A Short Commentary on "Assessment of Muscle Involvement in Patients with Duchenne Muscular Dystrophy via Segmental Multifrequency Bioelectrical Analysis"
Received: 12-Dec-2019 / Accepted Date: 27-Dec-2019 / Published Date: 03-Jan-2020 DOI: 10.4172/2476-2024.1000158
The Study
Duchenne muscular dystrophy (DMD) is an inherited X-linked recessive disorder affecting approximately 1 in 3500-5000 live male births. This disorder is caused by a mutation in the dystrophin gene and is characterized by muscle wasting and weakness, with early childhood onset that progresses to patients being unable to walk and becoming wheelchair bound by the second decade of life. Till date, there has been no effective therapy for DMD. However, many promising genetic strategies have emerged as potential therapies [1,2], including viral delivery of microdystrophin genes, stop-codon read through, and exon-skipping with antisense oligonucleotides, with several treatments currently entering clinical trials. Therefore, there is a pressing need for methods capable of frequently and easily assessing disease progression and treatment efficacy.
Bioelectrical impedance analysis (BIA) is used for the estimation of skeletal muscle mass in healthy populations [3]. It can be classified into single-frequency BIA and multi-frequency BIA (MBIA). The body’s impedance depends upon the frequency of the alternating current applied. The impedance of an applied low-frequency current primarily reflects extracellular water (ECW), whereas the impedance of an applied high-frequency current reflects the total tissue water (TTW) [4,5]. Yamada et al. showed that MBIA was superior to singlefrequency BIA for assessing skeletal muscle mass in elderly patients [4]. McDonald et al. reported high correlations between whole-body MBIA and dual-energy Xray absorptiometry in assessing the body composition of DMD patients [6].
We previously proposed the muscle density index (MDI) to be a novel index of BIA and demonstrated that it could accurately represent muscle development and muscle mass increases in healthy children [7]. The MDI can be calculated according to the following equation:
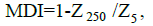
where Z is impedance in ohms and Z 5 and Z 250 represent impedance at 5 kHz and 250 kHz, respectively. MDI is believed to reflect the ratio of intracellular water (ICW) to TTW.
In our article titled, “ Assessment of muscle involvement in patients with Duchenne muscular dystrophy via segmental multifrequency bioelectrical analysis ” [8], we investigated the usefulness of segmental MBIA to assess muscle involvement in DMD patients. Bioelectrical impedance data of the upper arm, thigh, and lower leg were obtained from 29 boys with DMD and 41 healthy control subjects over a wide range of ages from infancy to the middle teen years. We obtained the following main findings from that study:
1. The MDI was lower in boys with DMD relative to the controls, with older DMD patients exhibiting a significant decrease in MDI.
2. The MDI of a patient’s thighs was significantly correlated with the percent muscle volume index, as measured by computed tomography.
3. MDI values for the upper arm, thigh, and lower leg were all significantly correlated with the Brooke and Vignos functional scales.
4. MDI was significantly greater in the glucocorticoid (GC)-treated group than in the untreated group in all regions.
Studies have shown that BIA values are altered in DMD [5,9]. The impedance of the tissue is affected by changes in the structure and composition of muscle, including muscle hypertrophy or atrophy, edema, inflammation, and replacement of muscle cells by fat and connective tissue. The results presented here are consistent with those of previous studies that demonstrated a significant increase in the ECW:ICW ratio in DMD relative to that in healthy children [5,6]. The MDI of healthy children showed a significant increase with increasing age, indicative of normal muscle growth and maturation. In contrast, the MDI of DMD patients showed a significant decrease with increasing age. However, the study used a cross-sectional design. Therefore, further longitudinal studies with serial examination would be needed to follow the changes in muscles because of DMD progression.
In this study, we measured bioelectrical impedance of segmental muscle instead of whole-body measurements. The severity and rate of progression of muscle involvement in DMD varies from site to site. Muscle weakness first becomes apparent in proximal muscles of the lower extremities and then spreads to distal muscles and the upper extremities. Segmental measurement makes it possible to directly evaluate the regional muscle involvement in detail. Particularly, because there has been a paucity of tools that can assess upper limb muscles in DMD, segmental MBIA will be useful for assessing muscle involvement of the upper limbs. Recently, electrical impedance myography (EIM) has been used to assess localized muscle [9]. There remains an important distinction between EIM and the technique described here in terms of the size of the area of interest.
GC medications have a beneficial effect on muscle strength and function in DMD [10,11]. The present study included both patients with and without GC treatment. The authors were able to identify a visual difference between the groups that was indicative of a difference in MDI between the DMD patients treated with GCs and those not treated with GCs. Segmental MBIA may offer an improved method for determining the efficacy of GC treatment.
A significant limitation of this study was the lack of a direct comparison between segmental MBIA and magnetic resonance imaging (MRI) findings. MRI is sensitive to disease progression and optimal for quantifying muscle pathology in DMD [12,13].
The degrees of fibro-fatty change and edema or inflammation in the skeletal muscle of DMD have been shown by improved imaging techniques, such as Dixon and MR spectroscopy. Studies comparing BIA with muscle characteristics measured by MRI would make it possible to determine which tissue characteristics most affect BIA measurements.
Segmental MBIA is an inexpensive, easy to use, non-invasive measurement tool. Examination by MBIA was well-tolerated, even by boys under 5 years of age. Moreover, MBIA can be performed repeatedly at short intervals because of its portability and convenient setup. Our results suggest that segmental MBIA could serve as an outcome measure in DMD.
References
- Welch EM, Barton ER, Zhuo J, Tomizawa Y, Friesen WJ, et al. (2007) PTC124 targets genetic disorders caused by nonsense mutations. Nature 447: 87.
- Cirak S, Arechavala-Gomeza V, Guglieri M, Feng L, Torelli S, et al. (2011) Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. The Lancet 378: 595-605.
- Lukaski HC (1990) Applications of bioelectrical impedance analysis: A critical review. Basic Life Sci 55: 365-374.
- Yamada Y, Ikenaga M, Takeda N, Morimura K, Miyoshi N, et al. (2013) Estimation of thigh muscle cross-sectional area by single-and multifrequency segmental bioelectrical impedance analysis in the elderly. J Appl Physiol 116: 176-182.
- Bedogni G, Merlini L, Ballestrazzi A, Severi S, Battistini N (1996) Multifrequency bioelectric impedance measurements for predicting body water compartments in Duchenne muscular dystrophy. Neuromuscular Disord 6: 55-60.
- McDonald CM, Carter GT, Abresch RT, Widman L, Styne DM, et al. (2005) Body composition and water compartment measurements in boys with Duchenne muscular dystrophy. Am J Phys Med Rehabil 84: 483-491.
- Uchiyama T, Nakayama T, Kuru S (2017) Muscle development in children evaluated by bioelectrical impedance analysis. Brain Dev 39: 122-129.
- Kuru S, Uchiyama T, Hattori A, Sato T, Murakami T, et al. (2019) Assessment of muscle involvement in patients with Duchenne muscular dystrophy via segmental multifrequency bioelectrical analysis. Neuromuscular Disord 29: 671-677.
- Zaidman CM, Wang LL, Connolly AM, Florence J, Wong BL, et al. (2015) Electrical impedance myography in Duchenne muscular dystrophy and healthy controls: A multicenter study of reliability and validity. Muscle Nerve 52: 592-597.
- Takeuchi F, Yonemoto N, Nakamura H, Shimizu R, Komaki H, et al. (2013) Prednisolone improves walking in Japanese Duchenne muscular dystrophy patients. J Neurol 260: 3023-3029.
- Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, et al. (2010) Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol 9: 77-93.
- Forbes SC, Willcocks RJ, Triplett WT, Rooney WD, Lott DJ, et al. (2014) Magnetic resonance imaging and spectroscopy assessment of lower extremity skeletal muscles in boys with Duchenne muscular dystrophy: A multicenter cross sectional study. PloS One 9: e106435.
- Kinali M, Arechavala-Gomeza V, Cirak S, Glover A, Guglieri M, et al. (2011) Muscle histology vs. MRI in Duchenne muscular dystrophy. Neurology 76: 346-353.
Citation: Kuru S (2019) Assessment of Muscle Involvement in Patients with Duchenne Muscular Dystrophy via Segmental Multifrequency Bioelectrical Analysis. Diagn Pathol Open 4: 158. DOI: 10.4172/2476-2024.1000158
Copyright: © 2019 Kuru S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 2467
- [From(publication date): 0-2019 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 1720
- PDF downloads: 747
