Research Article Open Access
A Short Motivational Program Based on Temporary Smoking Abstinence: Towards Increased Self-Efficacy to Quit in Psychiatric Inpatients
Ineke Keizer*, Marianne Gex-Fabry, Patrice Croquette and Aqal Nawaz Khan
Hôpitaux Universitaires de Genève, Département de santé mentale et psychiatrie, chemin du Petit-Bel-Air 2, Geneva, Switzerland
- *Corresponding Author:
- Ineke Keizer
Psychologist
Hôpitaux Universitaires de Genève
Département de santé mentale et psychiatrie
chemin du Petit-Bel-Air 2
Geneva, Switzerland
Tel: +4122 305 47 62
Fax: +4122 305 47 19
E-mail: ineke.keizer@hcuge.ch
Received date: June 17, 2016; Accepted date: July 25, 2016; Published date: July 31, 2016
Citation: Keizer I, Gex-Fabry M, Croquette P, Khan AN (2016) A Short Motivational Program Based on Temporary Smoking Abstinence: Towards Increased Self-Efficacy to Quit in Psychiatric Inpatients. J Addict Res Ther 7:289. doi:10.4172/2155-6105.1000289
Copyright: © 2016 Keizer I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Background: Specific approaches for smokers presenting with psychiatric disorders are scarce, even though the prevalence of smoking does not tend to decline in mental health settings, in contrast with general populations of most western countries.
Methods: Inpatient smokers (n=69) in a public mental health hospital participated in a multicomponent motivational intervention based on a temporary 26 h abstinence period. Evaluations, performed 1 week pre-, during and 1 week post-intervention, included cigarette consumption, carbon monoxide level, stage of change, craving, as well as anxiety, depression, well-being and smoking cessation self-efficacy.
Results: Carbon monoxide level significantly decreased during the intervention (median 16 to 6 ppm, p<0.001), with 76.8% of participants using nicotine replacement therapy. Craving decreased (MPSS 5 to 4, p=0.01), together with anxiety (STAI-State 47 to 38, p<0.001) and depression (BDI-21 18 to 13, p<0.001), whereas well-being increased (WHO-5 11 to 16, p<0.001). During the proposed 26 h abstinence period, 45.6% of participants successfully abstained from smoking, of which 58.1% subsequently attempted quitting. Ten participants (14.5% of 69) decided to stop smoking even though they had no intention to quit before the program. Self-efficacy for permanent cessation did not change, but self-efficacy for temporary abstinence increased (median 8 at pre- to 9 at post-evaluation, p=0.003).
Conclusion: A short multicomponent motivational intervention based on temporary abstinence can be a positive experience for patients with severe psychiatric disorders, contribute to increase self-efficacy and trigger quit attempts. The present study suggests that integration of such a program in mental health care is feasible and wellaccepted.
Keywords
Inpatients; Motivation; Program evaluation; Psychiatry; Self efficacy; Smoking cessation; Health psychology
Introduction
Smoking and poor mental health appear tightly entangled, as smoking prevalence, morbidity and mortality are clearly higher in psychiatric patients than in the general population [1,2]. Hospital stay can be considered as an opportunity to treat tobacco dependence and smoke-free environments requiring temporary abstinence are hypothesized to promote smoking cessation [3]. However, despite smoke-free policies, mental health settings still face tobacco-related difficulties. The number of smokers remains alarmingly high, and they seem to adapt to indoor smoking bans and continue smoking outdoors. Smoking rates decline less among individuals with mental health problems than in the general population [4-6].
Treating nicotine dependence in psychiatric patients is more difficult than in the general population, with a higher degree of dependence, frequent relapse often associated with psychiatric condition, and increased needs for support and nicotine replacement therapy (NRT) [7]. According to the trans theoretical model (TTM) or stages of change model, a large number of psychiatric patients are “precontemplators” who do not consider stopping [8]. The challenge is to help these smokers shift to the “contemplation” stage, introducing them to the idea of stopping. Special emphasis is needed on the largely neglected phase preceding the moment a smoker feels ready to engage in a quit attempt, or during initial phases of smoking history, when he does not consider himself as a smoker [9].
It is not uncommon to encounter barriers within the hospital staff when psychiatric patients express their intention to quit smoking. In our clinical practice, we observed that professionals sometimes advise patients not to quit because they fear of worsening psychiatric condition, for example when patients are not yet stabilized or just got stabilized after a severe episode. Onset of major depressive episodes on cessation have occasionally been reported in the literature and the hypothesis has been raised that smoking may act as self-medication against negative mood [10]. Anxiety and depression are part of usual nicotine withdrawal symptoms, underlining the very close interweaving between these elements and particular obstacles in the way to smoking cessation for psychiatric patients [11]. Together with established practice in psychiatric settings, this context partly explains why staff tends to neglect tobacco-related interventions [3].
To our knowledge, much support is available for smokers ready for a quit attempt, but there is a paucity of interventions for patients in the preceding, even more crucial stages, who are the majority of smokers in mental health care settings. To address this shortcoming, we developed a tailored intervention, consisting in a short 26 h smoking abstinence period, allowing patients to build up a positive experience and increase self-efficacy towards smoking cessation [12]. The objective of our study was to evaluate this intervention, by comparing data collected before and one-week after the program. We studied central issues related to smoking cessation, such as self-efficacy, motivational changes (TTM) and quit attempts. We also studied negative effects, to address the fears of potential increases of anxiety and depression, and positive effects, as we hypothesized that smoking abstinence and concomitant increase of well-being and self-efficacy might be important leverages towards cessation.
Materials and Method
Setting and participants
The intervention was designed in 2010 and its feasibility and acceptability were assessed in an earlier report [12]. The present study ran between June 2011 and July 2014. Approximately 4 sessions per year were proposed to inpatients at the Department of Mental Health and Psychiatry, Geneva University Hospitals (capacity of about 100 beds, 5 acute care units, 2 rehabilitation units).
Inclusion criteria were as follows: (a) being a smoker (widely defined as having smoked at least one cigarette in the past 3 days), ready to commit to a 26 h smoking abstinence period (“not a puff”); (b) being hospitalized, independently of psychiatric diagnoses; (c) presenting a stable clinical condition, compatible with study participation according to the nursing team and the attending psychiatrist. Exclusion criteria were insufficient level of French and cognitive impairment.
The program (described below) was presented to all inpatients by means of posters and oral information by mental health care providers. Potential participants were further informed during a preliminary interview and invited to provide written informed consent before entering the study. The study protocol was approved by the ethics committee of the Department of Mental Health and Psychiatry, Geneva University Hospitals (reference number 11-057).
Intervention
The multicomponent intervention comprises three distinct features. The first one, aimed to increase self-efficacy, is the patients’ commitment to succeed in not-smoking during 26 h. Emphasis is set upon succeeding: generous NRT is proposed and individual support is provided by staff with experience both in mental health and tobacco addiction. Additional boosting is provided by the group experience, the common challenge not to smoke, and a “diploma” for those who succeed. Repeated monitoring of expired carbon monoxide allows individual feedback on the on-going experience. A second essential component of the program relies on its positive content, i.e., activities aimed at enhancing relaxation and well-being (thermal baths or sport, music or occupational therapy) and showing that non-smoking is not necessarily painful or stressful. A third ingredient is smoke-related information during formal (sessions with tobacco specialists) and informal moments (support throughout the day).
The program begins on Thursday 8:30 am and involves group sessions, thermal baths, and lunch in a restaurant, interactive tobaccorelated information session, afternoon tea, music therapy, and final group meeting. Patients are back to their hospital units at 18:00. They return on Friday morning 8:30 am, have breakfast together and participate in a last group session. Nicotine replacement is provided as slow action products (patches of 7, 14 and 21 mg) and rapid action products (10 mg inhalers, 2 and 4 mg gums, 1 mg lozenges), with possible use of several products. A structured group setting is provided by having the same patients and staff together during the whole 26 h period (with the exception of the evening/night). The team comprises a medical doctor, a nurse, an occupational therapist and a psychologist, all working in inpatient psychiatric units. At each intervention, an additional staff member (preferably a smoker) joins the group. Tobacco specialists are in charge of the information session. The whole program is free of charge.
Study design
Participants were evaluated on 3 occasions, during individual interviews conducted by a psychologist (IK) and a trainee psychologist.
The first interview took place during the week preceding the intervention and allowed confirming participation, on the basis of clinical condition and acceptance of the program. It allowed patients to establish a personal contact with the coordinator and gain reassurance about their participation. Assessments included present characteristics of smoking, craving and motivation to quit, self-efficacy, anxiety, depression and well-being (described below), in addition to demographics and history of smoking. Clinical characteristics such as diagnosis and history of hospital stays were obtained from medical charts and discharge letters.
The second evaluation was performed immediately after the intervention (Friday morning), with the same instruments and satisfaction with the program.
The third assessment was made about one week after the program, with the same instruments as in the first 2 evaluations and a standardized substance use interview (details below). This last interview also provided an opportunity to encourage patients towards a cessation attempt and verify whether further specialized tobacco support was needed.
Expired carbon monoxide, use of nicotine replacement and possibly cigarettes were recorded on these 3 occasions and additionally on Thursday morning and afternoon, allowing monitoring of carbon monoxide (CO) on Thursday 9:15 am/Thursday 5:15 pm/Friday 10:00 am.
Main outcome variables were success rates for temporary 9h and 26h smoking abstinence, and motivational or behavioural changes about smoking between pre and 1 week post-intervention assessments.
Instruments
Baseline characteristics included demographics and illness-related variables (primary and comorbid diagnoses, number of hospital stays). Substance use was documented by searching medical charts and using the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST, [13]).
Positive and negative effects were measured using questionnaires for state anxiety (State-Trait Anxiety Inventory, STAI, state part [14]), depression (Beck Depression Inventory, BDI-21 [15]) and well-being (WHO-5 Well-being Index [16]). Satisfaction with the program was assessed with the question “Globally, I rate this intervention as: not at all satisfactory (1-2), little satisfying (3-4), quite satisfying (5-6), absolutely satisfying (7-8)”.
Use of nicotine replacement and cigarette consumption were self reported, with quit attempts considered as such if they exceeded 24 h. Expired carbon monoxide (CO) was measured using a piCO+ Smokerlyzer (Bedfont, UK). Values of 0-6 ppm correspond to nonsmokers, 7-15 ppm to low-dependence smokers and >15 ppm to strongly addicted smokers. The Heaviness of Smoking Index (HSI [17,18]) was calculated based on two questions of the Fagerström Test for Nicotine Dependence (FTND): “How many cigarettes a day do you smoke?” (Answers in 4 categories) and “How soon after you wake up do you smoke?” (4 categories). Two questions from the Wisconsin Predicting Patients’ Relapse questionnaire (WI-PREPARE [19]) were used to evaluate the time spent with smokers (“I’m around smokers much of the time”) and craving (“When I haven’t been able to smoke for a few hours, the craving gets intolerable”) on 7 point rating scales. Craving was also measured using the total score of 2 items of the Mood and Physical Symptoms Scale (MPSS [20]): “How much of the time have you felt the urge to smoke today?” and “How strong have the urges been today?” (0=not at all/no urges to 5=all the time/extremely strong). Withdrawal symptoms were measured using the Minnesota Nicotine Withdrawal Scale (MNWS-R [21,22]) that includes 9 items (frustration, anxiety, depression, craving, concentration, appetite, insomnia, restlessness, irritability) rated on 4-point Likert scales (0=not present to 3=severe) and a total score. Readiness to stop smoking was evaluated using Kahler’s Commitment to Quitting Smoking Scale and Biener’s Contemplation Ladder [23,24]. Stage of change was categorized as pre-contemplation (no intention to quit), contemplation (intention to quit in the next 6 months), preparation (intention to quit in the next 30 days, with possibly a quit attempt in the past year), and action (stopped smoking in the past month) [25].
Two questions evaluated self-efficacy or perceived confidence in remaining abstinent from smoking, either on a temporary basis (“If I would participate in this program again, I think I would be able not to smoke during 26 h”) or a permanent basis (“I’m convinced that one day, whatever happens, I will stop smoking”). Answers were given on 10 point scales (from “no, impossible” (1-2) to “yes, absolutely” (9-10)).
Data analysis
Frequency (% of valid cases) was used to describe categorical variables. Median (range) and mean (standard deviation, sd) were used for ordinal and continuous variables. Comparison of independent samples proceeded with the Mann-Whitney U test. Change over time was tested with the Wilcoxon signed-rank test (2 ordinal or continuous measures) or the Friedman test (>2 measures). The McNemar test was used for change of binary variables. Statistics were computed using SPSS version 22 (IBM Corporation, Armonk, NY, USA). All tests were two-tailed, with significance level at 0.05.
Results
Participation
After the first individual interview, 176 participants were admitted into the program, of which 110 met the study inclusion criteria. Reasons for exclusion were: second participation in the program (n=40); refusal to participate (4); cognitive impairment (5); worsening of clinical condition (12); language barrier (2); administrative reasons (2). One patient was excluded because he had stopped smoking 2 months before the intervention (he entered the program to reinforce motivation).
Nineteen patients were not present on the intervention day because of discharge from the hospital or worsened clinical condition. Four patients dropped out during the first morning because of acute symptoms or difficulty abstaining from smoking. Eighteen patients were not assessed at 1 week post-intervention, mainly because of hospital discharge. The study cohort thus comprised 69 patients assessed on 3 occasions (1-8 days before the program, second day of the intervention, 4-12 days after the program).
Baseline characteristics of participants
Participants were aged 17-64 (mean 34.3, sd 12.8). Sociodemographic and clinical characteristics are reported in Table 1. The sample comprised patients with severe psychopathology and social vulnerability (multiple diagnoses, several hospital stays, low education, living alone and depending upon disability pension or social aid). Only a minority had a principal ICD-10 diagnosis of substance-related disorders, but comorbidity was frequent. The sample had high level of nicotine dependence (mean HSI 3.3, sd 1.8) and long duration of tobacco consumption (mean 13.8 years, sd 10.4). At pre-intervention, mean level of carbon monoxide was 22.6 ppm (sd 16.8), whereas self reported mean number of cigarettes per day over the past 6 months was 19.0 (sd 11.3). A majority were not yet actively planning to stop tobacco use (mean score on the Biener’s 0-10 Contemplation Ladder 6.3, sd 2.8). More details of tobacco use are in Table 1.
| Socio-demographic characteristics | n | % | |
|---|---|---|---|
| Gender | Male | 41 | 59.4 |
| Female | 28 | 40.6 | |
| Age | <30 | 34 | 49.3 |
| 30-50 | 25 | 36.2 | |
| >50 | 10 | 14.5 | |
| Education | Compulsory basic or less | 33 | 47.8 |
| Secondary or professional degree | 31 | 44.9 | |
| University or similar degree | 5 | 7.2 | |
| Living condition | Alone | 36 | 52.2 |
| Couple | 7 | 10.1 | |
| With family or relatives | 18 | 26.1 | |
| Other | 8 | 11.6 | |
| Financial resources/occupation | Work | 11 | 15.9 |
| Training or studies | 16 | 23.2 | |
| Unemployment or social aid | 8 | 11.6 | |
| Disability pension | 31 | 44.9 | |
| Other | 3 | 4.3 | |
| Mother tongue | French | 46 | 66.7 |
| Bilingual French and other | 14 | 20.3 | |
| Other | 9 | 13 | |
| Clinical characteristics (Medical records) | n | % | |
| Number of hospitalizations | 1 | 15 | 21.7 |
| 2-3 | 14 | 20.3 | |
| 4-41 | 40 | 58 | |
| Length of hospital stay (In the last year) | <1 month | 17 | 24.6 |
| 1-2 months | 22 | 31.9 | |
| 2-3 months | 11 | 15.9 | |
| >3 months | 19 | 27.5 | |
| Primary diagnosis | Psychoactive substances | 6 | 8.7 |
| Psychotic disorders | 31 | 44.9 | |
| Mood disorders | 22 | 31.9 | |
| Other disorders | 10 | 14.5 | |
| Comorbidity | 1 diagnosis | 28 | 40.6 |
| 2 diagnoses | 22 | 31.9 | |
| 3 or more diagnoses | 19 | 27.5 | |
| Diagnosis of psychoactive substances (excluding tobacco) | Principal diagnosis | 6 | 8.7 |
| Comorbid diagnosis | 19 | 27.5 | |
| No substance related diagnosis | 44 | 63.8 | |
| Clinical characteristics (Structured interview) | n | % | |
| Substance use (ASSIST) | Tobacco (score>3) | 69 | 100 |
| Alcohol (score>10) | 15 | 21.7 | |
| Cannabis (score>3) | 20 | 29 | |
| Cocaine (score>3) | 7 | 10.1 | |
| Other: Opioids, amphetamine type stimulants, sedatives, hallucinogens, inhalants (score>3) |
14 | 20.3 | |
| Tobacco characteristics | n | % | |
| Fagerström Test for Nicotine Dependence items: | |||
| “How soon after you wake up do you smoke?” | Within 5 min | 27 | 39.1 |
| 6-30 min | 27 | 39.1 | |
| 31-60 min | 7 | 10.1 | |
| Later than 60 min | 8 | 11.6 | |
| “How many cigarettes a day do you smoke?” | 10 or less | 21 | 30.4 |
| 20-Nov | 21 | 30.4 | |
| 21-30 | 17 | 24.6 | |
| 31 or more | 10 | 14.5 | |
| Onset of smoking at age (n=68) | Less than 16 | 25 | 36.8 |
| 16-18 | 21 | 30.9 | |
| 19-25 | 11 | 16.2 | |
| More than 25 | 11 | 16.2 | |
| Quit attempt in past 6 months (n=67) | Yes | 17 | 25.4 |
| No | 50 | 74.6 | |
| NRT use in the past | No | 29 | 42 |
| Yes | 40 | 58 | |
| Wisconsin Predicting Patients’ Relapse questionnaire (WI-PREPARE) items: | |||
| “I’m around smokers much of the time” | No, not at all (Scores 1-2) | 7 | 10.1 |
| Medium (Scores 3-5) | 27 | 39.1 | |
| Yes, absolutely (Scores 6-7) | 35 | 50.7 | |
| “When I haven’t been able to smoke for a few hours, the craving gets intolerable” | No, not at all (Scores 1-2) | 14 | 20.3 |
| Medium (Scores 3-5) | 35 | 50.7 | |
| Yes, absolutely (Scores 6-7) | 20 | 29 | |
| Stage of change (n=68) | Pre-contemplation | 42 | 61.8 |
| Other stage | 26 | 38.2 |
Table 1: Socio-demographic, clinical and smoking characteristics of psychiatric inpatients participating in a temporary smoking abstinence program (n=69).
Observation over the 26 h abstinence period
Success with temporary abstinence
More than 80% of participants succeeded with 9 h abstinence, whereas 31 patients (45.6%) remained abstinent for the whole 26 h program (Table 2). Carbon monoxide level significantly decreased (Friedman test, p<0.001). A large majority of patients used NRT (76.8%).
| Abstinence1 | n | % |
|---|---|---|
| <9 h | 11 | 16.2 |
| 9 h | 26 | 38.2 |
| 26 h | 31 | 45.6 |
| Carbon monoxide (ppm)6 | Median | (Range) |
| Thursday morning1 | 16 | (1-66) |
| Thursday evening2 | 7 | (1-28) |
| Friday morning3 | 6 | (2-37) |
| Nicotine replacement | n | % |
| None | 16 | 23.2 |
| Slow | 4 | 5.8 |
| Rapid | 23 | 33.3 |
| Both slow and rapid | 26 | 37.7 |
| Craving Friday morning | Median | (Range) |
| MPSS4 | 4 | (0-9) |
| MNWS-R5 | 13 | (0-28) |
Table 2: Abstinence from smoking, NRT use and craving during the intervention (n=69), missing data: 1n=68; 2n=67; 3n=63; 4n=64, 5n=62, 6Friedman test for change, p<0.001
Self-efficacy for temporary abstinence, as measured before the intervention, was significantly higher for patients who succeeded with 26 h abstinence than for the ones who failed (median 9, n=30, vs. median 7, n=37; Mann-Whitney U test, p=0.003). No significant difference was observed for self-efficacy for permanent abstinence.
Craving and withdrawal symptoms
Craving did not increase during the abstinence period, but rather diminished between the pre- and during-intervention (Friday) assessments (MPSS median score 5, range 0-10 vs. 4, range 0-9; Wilcoxon signed-rank test, p=0.01). No increase of withdrawal symptoms was observed. On the contrary, the MNWS-R total score diminished significantly (median 18, range 0-35 vs. 13, 0-28; p<0.001).
Negative and positive affects
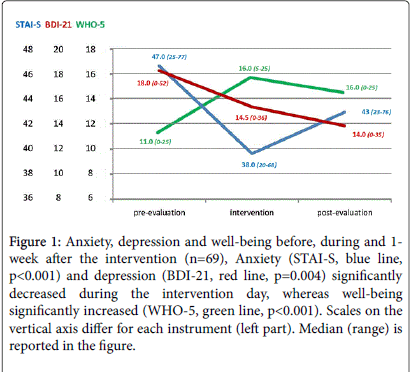
As shown in Figure 1, anxiety and depression severity significantly decreased during the intervention day (STAI-state median 47, range 25-77 vs. 38, 20-68; p<0.001; BDI-21 median 18, range 0-52 vs. 14.5, 0-36; p=0.004). Well-being significantly increased (WHO-5 median 11, range 0-25 vs. 16, 5-25; p<0.001). Satisfaction with the program was very high (mean 7.2, sd 0.9, range 5-8 on a 1-8 scale).
Figure 1: Anxiety, depression and well-being before, during and 1- week after the intervention (n=69), Anxiety (STAI-S, blue line, p<0.001) and depression (BDI-21, red line, p=0.004) significantly decreased during the intervention day, whereas well-being significantly increased (WHO-5, green line, p< 0.001). Scales on the vertical axis differ for each instrument (left part). Median (range) is reported in the figure.
Outcome after one week
Temporary abstinence and quit attempts
Among the 31 patients who succeeded with the 26 h abstinence period, 58.1% (n=18) decided to extend it and attempt quitting. After one week, 11 were still totally abstinent and 4 had resumed smoking but still intended to stop. Among participants who did not maintain the 26 h abstinence period, none engaged into a quit attempt. Thus, prevalence of smoking abstinence after 7 days was 15.9% (11 of 69).
Negative and positive affects
Depression, anxiety and well-being at one week confirmed the positive effects of the 26 h intervention (Figure 1).
Behavioral and motivational changes
As described in Table 3, behavioural changes were reflected by a significant decrease of cigarette consumption and carbon monoxide level that persisted up to one week. Subgroup analysis revealed that carbon monoxide level did not change significantly for participants who did not try quitting (n=50, median 21.5, range 4-100 vs. 24, range 2-66; p=0.55), but significantly decreased both for those who successfully stopped smoking (n=11, median 10, range 1-33 vs. 3, 1-7; p=0.005) or unsuccessfully tried to quit (n=7, median 16, range 3-25 vs. 10, 4-13; p=0.05).
| Pre-intervention | 1week Post-intervention | ||||
|---|---|---|---|---|---|
| Median | (Range) | Median | (Range) | P-value1 | |
| Cigarettes per day | 20 | (1-50) | 10 | (0-40) | <0.001 |
| Carbon monoxide (ppm) | 18 | (1-100) | 17 | (1-66) | 0.02 |
| Kahler’s commitment to quitting smoking scale (n=59) | 27 | (8-40) | 28 | (8-60) | 0.14 |
| Biener’s contemplation ladder | 7 | (0-10) | 8 | (0-10) | 0.17 |
| Self-efficacy for temporary abstinence | 8 | (3-10) | 9 | (3-10) | 0.003 |
| Self-efficacy for permanent abstinence | 8 | (1-10) | 8 | (3-10) | 0.84 |
| n | % | n | % | P-value2 | |
| Stage of change: Pre-contemplation (n=66) | 40 | 60.6 | 31 | 47 | 0.04 |
Table 3: Outcome 1 week after participation in a temporary smoking abstinence program (n=69), 1Wilcoxon signed-rank test; 2McNemar test.
Motivational changes, as measured by Kahler’s commitment to quitting scale and Biener’s contemplation ladder, were not significant. Nevertheless, a significant difference was observed for stage of change. Of 40 patients who were pre-contemplators before the intervention, 28 remained at the same stage 1-week after the intervention, but 12 reached contemplation or action stages. Of the 26 not in precontemplation before intervention, only 3 shifted back to precontemplation (Mc Nemar test p=0.04). Of 15 persons in “action” stage 1-week after the intervention, 6 were “pre-contemplators” at preevaluation, 7 were “contemplators” and 2 were “in preparation”: unlike in the traditional TTM, 40% had thus shifted directly from precontemplation to action. Ten participants (14.5% of 69) with no intention to quit smoking at pre-evaluation, but successful with the 26h abstinence period, took the decision to stop (8 in continuation of the program and 2 taking an appointment for individual support in the coming week).
Self-efficacy
Self-efficacy to quit permanently did not change, but self-efficacy for temporary abstinence significantly increased (Table 3). More than half (52.2%) of the participants had higher scores after one week, whereas about one third (32.8%) remained unchanged. Subgroup analysis according to success of abstinence during the intervention day showed that self-efficacy for temporary abstinence significantly increased for participants who succeeded with 26 h abstinence (median 10 vs. 9, n=30; Wilcoxon signed-rank test, p=0.003), but not for the ones who only achieved 9 h abstinence or failed to do so. After one week, self efficacy for temporary abstinence was significantly higher among patients who succeeded with the program (median10, n=31 vs. median 8, n=37; Mann-Whitney test, p<0.001).
Discussion
Results showed that the majority of smokers presenting with severe psychiatric disorders were able to comply with a 9 or 26 h smoking abstinence period, and that this experience did allow them to progress in motivational processes such as stage of change and self-efficacy. Thus, the main objective of the intervention was fully met. Furthermore, a quarter of patients made a quit attempt shortly after the program and 10% actually decided to stop smoking although this was not their intention before the intervention, suggesting that the program was able to trigger new perspectives.
The TTM model, postulating that step by step psychological changes lead to behavioral changes (stop smoking), although useful, has never been formally validated and about half of quit attempts seem to be unplanned [26,27]. In our study, some patients decided to stop even though they were in the pre-contemplation stage before the intervention. In the light of the TTM, one would expect smokers to have evolved in their cognitions to be ready for the action stage. Alternatively, one may postulate that behavioural events, such as temporary abstinence, might induce psychological changes and catalyse the stopping process. This is in agreement with the theoretical concept that positive consequences of an action (e.g. participation in the multicomponent intervention) are able to affect motivational processes that precede volitional or planning aspects of behaviour changes [28,29]. Intervention on motivational processes by means of positive experience seems crucial for the population targeted for our intervention, most of who are in pre-contemplation stage, not yet planning to stop smoking.
Several components of the proposed intervention are important. Firstly, generous NRT was proposed in order to reduce withdrawal symptoms. Our study indicated that craving (MPSS) was lower during the intervention than at pre-evaluation and that withdrawal symptoms significantly decreased (MNWS-R). This is of special interest, since the severity of withdrawal signs is generally highest within the first 3 h after cessation and decreases over time [30,31]. In our sample, only 16% failed to maintain abstinence for the first 9 h, suggesting that most smokers tolerated abstinence during that period. However, a minority felt that it was very difficult or uncomfortable to refrain from smoking; mainly the ones who refused NRT (e.g., increased auditory hallucinations in a schizophrenic patient not accepting NRT). Our clinical impression was that the large majority of participants felt fine, relaxed and proud about their participation, with acceptable craving related discomfort.
A second important component is the positive and pleasurable experience associated with the intervention, as shown by high satisfaction and increased well-being. Better oxygenation due to abstinence, light exercise and relaxation (thermal baths) may contribute to this effect, in addition to psychological effects (e.g. satisfaction of succeeding with the personal challenge of not smoking during 26 h). The program was indeed designed to be pleasurable and attractive. It is well known that nicotine activates reward pathways in the brain and induces pleasurable sensations. It was hypothesized that the association of abstinence with similarly pleasurable moments might contribute to enhance readiness for change with respect to smoking behaviour. Furthermore, the program was meant to be appealing to psychiatric inpatients who might not be willing to engage in usual smoking cessation programs.
Social and relational aspects are other important elements of the program. Many patients were socially isolated and appreciated the group experience and support from other participants and staff. The individualized relationship established between staff and participants during the pre-intervention session seems crucial in helping them overcome their fears and enrol. During the intervention, it helps them overcome their anxiety about spending a day in an unknown environment.
Formal information about smoking is the fourth ingredient of the program. Participants learn new facts about tobacco and get individual feedback about their carbon monoxide measurements. For many, this was an opportunity to become aware of a directly measurable effect of smoking in their lungs.
Finally, the proactive characteristic of the intervention needs to be emphasized, with personal commitment of patients towards temporary abstinence and supporting but non-directive attitudes of the staff. A proactive intervention to motivate smokers to quit was associated with improved results as compared to usual smoking cessation care [32]. Furthermore, patient empowerment, i.e. helping them to take autonomous, informed decisions, might be a fundamental element in the process towards quitting [33].
These different components of the program most likely contribute together to the motivational change, increased self-efficacy, and decision to attempt quitting, as observed in the present study. A review of predictors of cessation attempts showed the importance of motivation and self-efficacy, even for smokers not currently willing to quit [34,35]. Our results are in keeping with this finding, with significantly higher self-efficacy in patients who attempted to stop in continuation of the program. We also observed increased self-efficacy for temporary abstinence during the intervention and hypothesized that it might contribute to the decisional process towards cessation in a psychiatric population of mostly pre-contemplators.
In contrast with the fears of many clinicians that smoking cessation might be accompanied with worsening of patients’ condition, we observed a significant decrease of anxiety and depression during the intervention day that persisted up to one week later. Apart from somatic health benefits, growing literature points to improvement in mental health coupled with smoking cessation interventions [36]. In a randomized study, enrolment in tobacco cessation treatment showed a broad therapeutic effect with less frequent psychiatric rehospitalisation after 18 months [37]. A meta-analysis of 26 studies concluded that smoking cessation was associated with mental health benefits, with comparable effect sizes at 6 weeks, six months or longer [38]. A large epidemiological study also concluded that smoking cessation was associated with reduced risk for mood, anxiety and alcohol-related disorders, even among smokers with a pre-existing psychiatric disorder [39]. As mental and physical health is intertwined, interventions to promote smoking cessation deserve to be fully integrated within health care delivery systems [1]. Furthermore, techniques used in mental health care such as distress tolerance skills or working upon cognitive distortions might present combined benefits for smoking cessation too [40,41].
Limitations of the present study include the short duration of the intervention and follow-up period, in comparison with the long processes involved in smoking cessation, which may last for years if not decades. Research has shown that the first week of abstinence was highly predictive of long-term abstinence in non-psychiatric samples [42]. However, the long-term effects of the proposed intervention remain to be addressed. Another limitation is the absence of a control group, which will be needed in further studies aimed at confirming specific effects of the program. Indeed, all patients received psychiatric care which might have contributed to the decrease of symptoms one week after the intervention. The multicomponent structure of the intervention also renders the evaluation of single elements difficult.
Conclusion
Interventions for smokers presenting with severe psychiatric disorders are necessary and should target not only patients willing to quit but all smokers, independently of their motivation at any given time. The present study showed benefits of temporary smoking abstinence, as part of a global motivational intervention aimed at increasing self-efficacy and catalyzing the complex processes towards smoking cessation.
Acknowledgement
We would like to thank Dr Jean-Paul Humair (Division of Primary Care Medicine, University Hospitals of Geneva and CIPRET-Geneva) and Ms Patricia Borrero (Directorate of Nursing Services, University Hospitals of Geneva) for their participation in the program as tobacco treatment specialists. We are grateful to the hospital staff for helping us to introduce the intervention into the hospital setting. This study was not supported by any specific grant, and authors are employed by the University Hospitals of Geneva. They do not declare any competing interests.
References
- Colton CW, Manderscheid RW (2006) Congruencies in increased mortality rates, years of potential life lost and causes of death among public mental health clients in eight states. Prev Chronic Dis 3: A42.
- Glaus J, Vandeleur C, Gholam-Rezaee M, Castelao E, Perrin M, et al. (2013) Atypical depression and alcohol misuse are related to the cardiovascular risk in the general population. ActaPsychiatrScand 128: 282-293.
- Jochelson K (2006) Smoke-free legislation and mental health units: the challenges ahead. Br J Psychiatry 189: 479-480.
- Cook BL, Wayne GF, Kafali EN, Liu Z, Shu C, et al. (2014) Trends in smoking among adults with mental illness and association between mental health treatment and smoking cessation. Jama 311: 172-182.
- Steinberg ML, Williams JM, Li Y (2015) Poor mental health and reduced decline in smoking prevalence. Am J Prev Med 49: 362-369.
- Szatkowski L, McNeill A (2015) Diverging trends in smoking behaviors according to mental health status. Nicotine Tob Res 17: 356-360.
- Selby P, Voci SC, Zawertailo LA, George TP, Brands B (2010) Individualized smoking cessation treatment in an outpatient setting: Predictors of outcome in a sample with psychiatric and addictions co-morbidity. Addict Behav 35: 811-817.
- DiClemente CC, Fairhurst SK, Velasquez MM, Prochaska JO, Velicer WF, et al. (1991) The Process of smoking cessation: An analysis of precontemplation, contemplation and preparation stages of change. Journal of Consulting and Clinical Psychology 59: 295-304.
- Dickens GL, Staniford J, Long CG (2014) Smoking behaviour, motives, motivation to quit and self-efficacy among patients in a secure mental health service: Comparison with staff controls. J PsychiatrMent Health Nurs 21: 483-490.
- Ziedonis D, Hitsman B, Beckham JC, Zvolensky M, Adler LE, et al. (2008) Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine Tob Res 10: 1691-1715.
- Rigotti N, Rennard SI, Daughton DM(2016) Benefits and risks of smoking cessation.
- Keizer I, Bruegger A, Gex-Fabry M, Borrero P, Humair JP, et al. (2012) A brief motivational intervention based on positive experience and temporary smoking abstinence: Feasibility in a psychiatric hospital. Eur J Psychiat 26: 127-134.
- WHO ASSIST Working Group (2002) The alcohol, smoking and substance involvement screening test (ASSIST): development, reliability and feasibility. Addiction 97: 1183-1194.
- Spielberger CD (1983) State-trait anxiety inventory: A comprehensive bibliography. Consulting Psychologists Press, Palo Alto, CA.
- Beck AT, Epstein N, Brown G, Steer RA (1988) An Inventory for measuring clinical anxiety: Psychometric properties. J Consult ClinPsychol 56: 893-897.
- WHO (1999)Indice (en 5 points) de bien-être de l'OMS.
- Kozlowski LT, Porter CQ, Orleans CT, Pope MA, Heatherton T (1994) Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND and HSI. Drug Alcohol Depend 34: 211-216.
- Etter JF, Duc TV, Perneger TV (1999) Validity of the Fagerstrom test for nicotine dependence and of the heaviness of smokingindex among relatively light smokers. Addiction 94: 269-281.
- Bolt DM, Piper ME, McCarthy DE, Japuntich SJ, Fiore MC, et al. (2009) Thewisconsin predicting patients' relapse questionnaire. Nicotine Tob Res 11: 481-492.
- West R, Hajek P (2004) Evaluation of the mood and physical symptoms scale (MPSS) to assess cigarette withdrawal. Psychopharmacology (Berl) 177: 195-199.
- Hughes JR, Hatsukami D (1986) Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry 43: 289-294.
- Etter JF (2008) Minnesosta (MNWS-R) - Version française.
- Kahler CW, Lachance HR, Strong DR, Ramsey SE, Monti PM, et al. (2007) The commitment to quitting smoking scale: initial validation in a smoking cessation trial for heavy social drinkers. Addict Behav 32: 2420-2424.
- Biener L, Abrams DB (1991) The contemplation ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychol 10: 360-365.
- Dino G, Kamal K, Horn K, Kalsekar I, Fernandes A (2004) Stage of change and smoking cessation outcomes among adolescents. Addictive Behaviors 29: 935-940.
- Brug J, Conner M, Harre N, Kremers S, McKellar S, et al. (2005) The transtheoretical model and stages of change: a critique: observations by five commentators on the paper by Adams J and White M (2004) Why don't stage-based activity promotion interventions work? Health Educ Res 20: 244-258.
- Larabie LC (2005) To what extent do smokers plan quit attempts? Tob Control 14: 425-428.
- Parschau L, Fleig L, Warner LM, Pomp S, Barz M, et al. (2014) Positive exercise experience facilitates behavior change via self-efficacy. Health EducBehav 41: 414-422.
- Heckhausen H (1991) Motivation and action. Berlin, Germany: Springer.
- Brown J, Hajek P, McRobbie H, Locker J, Gillison F, et al. (2013) Cigarette craving and withdrawal symptoms during temporary abstinence and the effect of nicotine gum. Psychopharmacology (Berl) 229: 209-218.
- Hughes JR (2007) Effects of abstinence from tobacco: Valid symptoms and time course. Nicotine Tob Res 9: 315-327.
- Fu SS, van Ryn M, Sherman SE, Burgess DJ, Noorbaloochi S, et al. (2014) Proactive tobacco treatment and population-level cessation: A pragmatic randomized clinical trial. JAMA Intern Med 174: 671-677.
- Anderson RM, Funnell MM (2010) Patient empowerment: Myths and misconceptions. Patient EducCouns 79: 277-282.
- Vangeli E, Stapleton J, Smit ES, Borland R, West R (2011) Predictors of attempts to stop smoking and their success in adult general population samples: A systematic review. Addiction 106: 2110-2121.
- Jardin BF, Carpenter MJ (2012) Predictors of quit attempts and abstinence among smokers not currently interested in quitting. Nicotine Tob Res 14: 1197-1204.
- Banham L, Gilbody S (2010) Smoking cessation in severe mental illness: What works? Addiction 105: 1176-1189.
- Prochaska JJ, Hall SE, Delucchi K, Hall SM (2014) Efficacy of initiating tobacco dependence treatment in inpatient psychiatry: A randomized controlled trial. Am J Public Health 104: 1557-1565.
- Taylor G, McNeill A, Girling A, Farley A, Lindson-Hawley N, et al. (2014) Change in mental health after smoking cessation: Systematic review and meta-analysis. British Medical Journal 348: g1151.
- Cavazos-Rehg PA, Breslau N, Hatsukami D, Krauss MJ, Spitznagel EL, et al. (2014) Smoking cessation is associated with lower rates of mood/anxiety and alcohol use disorders. Psychological Medicine 44: 2523-2535.
- Trujillo MA, Khoddam R, Greenberg JB, Dyal SR, Ameringer KJ, et al. (2015) Distress tolerance as a correlate of tobacco dependence and motivation: Incremental relations over and above anxiety and depressive symptoms. Behav Med.
- Brown RA, Reed KM, Bloom EL, Minami H, Strong DR, et al. (2013) Development and preliminary randomized controlled trial of a distress tolerance treatment for smokers with a history of early lapse. Nicotine Tob Res 15: 2005-2015.
- Ashare RL, Wileyto EP, Perkins KA, Schnoll RA (2013) The first 7 days of a quit attempt predicts relapse: Validation of a measure for screening medications for nicotine dependence. J Addict Med 7: 249-254.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Relapse prevention
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 11575
- [From(publication date):
August-2016 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 10850
- PDF downloads : 725