A Search for Missing Pre-Diabetes Patients in a Highly Susceptible Population and a Review of the Dire Consequences of Failing to Identify Them
Received: 22-Dec-2022 / Manuscript No. jcmhe-22-84513 / Editor assigned: 26-Dec-2022 / PreQC No. jcmhe-22-84513 (PQ) / Reviewed: 09-Jan-2023 / QC No. jcmhe-22-84513 / Revised: 16-Jan-2023 / Manuscript No. jcmhe-22-84513 (R) / Published Date: 23-Jan-2023 DOI: 10.4172/2168-9717.1000796
Abstract
Type 2 diabetes mellitus (2DM) affects 10.5% and pre-DM touches 34.5% of the American population. The Center for Disease Control (CDC) estimates that 84% of that 34.5% are unaware that they have it. This study sought patients who are at risk for pre-DM who have yet to be informed of that risk or assessed for pre-DM, and to compare these findings to national averages.
In our federally qualified health center clinic (FQHC) a total of 101 patients were recruited from five cardiovascular groups and analyzed. Nine percent of patients, who had A1C values within the pre-DM range, were not informed of their risk and not undergoing treatment. Additionally, despite more T2DM in our non-Hispanic Black and Latinx populations, 58% of Black and 69% of Latinx patients with obesity were unaware of their risk for developing pre-DM. Furthermore, 42% of Black patients and 27% of Latinx patients with obesity were never investigated for DM. Future investigations should find whether this discrepancy exists among other FQHC clinics and seek the root cause behind such disparity.
Compared to the generalized American population, these data suggest that only 9% of our at risk patients live with pre-DM without being aware of it, compared to the 29% (84% of 34.5%) of American adults who have pre-DM but are unaware. The FQHC clinic involved in this study, though showing better awareness of the problem than those surveyed by the CDC, was still lacking in reaching the standard where the bar should be set.
Keywords: Pre-diabetes; Type 2 Diabetes mellitus; Peripheral neuropathies; Microvascular diseases; Diabetic nephropathy
Introduction
Diabetes mellitus (DM) is a metabolic disease characterized by persistent hyperglycemia [1]. Type 2 DM (T2DM) is common in the US, with 10.5% of the population bearing this diagnosis in 2020 [2]. T2DM has several distinct risk-factors, including obesity, hyperlipidemia, hypertension, family history, race, ethnicity, smoking status, and others [3,4]. A BMI>25 alone is a risk factor worthy of screening patients for pre-DM and T2DM [5]. T2DM is often referred to, like hypertension, as a “silent killer” since it often remains clinically quiet and therefore should be investigated in high-risk populations. DM also contributes to an increasing number of various acute and chronic conditions: hyperglycemic hyperosmolar state (HHS), diabetic ketoacidosis (DKA, particularly in T1DM), gastroparesis, coronary artery disease (CAD), nephrotic and hepatic disease, neuropathy, and microvascular trauma [1,6-8].
Pre-DM is a high-risk state for T2DM development, with 5%- 10% of patients with pre-DM progressing to T2DM per year [9]. Since 34.5% of the U.S. adult population of 259.4 million has pre-DM, a huge number of patients could progress annually to a life threatening illness [2]. The goal of this research is to identify patients at risk for pre-DM and to compare the sensitivity and performance of our clinical staff, concerning this important, chronic, manageable, healthcare issue, to national clinician statistics.
In 2017, the total cost of managing DM in the United States was $ 327 billion, lending to both the clinical and economic relevance of this study [10]. In terms of 2022 dollars, given an inflation rate of 3.58% per year between 2017 and today, and a cumulative price increase of 19.25%, the current cost of diabetes care totals $ 390 billion. Identifying pre-DM before it evolves into T2DM, and slowing or reversing its progression, can have significant effects on the ensuing micro vascular complications and the further inflation of healthcare dollars in the management of DM complications.
Methods
The 101 patients, ages 18 to 75 years old, in this study have been randomly selected from those being treated for diabetic risk factors at four adult FQHC clinics (One Health Ohio) in three NEO counties. These patients have not been identified as having pre-DM or are unaware that they have pre-DM based on blood glucose or HbA1C levels. To fit the study’s criteria, patients must have had ICD-10 codes representing one of the following five groups:
1. Obesity with a BMI greater than 39.9
2. Hypertension (HTN) plus obesity (BMI 30 to 39.9)
3. Hyperlipidemia (HLD) plus obesity
4. African American race plus obesity
5. Latino nationality plus obesity
A selection of these groups was based upon established risk factors for chronic hyperglycemia, along with possible social disparities and bias among groups at risk for chronic hyperglycemia [4,5]. Patients with existing T1DM, T2DM, or known pre-DM were excluded from the study.
Thirty patients from each risk group were randomly selected and called; if the patient did not answer, follow-up calls were placed twice. After three missed calls, patients were eliminated from the study. Patients unwilling to participate in the interview were immediately removed. Qualified patients subsequently participated in a phone interview. Patients were asked to report if they had ever been told that they are at risk for, or have pre-DM, and whether they are undergoing lifestyle modifications and/or metformin therapy to prevent progression into T2DM. Patients were then classified as to whether they fit in the pre-DM stage, where HbA1C ranges from 5.7 to 6.4, and analyzed accordingly. Patient data was anonymized for analysis. Data analysis and graphics presented were prepared using libraries on a Jupyter Notebook using Python 3.9.13.
Results
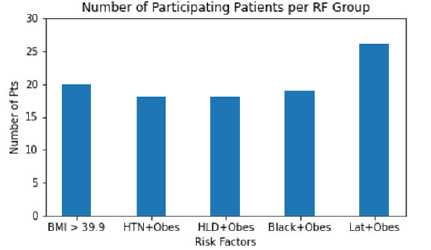
Out of the 101 patients examined in this study, 20 were in risk factor (RF) group 1 (BMI>39.9), 18 in risk group 2 (HTN+obesity), 18 in risk group 3 (HLD+obesity), 19 in risk group 4 (Black+obesity), and 26 in risk group 5 (Latinx+obesity), as displayed in Figure 1.
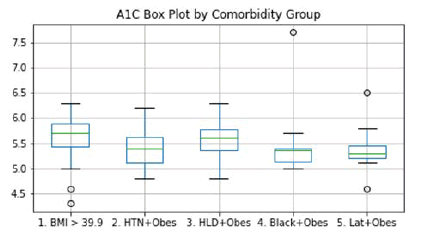
The mean A1C value among all groups was 5.5. Figure 2 reveals a box-whisker plot of A1C values by group. A one-way ANOVA test was performed on these groups and revealed no significant difference between the groups with p=0.6660 and with an F=0.5969.
Figure 2: A1C Box Plot by Comorbidity Group-This plot shows the A1C levels among patients based on their comorbidity group. A mean A1C of 5.5 among all patients leaves the BMI>39.9 and HLD + obesity groups above the average, and the other three groups below the average. No statistically significant differences among the groups were noted.
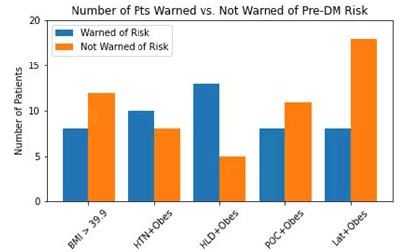
Figure 3 delves into the question of whether patients were informed by their clinicians that their obesity and other factors place them at higher risk of developing pre-DM or T2DM. It was found that a total of 54 patients were not informed of their risk: 12 in group 1, 8 in group 2, 5 in group 3, 11 in group 4, and 18 in group 5.
Figure 3: Number of Patients Warned vs. Not Warned of Pre-DM Risk-This plot shows how many patients recruited subjectively reported being informed by their clinician of increasedrisk for pre-DM/T2DM by risked factors compared to the number of patients who subjectively reported not being informed by their clinicians for their increased risk.
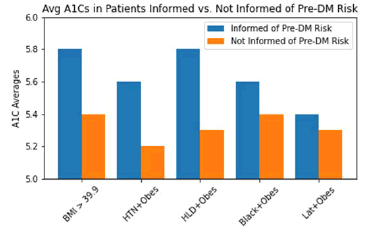
Figure 4 compares the patients informed by their clinicians of their increased risk for pre-DM and T2DM by their average A1C values. An unpaired t-test was performed on average A1Cs in each RF group whether 1. Warned or 2. Not warned by clinicians on increased risk for developing pre-DM or T2DM. Group 3 revealed significant differences in average A1Cs when informed compared to uninformed by clinicians of their increased risk (p=0.0051), as shown in Table 1. The other groups revealed no significant differences.
| Group | Avg A1C Informed | Avg A1C Not Informed | p-value |
|---|---|---|---|
| 1 | 5.8 | 5.4 | 0.0721 |
| 2 | 5.6 | 5.2 | 0.2166 |
| 3 | 5.8 | 5.3 | 0.0051 |
| 4 | 5.6 | 5.4 | 0.8365 |
| 5 | 5.4 | 5.3 | 0.5379 |
Table 1: Shows the average A1Cs by risk factor group whether warned or not warned of pre-DM risk by provider significance.
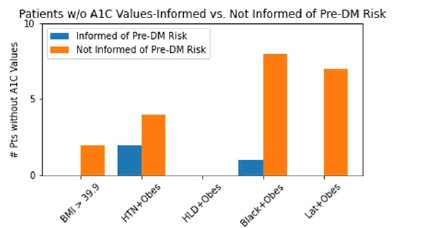
Figure 5 reveals significant differences in at-risk patients who had no A1C values on their charts. In total, 24 patients did not have A1C values (24% of patients surveyed). Among patients not informed of their risk for pre-DM, two fell into group 1, four in group 2, zero in group 3, eight in group 4, and seven in group 5. The other three patients who did not have A1C values were informed of their risk and are undergoing preventative lifestyle modifications.
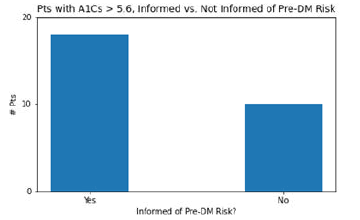
27 patients in this study, having A1C values of 5.7 or above, were not labeled with pre-DM in their charts. Of these 27 patients, 17 remember having discussions with their clinician about their increased risk for pre-DM, and 10 do not remember any such discussion. This data is in Figure 6.
Discussion
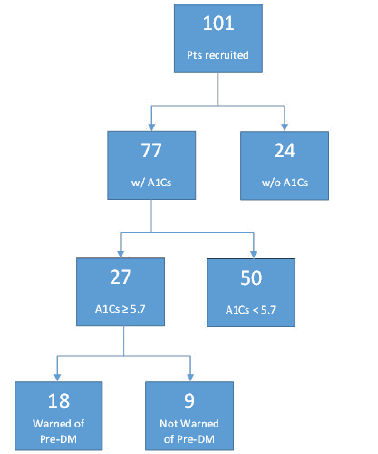
Approximately 34.5% of American adults have pre-DM; several studies have indicated that 84% of those individuals are unaware. As of 2022, 259.4 million Americans are age 18 years or older. Thus, 89.5 million possess pre-DM, implying that 75.2 million people (the 84% unaware) could be ignorant of that significant fact [11,12]. At the NEO FQHC studied, clinicians are discovering pre-DM and informing patients of their risk at a significantly better percentage than the national average. Of the 101 patient’s recruited based on risk factors for pre-DM without the diagnosis of pre-DM, 27 had A1C values in the pre-diabetic range (27%). Of these 27, nine patients were not warned by their clinician that they met criteria for the diagnosis of pre- DM. Of these nine patients, one saw a physician, and the other eight saw PA or NP clinicians; thus, the rate of patients missed was 5% among physicians and 16% among non-physicians. Data suggests that only 9% of surveyed patients with risk factors live with pre-DM without being aware of it, compared to the 29% (84% of 34.5%) of American adults who have pre-DM but are unaware. These findings are summarized further below in Figure 7.
Figure 7: Flowchart of Summarized Findings-This flowchart summarizes the findings in the patients surveyed throughout this study. Namely, 24% of patients at high risk for pre-DM/T2DM were found to have no A1C values on record, and 9 patients with A1Cs in the pre-DM range do not recall being informed by their clinicians of their increased risk. These 9 patients likely are missed and not undergoing any pharmacologic/lifestyle therapeutic intervention.
Despite the relative success of clinicians at this FQHC, 9% is still troublesome; the spontaneously progressive, chronic complications of DM, which are known to begin during the pre-DM, are not being therapeutically addressed. At the very least, patients should be undergoing lifestyle modifications, including smoking cessation, ethanol moderation, stress reduction techniques, dietary and exercise reformations, and weight loss. Additionally, there are some pre-DM patients who should be on treatment with metformin [13].
Unfortunately, certain consortia within the RF groups tended to be less informed by clinicians of their increased risk. Figure 2 revealed that the average A1C among RF groups was statistically insignificant. Figure 4 also reveals that, aside from the HLD+obesity group, the patient being warned of their risk for pre-DM did not correlate with a statistically significant change in A1C. This would imply that variance among the RF groups was not objectively available. However, when looking at Figures 3 and 5, social disparities may be present among clinicians. Figure 3 reveals that some clinicians failed to inform patients of their increased risk for pre-DM in 12/20 (60%) patients in group 1 (BMI>39.9), 11/19 (58%) patients in group 4 (Black+obesity), and 18/26 (69%) patients in group 5 (Latinx+obesity), compared to 8/18 (44%) in group 2, and 5/18 (28%) in group 3. Figure 5 reveals that clinicians failed to investigate pre-DM risk in 8/19 (42%) patients in group 4, and 7/26 (27%) patients in group 5, compared to 2/20 (10%) patients in group 1, 4/18 (22%) in group 2, and zero patients in group 3. Obesity alone warrants investigation of pre-DM with an A1C level; the additional RF further increases the danger of pre-DM and T2DM. Specifically, T2DM prevalence among the Latinx population is 12.5%, and the non-Hispanic Black population is 11.7%, compared to 7. 5% of non-Hispanic White patients [5]. In the surveyed patients, 58% of Black patients with obesity and 69% of Latinx patients with obesity were not aware of their increased risk for developing pre-DM.
Furthermore, 42% of Black patients with obesity and 27% of Latinx patients with obesity did not even have an A1C value checked. Additionally, further investigation of charts revealed that these patients lacked other, less common methods of screening for pre-DM (i.e., fasting plasma glucose and oral glucose tolerance tests). While it is possible that this discrepancy is due to random error or lack of clinician knowledge of specific risk factors, further investigation is warranted to determine clinician bias among other outpatient FQHC clinics.
Overview of sequelae in DM
The following compendium is an over-review of perilous sequelae of chronic hyperglycemia and hyperinsulinemia in pre- DM, T1DM, and T2DM. Microvascular changes have been noted as early as the pre-DM stage and are thus an important part of a clinician’s knowledge base to provide patients with appropriate, holistic care.
Peripheral neuropathies
Peripheral neuropathies are a major cause of disability in the United States. Half of those cases are secondary to DM, with the majority of those DM induced neuropathies appearing in the large fiber neuropathy group (LFN) [14]. In addition to the risk of eventual total disability, diabetics having peripheral neuropathies suffer from a decreased quality of life due to pain, the loss of proprioception resulting in gait instability with frequent injurious falls, foot ulcerations, and eventual amputation [15]. Small fiber neuropathies (SFN) develop insidiously and proceed unnoticed over months to years during the pre-DM phase, resulting in another form of diabetic peripheral neuropathy (DPN), and is already present in 20% of newly diagnosed type 2 DM [16]. SFN are also prone to develop in persons with the so-called metabolic syndrome [17].
Most patients with DPN have symptomatic large fiber involvement. Large sensory fibers are responsible for detecting and sending signals regarding joint position, vibration, and touch sensations. Clinically, patients complain of paresthesia and pain. Gait instability is a common manifestation of proprioceptive dysfunction, predominantly when walking in the dark or on uneven surfaces. LFN can lead to a progressive loss of protective pain sensation, even in the absence of the patients’ clinical awareness of sensory symptoms, thus placing those people at substantial risk of ulceration, Charcot joint derangements, and even amputation.
A minority of DPN patients experience neurological injury restricted to small fibers which carry cold and mechanical pain information; this is small fiber neuropathy (SFN). Though patients with SFN have reduced thermal and mechanical pain sensation, they have a relative sparing of vibratory and proprioceptive sensations. Neuropathic pain is particularly common, varyingly described as burning, stinging, shooting, aching, or pain on light touch. Nerve conduction studies (NCS) are frequently normal in patients whose DPN is limited to SFN. A diagnosis of SFN can be confirmed, in patients who are willing to undergo further testing using skin biopsy for assessment of intra‐epidermal nerve fiber density.
Once established, DPN is difficult, if not impossible, to reverse. Treatment should be started as early as possible during pre-DM. Given that SFNs are painful, patients often recognize them earlier. SF located in the epidermis regenerate with much greater ease than large diameter fibers. Therefore, the patients with pre-DM in this study represent an ideal opportunity to explore the potential therapeutic approaches noted above, which are directed at manageable risk factors. Weight and DMfriendly food stores would provide an excellent resource for accomplishing the desired results in this at-risk demographic of patient and should be of consideration in future, inner-city, community-based planning [18]. Addressing the LFN at an earlier stage, especially since they are not reversible, will likely result in fewer and less serious long-term LFN manifestations and would significantly impact the inflationary economic numbers of future DM healthcare [15].
Diabetic autonomic neuropathy (DAN) is a peripheral neuropathy. The most common and studied manifestation of DAN is cardiovascular autonomic neuropathy (CAN), owing to its’ lifethreatening complications (i.e., ventricular arrhythmias, silent myocardial ischemia, and sudden death) to its relationship with diffuse microvascular comorbidities [19]. CAN is defined as the impairment of autonomic control (sympathetic and parasympathetic) of the cardiovascular system. Early warning signs include reduced heart rate during a deep breath, prolongation of QT interval that is temporally followed by resting tachycardia, impaired exercise tolerance, and decreased baroreflex sensitivity with consequent orthostatic hypotension.
As life threatening as CAN is, the other troubling entity of DAN involves aberrations of the parasympathetic control of the gastrointestinal (GI) tract. It may reveal itself through the onset of gastroparesis, esophageal motility aberrations, constipation, nocturnal diarrhea, fecal incontinence, or gallbladder atony [7]. Gastroparesis induces upper GI symptoms of nausea, vomiting, early satiety, postprandial fullness, bloating, and abdominal pain. Furthermore, gastroparesis may delay glucose absorption, leading to frequent attacks of hypoglycemia.
Damage to the sacral parasympathetic fibers contributes to the frustrating DAN producing genitourinary (GU) dysfunction: impaired bladder sensation and detrusor muscle atony, leading to urine retention, dysuria, and nocturia. Incomplete bladder emptying predisposes to recurrent urinary tract infections and may be predictive for long-term development of renal failure.
Microvascular diseases
DM is further complicated by multiple organ malfunctions secondary to microvascular disease, because of chronic hyperglycemia, mixed hyperlipidemia, and or hyperinsulinemia [20,21]. Arterial complications of DM are classified as macrovascular or microvascular depending on the vessel size and the underlying pathophysiology [15]. The triad of retinopathy, nephropathy, and neuropathy due to microvascular complications is unique to DM. Most patients with DM will have one or more of these conditions presenting as overt or subclinical manifestations during the prolonged course of their disease. Microvascular disease is the result of a combination of direct glucose-mediated endothelial damage, oxidative stress due to elevated superoxide levels overproduced in glucose metabolism, and the production of sorbitol and advanced glycation end-products due to the prevailing state of hyperglycemia.
Microvascular disease in DM produces even greater numbers of disabling conditions than SFN and LFN combined. Microvascular disease is present long before the clinical onset of T2DM, during the pre-DM period. In pre-DM, microvascular damage and progressive organ dysfunction correlate more statistically with plasma insulin levels than the level of blood glucose. Since many people with pre-DM will not develop symptomatic, type-2 DM for as long as 10 years, microvascular disease can progress undetected. Regardless of the exact mechanism of injury resulting in microvascular disease in patients with DM, it is apparent that hyperinsulinemia plays a major roll. Thus, the control of that physiological impairment is of prime importance. There is a direct correlation between sedentary lifestyles resulting in obesity and hyperinsulinemia. Ergo, lifestyle changes focused on diet and exercise are the best hope of preventing both pre- DM and the T2DM. Metformin therapy also adds to the reduction of fasting insulin levels.
Given the fact that numerous studies have shown that SFN and microvascular diseases each progress undetected during the pre-DM phase of type 2 DM, it is essential that pre-DM be recognized and managed as early as possible in those persons who are known to be at the highest risk for the condition.
Diabetic nephropathy
Given our understanding that DM is a major cause of diabetic nephropathy (DN) and realizing that one of the most expensive treatments in chronic DN involves dialysis in patients with end-stage renal disease (ESRD), it behooves us to do everything clinically possible to limit the number of persons who progress to ESRD. To determine the significance of early intervention in the control of hyperglycemia during the pre-DM stage, a group of researchers tried to determine if pre-DM is an independent risk factor for glomerular hyper-filtration and high-normal urinary albumin-creatinine ratio (ACR) using measured GFR (mGFR) rather than estimated GFR. Their findings suggest that an independent role does exist for pre-DM in the development of glomerular hyper-filtration and albuminuria. Furthermore, pre-DM might be a target for early treatment to prevent DN.21 Here again, early detection of pre-DM and its’ attending hyperglycemia is critical if we are to ward off DN and its socioeconomic consequences.
At the end of 2018, there were over 500,000 diabetic patients in the U.S. receiving maintenance dialysis. Representing only 1% of the U.S. Medicare population, they account for approximately 7.2% of Medicare spending. The cost was $ 280 billion then, and given the inflation rate of 19.25% since 1918, the 2022 cost would be $ 334 billion; the cost of dialysis, for the Medicare portion (7.2%), is $ 24 billion. That should put an added ethical incentive in the minds of all clinicians who are currently failing to identify these, pre-DM, renal disease at-risk patients.
Conclusion
The importance of identifying persons with pre-DM who have neither yet been told they have it, nor are being treated for it, cannot be over-emphasized. In the presented patient sample, 41/101 of at-risk patients were either unaware of their risk or were not pursuing treatment for their pre-DM. Nine percent of these patients are included in the 41/101 (i.e., the other ~32 patients had HbA1C<5.7%, just RFs but not aware of their risk, or undergoing lifestyle modifications, to prevent pre-DM). All primary care clinicians (MD, DO, NP, or PA) need to be prepared to give more attention to screening for pre-DM in the at-risk population, thus being more likely to accomplish T2DM prevention. They must also be willing and able to discuss other known factors associated with cardiovascular risk reduction. Clinicians should be committed to discussing well established recommendations, regarding a proper diet and exercise program, and be prepared to discuss pharmacologic options in aiding a return of the HbA1C to normal while facilitating weight loss. It is also apparent that the risk of developing DM is likewise a family affair, not solely based on genetics, but also the influence of an obese friendly social environment. Additionally, diabetic and weight friendly food stores available in communities nationwide would provide readily available and affordable resources, to enable families to work together to stem the ever-rising tide of obesity and its attending DM in the United States. The authors strongly recommend that local politicians, community business leaders, and inner-city medical clinicians combine their respective influences and resources to see this vision come to fruition.
Limitations of this study include the subjective nature of the patients reporting, as to whether they were informed of their risk for pre-DM or not. The patients may have been told but forgot, misunderstood, or did not listen. It is important to consider this variable, as it could be an opportunity for clinicians to learn of better methods to provide clearer communication to their patients, and then with the patient’s cooperation, set obtainable goals to combat the copious sequelae of chronic hyperglycemia (pre-DM).
Another limitation was the small patient sample of the study within an FQHC with such a large demographic (30,000 patients). Better cooperation from clinicians in acquiring subjects for the study will provide more meaningful results. Future investigations could study whether the subjective reporting by patients was accurate or whether the clinicians provided sufficient lifestyle recommendations to these at-risk patients (by comparing SOAP notes to patients’ reporting). Another future investigation should determine whether there is a bias toward overlooking Black or Latinx patients with obesity that are at increased risk for pre-DM and therefore not properly screening or treating them.
When comparing One Health Ohio (OHO) statistics, regarding patient awareness and clinician response to the existence of pre-DM, with those reported nationally, our clinicians performed better. Where a national survey had shown that 29% of high-risk Americans are unknowingly living with pre-DM, we found that only 9% of our patients’ diagnosis of pre-DM had been missed. While that compares favorably, until it approaches 0%, more needs to be done. Having discovered that 9% of our high-risk patients are in the pre-DM stage but not yet aware nor undergoing treatment, it behooves us to assure that both the patients and the clinicians at OHO immediately set goals for finding missed cases and then either normalizing the A1C in those patients or at least reducing it through pharmacologic means, dietary referrals, and weight friendly groceries; the latter being strongly proposed by the authors. Such conversations with patients must also include beginning the process of eliminating other risk co-factors associated with pre-diabetes such as hyperlipidemia, sedentary lifestyle, hypertension, smoking, excessive consumption of alcohol and obesity.
Funding
No sources of funding were utilized for this research study.
Conflicts of Interest
All authors and major contributors are free of any conflicts of interest, real or perceived.
References
- American Diabetes Association (2005) Diagnosis and classification of diabetes mellitus. Diabetes Care 28(1): s37-s42.
- Centers for Disease Control and Prevention (2020) National diabetes statistics report.
- Kumar V, Encinosa W, Thakur K, Thakur H (2018) Just living with obese family members increases your risk of type 2 diabetes. Clin Diabetes 36(4):305-311.
[Crossref] [Google Scholar] [PubMed] [ResearchGate]
- Spanakis EK, Golden SH (2013) Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep 13(6):814-823.
[Crossref] [Google Scholar] [PubMed] [ResearchGate]
- US Preventive Services Task Force (2021) Screening for prediabetes and type 2 diabetes: Us preventive services task force recommendation statement. JAMA 326(8):736-743.
[Crossref] [Google Scholar] [PubMed] [ResearchGate]
- Cusi K, Roden M, Barritt AS, Firpi RJ, Clark V, et al. (2020) 1472-P: Clinical profile of nonalcoholic fatty liver disease in adults with type 2 diabetes mellitus. Diabetes 69(1):1472-P.
- Intagliata N, Koch KL (2007) Gastroparesis in type 2 diabetes mellitus: Prevalence, etiology, diagnosis, and treatment. Curr Gastroenterol Rep 9(4):270-279.
[Crossref] [Google Scholar] [PubMed] [ResearchGate]
- Olokoba AB, Obateru OA, Olokoba LB (2012) Type 2 diabetes mellitus: A review of current trends. Oman Med J 27(4):269-273.
[Crossref] [Google Scholar] [PubMed] [ResearchGate]
- Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M (2012) Prediabetes: A high-risk state for diabetes development. Lancet 379(9833):2279-2290.
[Crossref] [Google Scholar] [PubMed] [ResearchGate]
- American Diabetes Association (2018) Economic costs of diabetes in the U.S. in 2017. Diabetes Care 41(5):917-928.
[Crossref] [Google Scholar] [PubMed] [ResearchGate]
- Centers for Disease Control and Prevention (2020) Prediabetes-Chance to prevent type 2 diabetes.
- Mainous AG, Mansoor H, Rahmanian KP, Carek PJ (2019) Perception of risk of developing diabetes among patients with undiagnosed prediabetes: The impact of health care provider advice. Clinical Diabetes 37(3):221-226.
[Crossref] [Google Scholar] [PubMed] [ResearchGate]
- American Diabetes Association (2021) 3. Prevention or delay of type 2 diabetes: Standards of medical care in diabetes-2021. Diabetes Care 44(1):S34-S39.
[Crossref] [Google Scholar] [PubMed] [ResearchGate]
- Hicks CW, Selvin E (2019) Epidemiology of peripheral neuropathy and lower extremity disease in diabetes. Curr Diab Rep 19(10):86.
[Crossref] [Google Scholar] [PubMed] [ResearchGate]
- Elliott J, Tesfaye S, Chaturvedi N, Gandhi RA, Stevens LK, et al. (2009) Large-fiber dysfunction in diabetic peripheral neuropathy is predicted by cardiovascular risk factors. Diabetes Care 32(10):1896-1900.
[Crossref] [Google Scholar] [PubMed] [ResearchGate]
- Stino AM, Smith AG (2017) Peripheral neuropathy in prediabetes and the metabolic syndrome. J Diabetes Investigation 8(5):646-655.
[Crossref] [Google Scholar] [PubMed] [ResearchGate]
- Jolliffe CJ, Janssen I (2007) Development of age-specific adolescent metabolic syndrome criteria that are linked to the adult treatment panel III and international diabetes federation criteria. J Am Coll Cardiol 49(8):891-898.
[Crossref] [Google Scholar] [PubMed] [ResearchGate]
- McGowen CH, Vavrinak D, Duffett R (2019) A study of and a proposal for correcting non-compliance in the dietary management of type 2 diabetes in a select population. Am J Biomed Sci Clin Res 524-528.
- Serhiyenko VA, Serhiyenko AA (2018) Cardiac autonomic neuropathy: Risk factors, diagnosis and treatment. World J Diabetes 9(1):1-24.
[Crossref] [Google Scholar] [PubMed] [ResearchGate]
- Sprague RS, Ellsworth ML (2010) Vascular disease in pre-diabetes: New insights derived from systems biology. Mo Med 107(4):265-269.
[Crossref] [PubMed] [ResearchGate]
- Melsom T, Schei J, Stefansson VT N, Solbu MD, Jenssen TG, et al. (2016) Prediabetes and risk of glomerular hyperfiltration and albuminuria in the general non-diabetic population: A prospective cohort study. Am J Kidney Dis 67(6):841-850.
[Crossref] [Google Scholar] [PubMed] [ResearchGate]
Citation: McGowen CH, Vavrinak Davis D (2023) A Search for Missing Pre-Diabetes Patients in a Highly Susceptible Population and a Review of the Dire Consequences of Failing to Identify Them. J Community Med Health Educ 13:796. DOI: 10.4172/2168-9717.1000796
Copyright: © 2023 McGowen CH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2799
- [From(publication date): 0-2023 - Apr 21, 2025]
- Breakdown by view type
- HTML page views: 2367
- PDF downloads: 432