Review Article Open Access
A Review on Bioactive Porous Metallic Biomaterials
Kun Mediaswanti1*, Cuie Wen1, Elena P Ivanova2, Christopher C Berndt1, François Malherbe2, Vy Thi Hong Pham2and James Wang1
1IRIS, Faculty of Engineering and Industrial Sciences, Swinburne University of Technology, PO Box 218, Hawthorn, Vic. 3122, Australia
2Environment and Biotechnology Centre, Faculty of Life and Social Sciences, Swinburne University of Technology, PO Box 218, Hawthorn, Vic. 3122, Australia
- Corresponding Author:
- Kun Mediaswanti
IRIS, Faculty of Engineering and Industrial Sciences
Swinburne University of Technology
PO Box 218, Hawthorn, Vic. 3122, Australia
E-mail: kmediaswanti@swin.edu.au
Received date June 06, 2013; Accepted date July 04, 2013; Published date July 14, 2013
Citation: Mediaswanti K, Wen C, Ivanova EP, Berndt CC, Malherbe F, et al. (2013) A Review on Bioactive Porous Metallic Biomaterials . J Biomim Biomater Tissue Eng 18:104. doi:10.4172/1662-100X.1000104
Copyright: © 2013 Mediaswanti K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biomimetics Biomaterials and Tissue Engineering
Abstract
Porous metallic biomaterials have been extensively studied for many bone tissue engineering applications because porous structures provided space for bone in-growth and vascularisation. Improvement on mechanical properties also leads to the increased popularity of porous materials for bone substitute applications, especially for load-bearing implants. However, they usually lack sufficient osseointegration for implant longevity. In addition, their biocompatibility is also an important concern in these applications due to adverse reactions of metallic ions with the surrounding tissues after these metallic ions are released from the implant surfaces. One consideration to accelerate the healing process is surface treatment, including application of bioactive coatings, e.g. hydroxyapatite and biomimetic creation of surface. Surface treatments on biomaterials will determine surface chemistry and topography, whereas these surface characteristics influence osseointegration process.
To respond on the challenges of producing biocompatible and mechanical compatible biomaterials and lack of review studies on surface modifications on porous structures, a comprehensive literature review on surface modifications of various porous metallic materials is presented. This review covers various methods of surface treatment such as biomimetic, electrodeposition, alkali heat treatment, anodization and their effects on mechanical and structural properties which then provided insights into bone implants improvement studies. Biological responses (in vitro and In vivo) of porous material after surface treatment are thoroughly discussed.
Keywords
Porous; Biomaterials; Titanium; Hydroxyapatite; Bioactive
Introduction
Orthopaedic replacement surgery is a common medical practice that enhances life quality of human by alleviating pain and disability. People in both developing and developed countries are still facing a significant increase of bone and musculoskeletal problems, which consequently leads to the increasing demand of long term clinical performance of an implant. It was estimated that the demand of implants in the United States for 2005-2030 period will increase around 174% and 673% for hip and knee, respectively [1]. This trend will continue to increase in the foreseeable future. In addition to primary surgery, the number of revision surgery was also still high. According to the Australian orthopaedic association national joint replacement registry (AOANJRR) 2009 report, in Australia, the number of primary and revision hip surgery reached 224,390 procedures in 2008. This is higher by 32,217 procedures than the previous year [2]. Major reasons for orthopaedic revision surgery are loosening/lysis (56.4%), dislocation (14.5%), infections (11.1%) and fracture (8.8%) [2]. Furthermore, orthopaedic revision surgery might cause complicated issues, such as additional expense, social implications and sometimes revision surgery is a more difficult procedure than primary replacement surgery. Thus, a biocompatible, biomechanical compatible, long life implant with zero level of revision surgery is highly expected.
Metallic biomaterials (e.g. titanium, tantalum, magnesium) have been widely used in many load-bearing orthopaedic applications and showed good biocompatibility [3]. However, they usually lack sufficient osseointegration capacity for implant longevity, and their biocompatibility is also an important concern in these applications due to adverse reactions of some metallic ions (i.e. Al and V) with the surrounding tissues after these metallic ions are released from the implant surfaces [4]. Dense metallic materials have several drawbacks, such as mismatch on elastic moduli that causing stress-shielding, interfacial stability with host tissues, potentiality production of fibrous tissue on the implant surface, and low volumetric porosity [5,6]. These drawbacks have motivated many studies on porous materials for implant applications since the last two decades. It is also important to highlight that metallic implants have low capability of self healing and lack of ability to adapt with any new environments, such as blood flow, which is in contrast with natural bone or tissues where this characteristic is naturally embedded [7].
Surface topography and surface chemistry of biomaterials were convinced to influence the biocompatibility and osseointegration process of the material [8]. As a bio-inert material, porous metallic must undergo a surface modifications to enhance the bioactivity of the materials, so the direct bonding of the metallic material to bone could be achieved [9]. Various surface modification methods on porous biomaterials have been studied to achieve the desired bioactivity and biocompatibility, such as alkali-heat treatment [10,11], electrodeposition [12], biomimetic [13,14], and anodization [15]. However, to date a review on surface modifications effect on porous biomaterials performance is still lacking.
In respond to the above facts and challenges, a comprehensive literature review on surface modifications of various porous metallic materials is presented. The aim of this literature review is to compare various methods of surface modifications on porous metallic biomaterials (i.e titanium, tantalum, and magnesium). This review is organized into three major parts. The first part will cover a brief overview on porous biomaterials for bone implant applications. The second part will highlight surface modifications on porous materials and the third part a discussion on mechanical properties and in-vitro behaviour of these materials after surface modifications. Therefore, the ongoing studies are aiming to ensure excellent clinical performance of the implants for long term period.
Porous Biomaterials
Biomaterials are those whose surfaces will interact with biological system. These materials can be used to construct a device for a replacement of some part of a human body or for medical appliances in cardio-vascular device, maxillofacial, needle, catheters, tooth filling, and bone plate, etc. Studies on biomaterials started about five decades ago and continue to produce improved biomaterials. Crucial criteria of biomedical implant are the materials should be biocompatible, bioactive, and biomechanical compatible. Recent trend in implants shows a development of both bioactive and resorbable materials with emphasizing on the material capability to help the body to recover itself post-implantation [16].
A biologically compatible material is a material that will not cause inflammatory response on the tissue after implantation and noncytotoxic. Biocompatible materials should also have bioactivity and biointegration features. In the case of bone implants, after implantation the implant surface is expected to bond with the surroundings bones and adapt with the new environment. The surface material must be able to induce a carbonate apatite layer, so it will fulfil the bioactive criteria. It also should have excellent resistance on corrosion. Mechanical properties of ideal biomaterials for orthopaedic applications usually include high fatigue resistance, tensile strength, wear resistance and elastic modulus. Fatigue strength shows capability to response in cyclic loads and indicates the long term implants performance. In regards of elastic modulus properties, the dedicated biomaterials should have similar bone modulus value to prevent any detachment consequences and minimize bone resorption. Good wear resistance will reduce the production of debris [17].
Materials used for bone tissue engineering applications vary from ceramics, polymers, metallic, and/or composite of these materials. Ceramics, such as calcium phosphate and bioactive glass have been studied for bone graft scaffolds and showed promising bioactive features. For example, calcium phosphate ceramics shows excellent bioactivity, osseo-conductive and biocompatible. However, low strength, brittleness, in-elasticity, low impact resistance, and low toughness are some drawbacks for bone implant applications. Polymers such as polyurethane (PUR), poly (lactic co-glycolic acid) (PLGA), polylactide (PLLA), poly-DL-lactide (PDLLA) are also attractive materials for implant applications as they provide conducive environments for cell adhesion, attachment, proliferation and differentiation [18,19]. Studies on polyesters as biomaterials have been increased for bone regeneration applications due to their clinical success history. Biodegradable polymers are also favourable for scaffolds due to their flexibility to control the degradation rate through copolymerization. However, their bioactivity and mechanical strength are drawbacks for load bearing applications.
Metal and its alloys, such as titanium, stainless steel, tantalum, and magnesium showed high tensile strength which is suitable for load bearing bone substitutes. Table 1 provides elastic modulus and tensile strength values of bone and various materials commonly used for bone implant applications. In the case of dense titanium, the possibility of implant loosening due to the significant difference between the elastic modulus of titanium and natural bone is a major hindrance (i.e. 55- 110 GPa and 5-30 GPa, for titanium and bone respectively). One way to lower the elastic modulus is to produce a porous structure of the metallic by adjusting its porosity and introducing β alloying elements. Studies on fabricating porous metallic led to many improvements in decreasing the elastic moduli and simultaneously also showing sufficient mechanical strength for bone substitute [20].
| Material | Elastic modulus GPa | Tensile strength (10-3) GPa |
|---|---|---|
| Cortical bone | 20 | 150 |
| Cancellous bone | 3 | 5 |
| Stainless steel | 200 | 700 |
| Co-Cr-Mo alloys | 230 | 500 |
| Titanium | 110 | 500 |
| Ti-6Al-4V | 110 | 950 |
| Hydroxyapatite ceramics | 20 | 100a |
| Glass ceramics | 30 | 200a |
| PMMA | 3 | 80 |
aBend strength values
Table 1: Mechanical properties of bone and bone implant materials [21].
Porous structures on metallic materials will provide several advantages for long-term clinical performances. Firstly, the porous structure allows bone to grow into the pores and lock the artificial implant for better fixation. The interconnected structure will promote cell adhesion and maintain cell growth. The rough surface of porous material also provides a platform where stress can be transferred from an implant to bone. As an example, porous structure of tantalum was also applied to the cervical and lumbar spinal arthrodeses [21,22]. In vivo study of porous tantalum used for lumbar spinal fusion showed bone in growth.
Pore size and porosity
Pore size and porosity are the major concern in producing porous scaffolds as they influence the mechanical properties of an implant (e.g. elastic moduli) and also the biological performance of the implant material. It was believed that the optimal pore size to ensure vascularization and bone in-growth is in the range of 100-400 μm [23].
Porosity will enhance interlocking process for stability, and immobility of the new implant. Porosity is influenced by several factors, namely the particle size of metallic powder and sintering pressure. The particle size will influence the surface energy and apatite inducing ability. Decreasing the particle size will reduce the porosity due to surface energy per unit volume. Small diameter particles with high specific surface area have higher energy, thus sintering process of the particles will be more rapid. Chen et al. reported that by reducing the titanium particle powder size the surface energy increased and subsequently high surface energy led to higher apatite inducing ability [24]. On the other hand, raising the pressure will decrease the porosity due to more plastic deformation of titanium powder at contact area. Studies revealed that the optimum porosity of an implant for bone in growth is in the range 20-50% [24].
The porosity of the scaffold is characterised by gravimetry using the formula [25]:
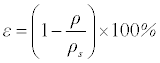 (1)
(1)
where ρ and ρs are the density of the porous alloy and its corresponding theoretical density, respectively.
The interconnectedness is another ultimate parameter for successful bone in-growth. Connectivity between the pores provides sufficient area for bodily fluid to flow throughout the new tissue which leads to enhancement in the nutrients transportation. Interconnectivity could be determined by using paraffin method [25]. Basic principle of this method is to boil the samples in paraffin in vacuum and measure the weight of paraffin penetrating the scaffolds. The interconnectivity can be calculated using the formula [25]:
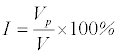 (2)
(2)
where Vp and V are volume of paraffin penetrating into porous structure and overall pore volume, respectively. This method will give a reasonable estimate of what percentage of open pores (interconnectivity).
Elastic modulus
Elastic modulus plays a significant role in biomechanical compatibility of implant materials. New materials have been developed to focus on achieving elastic modulus values close to that of natural bone. Reducing the elastic modulus to mimic the bone is critical, as it could reduce implant mobilization risk. Attempts have been made to develop various models to predict the elastic modulus of porous materials, namely analytical models, finite element models, and X-ray tomography models [26]. Analytical model could be described as accounting material responses under idealized conditions. An example of analytical models is modelling cell walls as beams. Finite element model is based on more realistic structures, such as level cut Gaussian model. Many studies have shown that elastic modulus and compressive strength of porous material will decrease as the porosity increases. The relationship between elastic modulus and relative density can be expressed as follows [26]:
E/Es = (ρ/ρs) (3)
σpl / σys = 0.3 (ρ/ρs)3/2 (4)
where E is the elastic modulus of the porous material, Es is the elastic modulus of the cell edge solid material, σpl is the plateau stress of the porous material, and σys is the yield strength of the cell edge solid material. Wang et al. reported that it is possible to achieve both the desired mechanical properties and high porosity if the yield strength of cell edge solid material is high.
Porous titanium and titanium alloys
Porous titanium development is one solution to overcome stress shielding problems on dense titanium, which often leads to aseptic loosening. One successful example of porous titanium fabrication was conducted by Wen et al. The elastic modulus of porous titanium was approximately 4 GPa which is significantly lower compared to the dense titanium with 110 GPa [23].
Titanium alloys with aluminium and vanadium as a stabilizer element (Ti6Al4V) have been widely used as implant materials; however some studies revealed that the release of the ions i.e., Al and V can be harmful to human body [3,4]. Thus, other alloy stabilizers were explored to reduce the biological adverse impacts. Additionally, introducing biocompatible alloying element into titanium alloys might improve the mechanical properties of the implant.
To date several α + β and β – type titanium alloys have been developed. Alloying elements that attract titanium alloys development studies are tantalum (Ta), tin (Sn), niobium (Nb), and zirconium (Zr) [27]. These non-cytotoxicity elements have shown good biocompatibility, high corrosion resistant and completely soluble in titanium [28,29]. These alloying elements were classified into three types of alloying elements: α stabilizers, β stabilizers and neutral. It is also believed that titanium alloy with the combination of α + β phase is preferred due to its high strength. However, many studies also focused on β – type titanium alloys due to its desired elastic modulus. Study conducted by Obbard et al. demonstrated that adjusting the concentration of β stabilizer (Ta and Sn) could reduce the elastic modulus [30]. Based on the study of cytotoxicity of alloying elements; it is observed that titanium, tantalum, zirconium, niobium and tin are biocompatible, while molybdenum and silicon are cytotoxic in a certain level of ion concentrations [27].
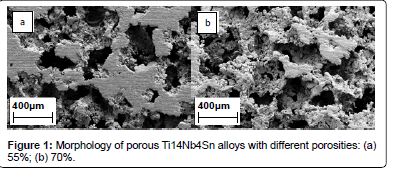
Many studies on the microstructure of porous Ti alloys showed a combination of both macropores and sometimes micropores on the cell walls. It has been reported that based on space-holder method, there are two types of pores, macro-pores which are determined by the size of the space holders and micro-pores which are dependent on the dimension of the titanium powder particles [31]. As shown on Figure 1, porous titanium alloys (Ti14Nb4Sn) exhibited both micro- and macropores [32].
It has been noted that the development of porous titanium alloys with a variety of alloy components have brought many improvements in bio-mechanical properties. Porous Ti10Nb10Zr alloys with 69% porosity exhibited mechanical strength of 67 MPa, while pure Ti and pure Ta scaffolds with the same porosity showed lower strength of 53 MPa and 35.2 MPa, respectively [25]. Table 2 provides information on elastic modulus values of various metallic materials [33-36].
Porous magnesium
Magnesium is a well known mineral in our everyday lives and plays an essential role in human metabolism. Magnesium is known as light weight metal and its good fracture toughness. Magnesium shows a lower elastic modulus than titanium (41-45 GPa and 110 GPa for magnesium and titanium, respectively). It is believed that magnesium biodegraded more rapidly than other materials (e.g. natural polymer and ceramics), thus reducing the necessity for revision surgery. This favourable magnesium feature attracts more studies for bone tissue implants.
| Material | Porosity (%) | Elastic Modulus (GPa) |
|---|---|---|
| Bone | 5-90 | 0.1-30 |
| Dense Ti | 0 | 80-130 |
| Porous Ti | 78 | 5.3 |
| Porous Ti6Ta4Sn | 75 | 4.6 |
| Porous TiNbSn | 30-60 | 10.8-33.2 |
| Porous Ti10Nb10Zr | 59 | 5.6 |
| Porous TiZr | 70 | 15.3 |
| Porous Ti15Mo5Zr3Al | 26 | 20 |
| Dense Ta | 0 | 185 |
| Porous Ta | 75-85 | 2.5-3.9 |
It has been demonstrated that the mechanical properties of an alloy are in part dependent on its porosity. Wen et al. reported that the prepared magnesium foam with a porosity of 50% showed a compressive strength of 2.33 MPa, Young’s modulus of 0.35 MPa, respectively [37]. Zhuang et al. also evaluated the mechanical properties of porous magnesium which fabricated using powder metallurgy method. Porous magnesium scaffolds with porosities of 36-55 % showed a Young’s modulus value in the range of 3.6-18.1 GPa, which is closer to that of natural bone [38]. They also investigated the effect of porosity on biodegradation behaviour. In their study it was reported that higher porosity degraded faster, due to higher interconnectivity and conditions that enhanced chemical reactions.
Since the degradation kinetics could be adjusted via manipulating surface and subsurface properties through the manufacturing process, the porosity level of porous alloys for specific applications must be optimised to give optimum mechanical properties and in vitro biodegradation behaviour for their enhanced performance.
Despite many favourable properties, magnesium alloys exhibit low corrosion resistance once implanted into body whose fluid is highly alkaline. This may bring hindrance to their medical applications [39].
Porous tantalum
Tantalum is a lustrous transition metal and shows low stiffness and good ductility [35]. Tantalum has been widely used for various medical devices, including pacemaker electrodes, radiopaque markers, foil and mesh for nerve repair, cransioplasty plates, and femoral endoprotheses [7]. Tantalum implants exhibited distinctive biocompatibility properties that have been proven in orthopaedic, dental, and cranio-facial applications [35,40]. However, dense tantalum presents low friction properties and high elastic moduli compared with natural bones. Thus, porous tantalum alloys have been developed to counter this mismatch, and their potential applications in bone tissue engineering have been explored. Studies revealed porous tantalum distinctive properties are highly corrosion resistant, excellent bioactivity In vivo, low elastic modulus and high surface frictional properties [41]. Porous tantalum consists of repeating dodecahedrons and has a similar appearance of cancellous bone. Elastic modulus of porous tantalum is in the range 2.5-3.9 GPa and yield strength around 35-51 MPa. Fatigue strength of porous tantalum is greater than cancellous bone. These mechanical properties of porous tantalum demonstrate its potentiality as an orthopaedic material [35].
One commercially available porous tantalum is Trabecular Metal produced by Zimmer and has been widely used in orthopaedics implants. Commercial products of trabecular metal include acetabular cup, revision shell, acetabular restrictor and augment, humeral stem, osteonecrosis intervention implant, and reverse shoulder system [42]. Trabecular metal exhibits a high strength to weight ratio and could provide withstanding physiologic loading. In addition, it provides greater volumetric porosity than solid metals and low stiffness that is similar to bone. Low stiffness of this material could minimize stress shielding. For stability reason, trabecular metal shows excellent frictional characteristics compared to solid metal material. Moreover, the mechanical properties of trabecular metal will not degrade with time [42]. A study on comparison of tantalum and titanium cups in revision hip arthroplasty, conducted by Jafari et al. revealed that in the situations with severe bone deficiency, tantalum cup performed better than titanium cup [3]. The elastic modulus of porous tantalum could be tailored between 1.5 and 20 GPa by adjusting the pore volume fraction [42].
In vitro test of porous tantalum, using MTT assay and immunochemistry study, exhibited excellent cell attachment, proliferation and differentiation. This indicated that porous tantalum could promote early biological fixation. They suggested that porous tantalum performance was attributed to its chemistry, high wettability and higher surface energy [42].
An In vivo test on porous tantalum demonstrated a bone growth after 8 weeks of implantation. This suggests that trabecular metal exhibited excellent biocompatibility and infiltration and subsequently, osseointegration process could be accelerated. Likewise, studies of soft tissue attachment onto trabecular metal reported soft tissue was adhered after 4 to 8 weeks after surgery and extensive tissue ingrowth occurred [42].
Surface Modifications
Bioactive coatings
Surface modification is a process to change the composition, structure and morphology of a surface while the mechanical properties still remain. The aim of surface modification is to improve the bioactivity of the biomaterials, so biomaterials could demonstrate high apatite inducing ability which leads to rapid osseointegration. After surface treatment, it is expected that the surface implant could form an active apatite layer. The role of apatite thin layer on the surface implant is to act as bonding interface [9], and the bone apatite and collagen production took places on the apatite layer [43]. It is believed that the alteration in nanostructured surface morphology influences the apatite inducing ability, and could improve osteoblast adhesion and differentiation [44].
Calcium phosphate coatings
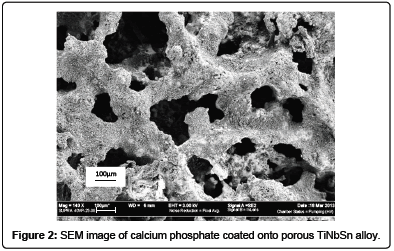
Calcium phosphate is a synthetic ceramic which has been proven to support bone apposition and enhances its osteoconductive ability. Calcium phosphate ceramics commonly used for bone implants applications include tricalcium phosphate (TCP), octocalcium phosphate (OCP), hydroxyapatite (HA), and biphasic calcium phosphate (BCP) [45]. These ceramics accelerate healing process and have been widely used in conjunction with metallic material as a bioactive coating material. However, hydroxyapatite is the most suitable materials for adding bio-function as it have similar calcium to phosphate ratio with human bone minerals [46]. Figure 2 exhibited the SEM image of calcium phosphate coated of porous TiNbSn fabricated via powder metallurgy.
Hydroxyapatite has functions of promoting osteoblast adhesion, migration and differentiation and proliferation, which are used in bone regeneration. It also has the ability to bond directly into bone. The bioactivity of HA has made this ceramics favourable in implant applications and HA nanoparticles could induce cancer cell apoptosis [47]. Crystalline form of HA exhibits biointegration and no formation of fibrous tissue. The desirable coating is crystalline HA, due to its ability to provide a better substrate for a different cell line than amorphous HA [48]. Amorphous HA tends to dissolve in human fluid more easily and leads to loosening. Moreover, nanocrystalline should be more relevant than micron size HA because of its structural similarity with apatite [49].
Various techniques have been applied for HA coatings onto metallic substrates namely electrodeposition, chemical vapour deposition, alkali heat treatment and sol gel. Surface modifications techniques with employing calcium phosphate onto porous biomaterials in order to enhance their bioactivity can be described as follow:
Electrodeposition
Lopez-Heredia et al. electrodeposited calcium phosphate onto porous titanium alloys. Titanium, platinum mesh and supersaturated calcium phosphate solution were used as the cathode, electrode and electrolyte, respectively. The calcium phosphate ratio was 1.65 which fulfilled the desired Ca/P ratio range. The calcium phosphates were homogenously coated and covered the entire titanium surface. The HA coating thickness was approximately 25 μm and showed a bond strength 25 MPa [28].
Garbuz et al. [50] coated 5 μm thick calcium phosphate and alendronate onto porous tantalum (Trabecular Metals) using electrolytic technique. The alendronate-calcium phosphate coatings have been reported to significantly enhance bone ingrowth. Adamek et al. prepared a HA bioactive surface on porous Ti6Al4V using electrochemical etching and cathodical deposition technique [51]. Large pores and nanolamellas were observed in the HA layer [51]. The cathodical deposition method was promising route for enhancing the bioactivity of the surface.
Biomimetic
There are two major steps involved in this biomimetic technique. The first step is to conduct a preliminary treatment of the implant surface with the aim of creating functional layer to induce effective apatite formation. Studies revealed that material without any treatment prior to immersing led to no apatite layer formation [12]. Preliminary treatment usually includes hydrothermal, sol-gel, alkali heat treatment and micro arc, etc. The second step is to immerse biomaterials into a simulated body fluid (SBF). SBF is a solution that mimics human body fluid which has similar ion concentration and pH to human blood plasma. In this step the bone apatite layer is formed on the surface. The high apatite forming ability of titanium is due to the formation of a hydrated titanate surface layer during chemical treatment. Competitive advantages of biomimetic are flexibility in controlling chemical composition of the coating, ability to produce homogenous film, relative lower temperature, bioactive bone like apatite coatings easily formed, ability to coat 3D geometries and similar structure to bone apatite [13].
Wang et al. [31] performed a modified biomimetic approach to improve bioactivity of porous titanium alloy scaffolds. In their experiment, porous Ti10Nb10Zr was alkali heat treated prior soaking into SBF solution. They used two different NaOH concentrations with 5 M and 0.5 M each. In the soaking stage, they used 1.5x SBF recipes and soaked the sample for 1 week. Surface morphologies of porous TiNbZr after alkali soaking and heat treatment showed a nanofiber layer. This layer was believed to be consisting of sodium titanate. Parameters that influenced the morphology and thickness of sodium titanate were reaction temperature and NaOH concentration. Calcium phosphate was successfully developed at the surface of the porous TiNbZr. The calcium phosphate layer was uniformly and homogenously spread onto the entire surface.
Xiong et al. [10] managed to pre treat the porous Ti18Nb4Sn using alkali heat treatment. In this treatment the material was soaked into NaOH solution and subsequently heated to 600°C and kept at this temperature for 1 hour. The biomimetic process began with soaking the treated alloy into SBF solution and incubating at 37°C without stirring. Other biomimetic studies on hyroxyapatite coating conducted by Habibovic et al. which showed a thick and homogenously crystalline HA coated on all pores and resembled bone mineral [52]. From the above example indicated that biomimetic could induce bone formation. Other attempt to enhance bioactivity is to coat other form of calcium phosphate (i.e. octacalcium phosphate) onto porous metallic material. Habibovic et al. [52] also studied the influence of biomimetically OCP on porous titanium alloys. To speed up biomimetic coating they utilized more concentrated SBF and used simulated calcifying solution to produce crystalline coatings. An In vivo study revealed that the coated OCP showed an inductive behaviour.
Nitric acid has also been utilized for pre-treatment of biomaterials. The basic principle is to immerse the sample into HNO3 solution. Turkan et al. [53] conducted experiment using HNO3 (65%) and H2O with volume ratio 1:1 for 5 h and reported that nitric acid treatment could be used as an alternative pre-treatment. However, they also noted that there should be further investigations on the adhesion of calcium phosphate coatings.
Osteoinductivity is an ability of implant to induce osteogenesis and is also one indicator of an ideal implant. Takemoto et al. [54] studied the osteoinductivity of the HCl treated material implanted in a back muscle of a dog and also performed an in vivo assessment. Their study showed that subsequent HCl treatment also enhanced apatite forming ability. In vivo assessment revealed excellent osteoinductive ability. Within 3 months bone formation was observed, while for the non HCl treatment, there was no bone formation detected. New bone area produced for the HCl treatment was significantly higher than that for the non-HCl treatment. The HCl treatment also caused an etching effect which leads to the formation of small and large micropores. These variety sized micropores provided larger surface areas for improving cell attachment and proliferation, and finally enhancing osteoinductive ability. Both surface and topography had an influence on the osteoinductivity enhancement. Alkali heat treatment with dilute HCl treatment on the porous material was proven to be effective to remove sodium from the sodium titanate formed, contribute to the formation of titania, and therefore improve the osteoinductive ability and apatite forming ability [54]. Chemical and heat treatment is one of the techniques to modify the implant surface topography and chemistry so it becomes bioactive. The process of alkali heat treatment is firstly to produce a sodium titanate layer, followed by removal of sodium [55]. Studies revealed the powerful HCl dilution treatment could completely removed sodium throughout the entire pore.
Sol-gel method
Sol-gel method has been widely used in coating calcium phosphate onto dense or porous metallic. There are two routes of sol gel deposition, namely inorganic and organic, using reagents consisting of a colloidal suspension solution of inorganic or organic precursors. Usually reagents react at low temperatures in order to form thin films of CaP. Gan et al. applied both inorganic and organic routes onto poroussurfaced implants and performed an In vivo assessment to evaluate the effectiveness of these routes on osseointegration acceleration [56,57]. To produce the films, they used calcium nitrate tetrahydrate and ammonium dihydrogen phosphate as an inorganic percusor solution. Alternatively as an organic percusor solution, they used calcium nitrate tetrahydrate and triethyl phosphite. Inorganic route showed the top layer of the CaP film was less dense and composed of nanoscaled particles with 20-30 nm size. The CaP films produced by organic route were observed closely to the substrate thermal etch pattern. The film thickness was around 1 μm and 1.5 μm for organic and inorganic routes respectively and showed no crack. Organic route exhibited dense film with higher crystallinity compared with the inorganic. Both methods showed that the calcium phosphate films were uniform and fully covered the adjacent substrate and the innermost sintered particles. Ca/P ratio showed a significant difference value, 1.46 and 2.10 for inorganic and organic route respectively.
Micro-arc oxidation (MAO)
Micro-arc oxidation is a versatile method to form oxide ceramic coatings. MAO utilized a stainless steel plate as a cathode and a porous material as an anode. NaOH is usually used as an electrolyte. The advantages of this method are its flexibility in coating complex geometry and a strong bond coating produced in situ on titanium surface. Sun et al. reported a high roughness and nanoporous which led to a strong chemical bonding at the bone implant interface [58].
Metal Organic Chemical Vapour Deposition (MOCVD)
Metal Organic Chemical Vapour Deposition (MOCVD) has been used to deposit hydroxyapatite onto metal and has known to produce thin film with good adhesion [59]. Based on this technique, Hartshorn et al. developed a modified process called pulsed pressure MOCVD (PP-MOCVD) using timed injections of liquid precursor with ultrasonic atomization and no carrier gas. They used methanolic MOCVD precursor solutions of calcium dibenzoylmethane and trimethylphosphate. Their study indicated that calcium phosphate coatings were spread onto porous tantalum, although whether the coatings resembled HA composition is still need further investigation [59].
TiO2 coating
Titanium dioxide (TiO2) is a bioactive compound that has been applied as a coating material and scaffold material for biomedical applications. For scaffold applications, TiO2 showed a sufficient compressive strength which suitable for load bearing applications. Several deposition methods were used to apply TiO2 coating, namely electrophoretic sol gel coating, anodization and slurry coating. In a study conducted by Verket et al. [60], they compared the protein release from normal human osteoblast on three different materials for coating (i.e. TiO2, SiO2 and calcium phosphate). They reported that TiO2 coatings secreted higher bone markers than silica and calcium phosphate which indicated TiO2 supports osteoblast growth and bone remodelling.
Electrophoretic sol gel coating
Anatase-type TiO2 was believed has a superior biocompatibility. To produce TiO2 layer, electrophoretic sol-gel coating method on porous SUS 304 substrates was carried out by Inoue et al. In this process sol gel transformation was induced on an electrode (substrate) by using homogenous sols as the electrophoresis medium. The transparent sols of the precusor were prepared by deflocculating colloidal solutions formed by hydrolysis of titanium tetraisopropoxide using CaCl2 as an agent. The gel film was produced by applying a dc voltage between the substrate and counter electrode in the solution. As a result, they successfully produced anastase TiO2 coating layer doped with Ca2+ ions after water soaking and annealing at 400°C [61].
Anodization
Anodization is a cost-effective deposition method that utilizes an electrolyte solution to produce surface oxide layers [62]. This method depends on factors such as solution concentration, composition and electrolyte temperature etc. It is reported that the anodized film enhances the anchorage of the implants to the bone. Das et al. modify the surface of laser- processed porous titanium by coating TiO2 via anodization in order to enhance the osteoconductive properties [63]. In this study, the TiO2 film produced had a nanoporous structure with pore diameter of 50 nm and coating thickness was 300 nm. It is important to highlight that after anodization, the contact angle were significantly decreased from 70° to 4°. The new hydrophilic feature suggests a high surface energy of anodized films with nanotube structure. Hence, these features improved the apatite-forming ability in SBF.
Lee et al. developed a novel method to produce porous titanium using polymeric sponge replication and carried out anodization to enhance its biocompatibility [64]. They succeeded in creating an elongated porous structure. The basic fabrication process started with stretching polyurethane sponges to an elongation of 50% and followed by heat treatment. Subsequently sponges underwent coating process using titanium hydride and were heat treated. Finally, anodization was performed to coat bioactive TiO2 onto the porous Ti scaffolds. The TiO2 coating layer was amorphous and uniformly covered throughout the titanium scaffolds. The mean size of TiO2 nanotubes was approximately 45 nm, and the TiO2 layer thickness was 1.65 μm. Energy dispersive spectroscopy (EDS) analysis showed the presence of Ti and O, and indicated the formation of the TiOx layers.
In vitro biocompatibility assessment was carried out to investigate the influence of nanoporous TiO2 coating onto Ti scaffolds. In vitro study using MC3T3-E1 cells showed a cell spread and activity through the cytoskeletal process on Ti scaffolds with TiO2 coating and indicated an improvement in the osteoblastic activity [64].
Effect of Surface Modifications on Physical and Mechanical Properties
Mechanical property is one of the most critical parameters that determine the performance of a designed implant. It mainly depends on the process, and structural properties of the biomaterials. Therefore, it is possible to achieve desired mechanical properties through modifying the structural characteristics of a biomaterial.
Surface roughness
Surface roughness is believed to influence the activity of bone cells, bone formation at the bone-implant interface, and bone apatite nucleates. Studies revealed that pre-treatment prior to any deposition might increase the surface roughness. Lopez-Heredia et al. [28] reported the porous titanium was cleaned by grit blasting using biphasic calcium phosphate and acid etching. After surface treatment, rougher morphology and a micro and macro texture was reported. Turkan et al. also investigated the effect of surface treatment on surface roughness. They performed three surface treatments on Ti6Al4V open cell foams (60% porous) using alkali, nitric acid treatment, and acid etching and subsequently SBF immersion for 14 days [58]. After alkali treatment the surface roughness significantly increased. In contrast, nitric acid treatment did not show to affect the surface roughness. They also reported that alkali treatment induced continuous, uniform CaP, while the acid etching treatment did not produce a continuous coating layer. After 14 days SBF immersion, the coating thickness was measured as 3 μm and 0.6 μm in alkali treatment and nitric acid treatment respectively. The results indicated a rougher surface roughness could enhance the adhesion of calcium phosphate coating and cell adhesion. It is also reported that after SBF immersion, the surface roughness of all foam specimens were also increased.
Mechanical and bond strength
Mechanical strength is an ultimate parameter in orthopaedic implant performance. Lin et al. investigated the influence of alkali heat treatment on the strength of porous Ti alloy (75% porous). They used two different concentrations of NaOH (5 M and 0.5 M NaOH) for surface modifications and reported deterioration in mechanical strength. The significant decrease in mechanical strength was mainly caused by corrosion of the struts of the porous titanium and micro cracks, as a corrosive product was observed on the surface of the sample. In their observation, the plateau stress was gradually decreased, as the concentration of NaOH increased. In their study, it was reported that plateau stress of as sintered porous titanium without any surface treatment was 31.5 MPa. While after 1 M NaOH, 2 M NaOH and 5 M NaOH immersion and heat treatment, the porous titanium exhibited 24.0 MPa, 21.0 MPa and 20 MPa respectively. Elastic modulus was also slightly decreased. This indicates that a relatively low concentration of alkali solution would be more effective for enhancing the bioactivity of implant material [65].
Lopez-Heredia et al. electrodeposited calcium phosphate onto the titanium surface and reported a bonding strength between them of 25.5 MPa. Bond strength using biomimetic method with alkali heat treatment showed a value of 21 MPa [28]. These values are lower than the loading stress on the hip joint during gait which is less than 35 MPa. Therefore, further investigations on the improvement in bond strength between implant surface and coatings are highly demanded.
Effect of surface modifications on biological performances
Biological behaviour of cells assessment after surface modifications is required to check its biocompatibility and bioactivity. The effect of various surface modifications techniques will be explored based on the type of surface treatments.
A bioactive TiO2 coating using anodization technique onto porous titanium was carried out by Lee et al. In vitro biocompatibility assessment was conducted and the results showed that the MC3T3-E1 cells were spread through the samples which indicated an improvement in the osteoblastic activity [64].
An in vitro study using biomimetic method onto porous Ti10Nb10Zr showed that a higher number of cells adhering to porous structure after surface modification compared to the non-treated porous samples. They reported that the surface of porous titanium alloys with calcium phosphate coatings showed the highest number of cells attached.
Three types of surface modifications technique were subsequently employed in the study conducted by Li et al. [41], which are alkali heat treatment, alkali heat treatment plus simulated body fluid soaking and sol gel method. The biological behaviour of porous Ti6Ta4Sn after surface modification showed that the cell density using the sol gel method exhibited the highest increase compared to the other two methods. It can be suggested that hydroxyapatire coatings are more favourable for osteoblast cell proliferation. A similar outcome was shown in Gan et al. study, which they also employed calcium phosphate coating using the sol gel method. Based on their In vivo assessment, it was suggested both routes and coatings significantly accelerate osseointegration [57].
Conclusion
Studies on porous metallic biomaterials have been growing since the last two decades due to their excellent features such as low elastic modulus, excellent strength, and biocompatible. Studies have reported various fabrication and surface modification techniques to achieve the desired properties with subsequent mechanical and biological assessment. In this review, it can be suggested that surface modifications have significant influence towards the osseointegration process and mechanical performance of the implants. Factors that influenced the biological performances of porous metallic material are porosity, pore size and surface modification. Challenges on surface modification for porous biomaterials are the technique chosen should not cause any damage on mechanical properties and accelerate the healing process after implantation. It also important to highlight that hydroxyapatite coating via sol-gel method is still a promising route to enhanced bioactivity of the porous metallic materials.
References
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M (2007) Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 89: 780-785.
- National Joint Registry Annual Report 2009. Australian Orthopaedic Association National Joint Replacement Registry.
- Jafari SM, Bender B, Coyle C, Parvizi J, Sharkey PF, et al. (2010) Do tantalum and titanium cups show similar results in revision hip arthroplasty? Clin Orthop Relat Res 468: 459-465.
- Okazaki Y, Rao S, Asao S, Tateishi T, Katsuda SI, et al. (1998) Effects of Ti, Al and V Concentrations on Cell Viability. Mater Trans JIM 39: 1053-1062.
- Wen CE, Yamada Y, Hodgson PD (2006) Fabrication of Novel TiZr Alloy Foams for Biomedical Applications. Mater Sci Eng C 26: 1439–1444.
- Wen CE, Yamada Y, Shimojima K, Chino Y, Asahina T, et al. (2002) Processing and mechanical properties of autogenous titanium implant materials. J Mater Sci Mater Med 13: 397-401.
- Levine BR, Sporer S, Poggie RA, Della Valle CJ, Jacobs JJ (2006) Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials 27: 4671-4681.
- Wang X, Lin CJ, Hu R (2009) Applied Surface Science 255: 4074-4081.
- Chen X, Nouri A, Li Y, Lin J, Hodgson PD, et al. (2008) Effect of surface roughness of Ti, Zr, and TiZr on apatite precipitation from simulated body fluid. Biotechnol Bioeng 101: 378-387.
- Xiong J, Li Y, Wang X, Hodgson P, Wen C (2008) Mechanical properties and bioactive surface modification via alkali-heat treatment of a porous Ti-18Nb-4Sn alloy for biomedical applications. Acta Biomater 4: 1963-1968.
- Wang XJ, Xiong JY, Li YC, Hodgson PD, Wen CE (2009) Apatite Formation on Nano-structured Titanium and Niobium Surface. In: Lu J (Ed.), Advanced Materials Science and Technology.
- Zhang Q, Leng Y, Xin R (2005) A comparative study of electrochemical deposition and biomimetic deposition of calcium phosphate on porous titanium. Biomaterials 26: 2857-2865.
- Habibovic P, Barrère F, Van Blitterswijk CA, De Groot K, Layrolle P (2003) J Am Ceram Soc 85: 517-522.
- Chen X, Li Y, Hodgson PD, Wen C (2009) Microstructures and bond strengths of the calcium phosphate coatings formed on titanium from different simulated body fluids. Materials Science and Engineering C 29: 165-171.
- Park IS, Woo TG, Jeon WY, Park HH, Lee MH, et al. (2007) Surface characterization of titanium anodized in the four different types of electrolyte. Electrochimica Acta 53: 863-870.
- Hench LL, Polak JM (2002) Third-generation biomedical materials. Science 295: 1014-1017.
- Geetha M, Singh AK, Asokamani R, Gogia AK (2009) Ti based biomaterials, the ultimate choice for orthopaedic implants—a review. Progress in Materials Science 54: 397–425.
- Pantojas VM, Velez E, Hernández D, Otaño W (2009) Initial study on fibers and coatings for the fabrication of bioscaffolds. P R Health Sci J 28: 258-265.
- Advincula M, Fan X, Lemons J, Advincula R (2005) Surface modification of surface sol-gel derived titanium oxide films by self-assembled monolayers (SAMs) and non-specific protein adsorption studies. Colloids Surf B Biointerfaces 42: 29-43.
- Hollister SJ (2005) Porous scaffold design for tissue engineering. Nat Mater 4: 518-524.
- LeGeros RZ, Craig RG (1993) Strategies to affect bone remodeling: osteointegration. J Bone Miner Res 8 Suppl 2: S583-596.
- Zou X, Li H, Bünger M, Egund N, Lind M, et al. (2004) Bone ingrowth characteristics of porous tantalum and carbon fiber interbody devices: an experimental study in pigs. Spine J 4: 99-105.
- Bobyn JD, Pilliar RM, Cameron HU, Weatherly GC (1980) The optimum pore size for the fixation of porous-surfaced metal implants by the ingrowth of bone. Clin Orthop Relat Res : 263-270.
- Chen XB, Li YC, Hodgson PD, Wen C (2009) The importance of particle size in porous titanium and nonporous counterparts for surface energy and its impact on apatite formation. Acta Biomater 5: 2290-2302.
- Wang X, Li Y, Xiong J, Hodgson PD, Wen C (2009) Porous TiNbZr alloy scaffolds for biomedical applications. Acta Biomater 5: 3616-3624.
- Niu WJ, Gill SP, Dong HB, Bai CG (2010) A two-scale model for predicting elastic properties of porous titanium formed with space-holders. Computational Materials Sciences 50: 172-178.
- Li Y, Wong C, Xiong J, Hodgson P, Wen C (2010) Cytotoxicity of titanium and titanium alloying elements. J Dent Res 89: 493-497.
- Lopez-Heredia MA, Sohier J, Gaillard C, Quillard S, Dorget M, et al. (2008) Rapid prototyped porous titanium coated with calcium phosphate as a scaffold for bone tissue engineering. Biomaterials 29: 2608-2615.
- Oshida Y (2007) Bioscience and Bioengineering of Titanium Materials, Oxford, Elsevier.
- Obbard EG, Hao YL, Akahori T, Talling RJ, Niinomi M, et al. (2010) Mechanical of Susperelasticity in Ti-30Nb-(8-10)Ta-Zr Alloy. Acta Materialia 58: 3557-3567.
- Wang X, Li Y, Hodgson PD, Wen C (2010) Biomimetic modification of porous TiNbZr alloy scaffold for bone tissue engineering. Tissue Eng Part A 16: 309-316.
- Mediaswanti K, Truong VK, Hasan J, Li YC, Wen C, et al. (2012) Key Engineering Materials 520: 214-219.
- Nomura N, Kohama T, Oh IH, Hanada S, Chiba A, et al. (2005) Mechanical properties of porous Ti–15Mo–5Zr–3Al compacts prepared by powder sintering. Materials Science and Engineering C 25: 330-335.
- Xiong JY, Li YC, Hodgson PD, Wen CE (2008) Mechanical properties of porous Ti-26Nb alloy for regenerative medicine. In: Fan JH, Chen HB (Ed.), Advances in Heterogeneous Material Mechanics 2008: 630-634.
- Balla VK, Bodhak S, Bose S, Bandyopadhyay A (2010) Porous tantalum structures for bone implants: fabrication, mechanical and in vitro biological properties. Acta Biomater 6: 3349-3359.
- Li Y, Xiong J, Wong CS, Hodgson PD, Wen C (2009) Ti6Ta4Sn alloy and subsequent scaffolding for bone tissue engineering. Tissue Eng Part A 15: 3151-3159.
- Wen CE, Mabuchi M, Yamada Y, Shimojima K, Chino Y, et al. (2001) Processing of Biocompatible Porous Ti and Mg. Scripta Materialia 45: 1147-1153.
- Zhuang H, Han Y, Feng A (2008) Preparation, mechanical properties and in vitro biodegradation of porous magnesium scaffolds. Materials Science and Engineering C 28: 1462-1466.
- Witte F, Kaese V, Haferkamp H, Switzer E, Meyer-Lindenberg A, et al. (2005) In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 26: 3557-3563.
- Fernandez-Fairen M, Querales V, Jakowlew A, Murcia A, Ballester J (2010) Tantalum is a good bone graft substitute in tibial tubercle advancement. Clin Orthop Relat Res 468: 1284-1295.
- Li Y, Xiong J, Hodgson PD, Wen C (2010) Cytotoxicity of titanium and titanium alloying elements. Journal of Alloys and Compounds 494: 323-329.
- Levine B, Sporer S, Della Valle CJ, Jacobs JJ, Paprosky W (2007) Porous tantalum in reconstructive surgery of the knee: a review. J Knee Surg 20: 185-194.
- Kokubo T (1991) Bioactive glass ceramics: properties and applications. Biomaterials 12: 155-163.
- Li J, Habibovic P, Yuan H, van den Doel M, Wilson CE, et al. (2007) Biological performance in goats of a porous titanium alloy-biphasic calcium phosphate composite. Biomaterials 28: 4209-4218.
- Suzuki Y, Nomura N, Hanada S, Kamakura S, Anada T, et al. (2007) Key Engineering Materials 330-332: 951-954.
- Shi D (2004) Biomaterials and Tissue Engineering.
- Hu S, Li S, Yan Y, Wang Y, Cao X (2005) Journal Wuhan University Of Technology, Materials Science Edition 20: 13-15.
- Hu Q, Tan Z, Liu Y, Tao J, Cai Y, et al. (2007) J Mater Chem 17: 4690-4698.
- Dumbleton J, Manley MT (2004) Hydroxyapatite-coated prostheses in total hip and knee arthroplasty. J Bone Joint Surg Am 86-86A: 2526-40.
- Garbuz DS, Hu Y, Kim WY, Duan K, Masri BA, et al. (2008) Enhanced gap filling and osteoconduction associated with alendronate-calcium phosphate-coated porous tantalum. J Bone Joint Surg Am 90: 1090-1100.
- Adamek G, Jakubowicz J (2010) Materials Chemistry and Physics 124: 1198-1204.
- Habibovic P, van der Valk CM, van Blitterswijk CA, De Groot K, Meijer G (2004) Influence of octacalcium phosphate coating on osteoinductive properties of biomaterials. J Mater Sci Mater Med 15: 373-380.
- Turkan U, Guden M (2010) Ceramics International 36: 1805-1816.
- Takemoto M, Fujibayashi S, Neo M, Suzuki J, Matsushita T, et al. (2006) Osteoinductive porous titanium implants: effect of sodium removal by dilute HCl treatment. Biomaterials 27: 2682-2691.
- Tanaka K, Takemoto M, Fujibayashi S, Kawanabe K, Matsushita T, et al. (2009) Key Engineering Materials 396-398: 353-356.
- Gan L, Wang J, Tache A, Valiquette N, Deporter D, et al. (2004) Calcium phosphate sol-gel-derived thin films on porous-surfaced implants for enhanced osteoconductivity. Part II: Short-term in vivo studies. Biomaterials 25: 5313-5321.
- Gan L, Pilliar R (2004) Calcium phosphate sol-gel-derived thin films on porous-surfaced implants for enhanced osteoconductivity. Part I: Synthesis and characterization. Biomaterials 25: 5303-5312.
- Sun J, Han Y, Cui K (2008) Microstructure and apatite-forming ability of the MAO-treated porous titanium. Surface and Coatings Technology 202: 4248-4256.
- Hartshorn R, Stockwell S, Lebedev M, Krumdieck S (2007) Surface and Coatings Technology 201: 9413-9416.
- Verket A, Tiainen H, Haugen HJ, Lyngstadaas SP, Nilsen O, et al. (2012) Enhanced osteoblast differentiation on scaffolds coated with TiO2 compared to SiO2 and CaP coatings. Biointerphases 7: 36.
- Inoue M, Hyun SK, Suganuma K, Nakajima H (2006) Materials Transactions 47: 2161-2166.
- Yao C, Webster TJ (2006) Anodization: a promising nano-modification technique of titanium implants for orthopedic applications. J Nanosci Nanotechnol 6: 2682-2692.
- Das K, Balla VK, Bandyopadhyay A, Bose S (2008) Scripta Materialia 59: 822-825.
- Lee JH, Kim HE, Shin KH, Koh YH (2010) Materials Letters 64: 2526-2529.
- Lin JG, Li YC, Wong CS, Hodgson PD, Wen CE (2009) Degradation of the strength of porous titanium after alkali and heat treatment. J Alloys Compd 485: 316-319.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 22366
- [From(publication date):
July-2013 - Apr 19, 2025] - Breakdown by view type
- HTML page views : 17468
- PDF downloads : 4898