepressant use in a national sample.Arc`h Gen Psychiatry 65:94-101.
- Libby AM, Orton HD, Valuck RJ (2009). Persisting decline in depression treatment after FDA warnings. Arch Gen Psychiatry66:633-639.
- Katz LY, Kozyrskyj AL, Prior HJ (2008).Effect of regulatory warnings on antidepressant prescription rates, use of health services and outcomes among children, adolescents and young adults. CMAJ178:1005-1011.
- Sunaryo PL, Colaco M, Terlecki R (2014). Penile Prostheses and the Litigious Patient: A Legal Database Review. J Sex Med.
- Hong SS, Yheulon CG, Wirtz ED, Sniezek JC (2014) Otolaryngology and medical malpractice: A review of the past decade, 2001-2011. Laryngoscope124:896-901
- Eloy JA, Svider PF, D'Aguillo CM, Baredes S, Setzen M, et al. (2013). Image-guidance in endoscopic sinus surgery: is it associated with decreased medicolegal liability? Int Forum Allergy Rhinol3:980-985.
- Pasricha PJ, Pehlivanov N, Sugumar A, Jankovic J (2006). Drug Insight: from disturbed motility to disordered movement--a review of the clinical benefits and medicolegal risks of metoclopramide. Nat ClinPractGastroenterolHepatol 3:138-148.
- Carroll AE, Parikh PD,Buddenbaum JL (2012) The impact of defense expenses in medical malpractice claims. J Law Med Ethics.Spring 40:135-142.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 17174
- [From(publication date):
February-2015 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 12607
- PDF downloads : 4567
