A Review on 3D Oral Printed Tablets: A Breakthrough Tool for Individualized Therapy According To Each Patient's Individual Circumstances with Different Techniques and Polymers Used For Formulation
Received: 07-Nov-2022 / Manuscript No. ijrdpl-22-82547 / Editor assigned: 09-Nov-2022 / PreQC No. ijrdpl-22-82547 / Reviewed: 23-Nov-2022 / QC No. ijrdpl-22-82547 / Revised: 28-Nov-2022 / Manuscript No. ijrdpl-22-82547 / Accepted Date: 04-Dec-2022 / Published Date: 05-Dec-2022 QI No. / ijrdpl-22-82547
Abstract
When treating patients from various origins with distinct cultures, metabolisms, and needs, interindividual variability is becoming a more widespread concern. Because dose modification typically relies on empirical techniques, there is a strong probability that undesirable side effects may manifest.The pharmaceutical industry is being revolutionized by three-dimensional (3D) printed pharmaceuticals as prospective instruments to create individualized therapies tailored to each patient's unique needs,taking into consideration their age, weight,pharmacogenetic,comorbidities, pharmacogenetic,and pharmacokinetic features. Only three of the various processes used in additive manufacturing, or 3D printing, are primarily employed in the 3D printing of pharmaceuticals: printing-based inkjet systems, nozzle-based deposition systems, and laser-based writing systems. Each method has a number of downsides, and the kinds of polymers thatare easily accessible don't necessarily have the best qualities for each medicine. The purpose of this study is to provide an overview of the current methods used in 3D printing medications, highlighting their advantages and shortcomings as well as the necessary polymers and medications for a successful print. Also covered will be the main applications
of these approaches.
Keywords
3D printing; Novel drug delivery system; Approach; Materials; Polymers
Introduction of 3D Printed Oral Tablets
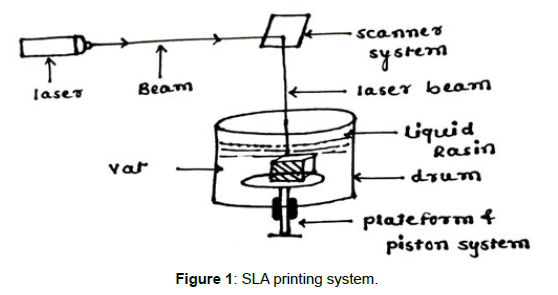
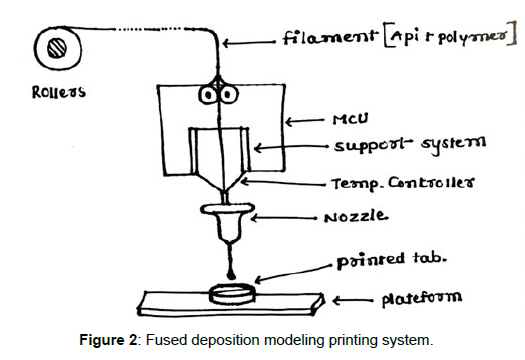
Such variation has always been recognised as a necessary component of treatment, but modern technologies enable treatment optimization based on population subgroups and pharmacokinetic and pharmacokinetic profiles [1]. When treating patients from various origins with distinct cultures, metabolisms, and needs, interindividual variability is becoming a more widespread concern. Currently, dosage modification is typically relied on empirical techniques, which increases the likelihood of unfavourable side effects. For instance, it is anticipated that each year in the United States, millions of adverse effects will res ult in over 100,000 fatalities [2]. The aforementioned research tries to go beyond the Pharmaceutical Industry's conventional preference for producing medications in accordance with the broadest and most representative therapeutic response profile. When designing medications while taking into consideration all of the unique characteristics of each individual, drug mass manufacturing is a hindrance. Due to this characteristic, therapy dosages for paediatric and geriatric groups are often insufficient, increasing the likelihood of side effects. To obtain the intended therapeutic effect and enhance the efficacy/toxicity balance, dosage must be adjusted in accordance with pharmacogenetic and pharmacokinetic features, weight, and age. Similarly, changing the colours, tastes, or even the shape of solid dose forms will significantly improve treatment compliance in both children and seniors [1]. However, polimedicated patients, those who have been prescribed more than five different types of medications, are also at risk of therapeutic failure and the promotion of side effects and drug interactions due to poor patient compliance and treatment adherence. Polimedicated patients suffer from the inadequacy of solid dosage forms tailored to their needs, just as children and the elderly do. The administration of a single tablet containing all of the medications the patient needs would simplify pharmacological therapy, increase adherence to the recommended course of action, and presumably improve the efficacy/toxicity balance [1]. But Rapid Prototyping (RP), which was created with the objective of lowering the arduous periods of time and the massive expenses in drug research and production, is the trend that is actually transforming the world of pharmaceutical technology. RP is the process of creating physical models from threedimensional computer-aided design (CAD) data. The term "additive manufacture" (AM) or the "manufacture of solids of free form" (SFF) encompasses a variety of quite distinct technologies, including one of the most cutting-edge ones: three-dimensional (3D) printing. This innovative approach places the pharmaceutical industry in a new light and opens the door to personalised medicine [1]. Inkjet printing a binder solution onto a powder bed, binding the particles together as a result of the semi-liquid binder solution, was the first 3D printing method utilised in pharmaceutics. Until the required structure was achieved, the procedure was repeated. This initially occurred at the MIT (Massachusetts Institute of Technology), founded by Sachs et al., in the early 1990s. Spritam, the first 3D-printed medication authorised by the Food and Drug Administration (FDA) and made available on the market by Aprecia Pharmaceuticals in the summer of 2016, was created using inkjet printing, (Figure 1) demonstrating how far the technology has advanced in recent years [1]. With fused (Figure 2) deposition modelling (FDM), extruded polymer filaments heated to a semi-liquid state were extruded via a heated nozzle and placed onto a build platform layer by layer to solidify; Scott Crump submitted a patent in the previous decade in 1989 [1].Since then, several other 3D printing methods have been created.
Clinical 3D printing using polymers
Polyvinyl alcohol (PVA)
PVA is a synthetic thermoplastic polymer that dissolves well in water but poorly in ethanol and is insoluble in a wide range of organic solvents. It has high mechanical qualities and is bland and odourless. By removing the acetate groups from polyvinyl acetate, it is created by either partial or complete hydrolysis. The degree of hydroxylation affects the polymer's mechanical, chemical, and physical characteristics. The melting temperature (Tm) of PVA varies from 180 °C (partially hydrolysed) to 220 °C depending on how much the acetate groups have been hydrolysed (fully hydrolysed). The viscosity scale of the polymer depends on the degree of hydrolysation; it ranges from 3.4 to 52 mPa for moderately hydrolysed PVA to 4 to 60 mPa for completely hydrolysed PVA.1 The more easily PVA crystallises and the less hydrolyses and polymerizes, the more water it is soluble in additionally, when the degree of hydrolysation is smaller, the polymer's molecular weight is larger. PVA's deterioration temperature varies from 350 to 450 °C, whereas its glass transition temperature (Tg) is 85 °C.
Poly (Lactic Acid) (PLA)
The United States Food and Drug Administration (FDA) has designated the biodegradable polymer poly(lactic acid) as safe (GRAS), making it appropriate for use in a variety of medical applications including orthopaedic, fixation devices, stents, tissue engineering, and regenerative medicine. Direct and ring opening polymerization is the basis for the primary manufacturing methods for this polymer. The qualities change based on the PLA's molecular weight, processing temperature, ratio of isomers, and crystallinity. The proportion of crystalline and amorphous areas in a polymer is referred to as its crystallinity, and it affects properties including stiffness, hardness, and tensile strength. The melting point and Tg of PLA homopolymer are 150–175°C and 55°C, respectively [2] According to reports, PLA has a melt viscosity of 1000 Pa at 200°C, but under shear stress and at high temperatures, it may reach 5100 Pa. In dioxane, acetonitrile, chloroform, methylene chloride, 1, 1, 2-trichloroethane, and dichloroacetic acid, PLA and its derivatives dissolve well. When heated to boiling temperatures, the solubility of all of them increases. They are all poorly soluble in cold ethyl benzene, toluene, acetone, and tetrahydrofuran. Water, alcohols (such as methanol and ethanol), propylene glycol, and unsubstituted hydrocarbons have all been found to have poor solubility (e.g., hexane and heptane) [3].
Poly (Caprolactone) (PCL)
Poly (caprolactone) is a hydrophobic semi-crystalline polymer whose crystallinity tends to rise with decreasing molecular weight. It has a Tg of 60 °C and a melting point that fluctuates from 59 to 64 °C. At room temperature, PCL dissolves well in benzene, toluene, cyclohexanone, dichloromethane, chloroform, and 2-nitropropane. In contrast, it is poorly soluble in alcohol, petroleum ether, and diethyl ether and insoluble in acetone, 2-butanone, ethyl acetate, dimethylformamide, and acetonitrile. Poly (vinyl chloride), poly (styrene-acrylonitrile), poly (acrylonitrile butadiene styrene), and poly (bisphenol-A) are just a few of the polymers that PCL mixes well with. It is also mechanically compatible with other polymers like polyethylene, polypropylene, natural rubber, poly (vinyl acetate), and poly (ethylene-propylene) rubber. It may be made via the ring opening polymerization (ROP) of e-CL and the condensation of 6-hydroxycaproic (6-hydroxyhexanoic) acid [3].
Applications of 3D Printed Drugs
Drugs developed in 3D and approved for sale: Spritam®
The first 3D printed medication, Spritam® (Aprecia Pharmaceuticals, East Windsor, NJ, USA), which contains the antiepileptic API levetiracetam, received FDA approval in 2015. The pharmacological efficacy was shown to be comparable to that of traditional tablets, but with the noteworthy enhancement that, because to the porous and soluble matrix composition, the solubilization period was (Table 1) greatly shortened. Aprecia Pharmaceuticals trademarks Spritam® utilising the ZipDose method, which is based on the layer-bylayer manufacturing methodology and powder bed fusion. The active component and all of the excipients required to make the matrix tablet are found in the top layer. A binder liquid is then used to ensure flawless aggregation and integration between all of the succeeding and identical layers. The outcome is an orodispersible tablet with a dose capacity of up to 1000 mg of API, which dissolves in a few seconds and with a very tiny amount of water [3].This innovation shows how this technique may be used to create specific dosage forms with characteristics that are not possible to achieve through compression or other traditional production processes.
| 3D Printer | CIJ | FDM | PAM | SLA | Remarks |
|---|---|---|---|---|---|
| Polymer | Polymer Stabilizer Liquid | Material heat-resistant as melted metals, photo-polymerizable resin and thermoplastic materials | Semi-liquid viscous material | Liquid photopolymer which rapidly solidifies with UV light, as low molecular weight polyacrylate macromers | |
| Polymer Example | Tween 20 | PVA, PLA, Nylon, ABS, Polyvinyl chloride | Hydroxypropyl methylcellulose (HPMC), Polyacrylate Methocel® E5 | PEGDA (liquid photosensitive resin), Propiophenone 2-hydroxy-2-methyl (initiator) | |
| Drug Type | Slightly soluble in water and organic solvents | Thermorresistant molecule | Wide variety Non-specific type |
Proteins and Peptides | |
| Drug Example | Folic Acid | Prednisone, Theophylline, 5-ASA | Nifedipine, Glipizide | BSA (Bovine Serum Albumin) | |
| Advantage | Works in continuous | Lowest cost, Good mechanical resistance | Manufacture of complex drug delivery systems | Smooth surface due to the use of liquid photopolymers, Manufacture of micro-structures | |
| Disadvantage | High energy expenditure and waste generation | Low adequate thermoplastic materials., API degradation due to high temperatures | Use of organic solvents, toxicity and loss of stability | Lack of FDA-approved photosensitive polymers |
Table 1: Type of polymers most commonly employed in 3D printing medicines.
3D Printed Polypill
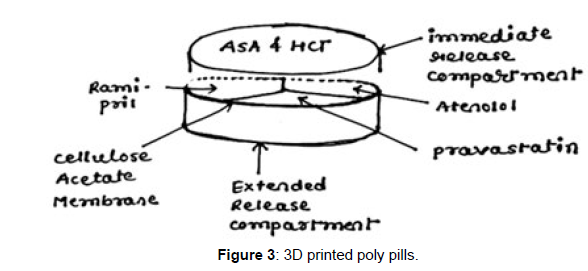
A "polypill" is a single tablet that contains the mixture of numerous medications. As a result, it offers significant advantages to polymedicated patients, including the elderly. Different polypills have been successfully produced using 3D extrusion printing. For instance, 3D printing has made it possible to produce pills containing the drugs captopril, nifedipine, and glipizide, which are used to treat type 2 diabetes, hypertension, and hypertension. The technology has advanced, and at this time, prototypes have been created that include five distinct types of APIs with various release profiles. The prolonged release compartment included three APIs (pravastatin, atenolol, and ramipril), which were physically separated from one another by a permeable membrane made of hydrophobic cellulose acetate. On top of the aforementioned compartment, a quick release container containing aspirin and hydrochlorothiazide was placed.
Devices for Personalized Topical Treatment
The fabrication of personalised, drug-filled devices that are sized and shaped specifically for each patient is also possible thanks to the 3D printing revolution. Salicylic acid-filled nose-shaped masks for anti-acne treatments have been quickly and effectively produced. The patient's face was scanned, and the picture that was obtained was exported to the AutoCAD software, where the nose part was chosen. It was important to leave the inside section hollow in order to construct the three-dimensional model with absolute precision and guarantee the best possible fit to the patient's face (Figure 3). The geometric model was created, and then it was printed using two distinct processes—FDM and SLA—to see which one was better for (Figure 1) manufacturing, object morphology, drug release, and printing stability. Due to the better device resolution, which permits a larger drug loading as well as the little degradation of salicylic acid during 3D printing, SLA was the most promising approach for the mask fabrication [1]. The most notable aspect of this discovery is the broad view that comes from combining the scanner with 3D printing in the era of personalised medicine based on dose, size, and form of certain devices to treat particular illnesses.
3D Printing for Cancer Treatment
Traditional chemotherapy has trouble getting to the tumour location in therapeutic concentrations. This is because the majority of chemotherapy medicines have a low solubility in aqueous fluids, making it impossible to acquire the necessary concentrations at the tumour site using traditional procedures such intravenous injection or oral delivery. Additionally, antineoplastics frequently build up in pertinent organs including the liver and heart, leading to negative side effects. Local delivery methods would thus be very helpful in overcoming the drawbacks of traditional chemotherapy. Currently, patches containing PCL, poly (lactic-co-glycolic acid), and 5-fluorouracil have been successfully printed and inserted into a pancreatic malignancy. The drug release was maintained for a total of four weeks by manipulating the patch's shape and release kinetics. The patch biodegraded in the body after that time [5].
3D Printed drugs facing Challenges
The requirements for excipients, the development of printing software and instrumentation, optimizing the mechanical properties of products, and the regulatory environment are the four challenges impacting application, despite the potential of 3D-printed technologies to advance the pharmaceutical industry. Excipients for use in pharmaceutical 3D printing are typically more constrained than they are in traditional production methods, particularly for specialized dosage forms and heat-based technologies. Further research on nontoxic, biodegradable, biocompatible, and stable excipients is needed to support its application by the pharmaceutical industry. The modelling and slicing software used to develop and provide information for the manufacture of a dosage form must be regularly updated as the intricacy of the structure gets more sophisticated. To satisfy the requirements of the various processes, whether to avoid clogging or encourage product consistency, the mechanical equipment, operating methods, and control system must also be updated and optimised. Currently, 3D printers used to create pharmaceutical formulations do not adhere to good manufacturing practices (GMP) requirements; as a result, they must be verified to make sure they do.
Conclusion
Three-dimensional printing is now a practical and prospective tool for the pharmaceutical industry, opening the door to individualised treatment according to the demands of the patient. Since an RP may be completed in a matter of minutes, it has many benefits, including improving cost effectiveness and production speed. To assure that 3D printed medications have the same efficacy, safety, and stability as those traditionally produced by the pharmaceutical industry, there is still a sizable obstacle to overcome. Given the conventional demands of the pharmaceutical industry, it is a significant issue for the regulatory authorities to develop rules, legislation, quality systems, and safety of use and consumption of 3D printed medications. However, the regulatory bodies' viewpoint is quickly evolving to reflect the demands of patients and the actual world. Technical Considerations for Additive Manufactured Devices is a new FDA guidance document that was created in 2016 to provide the FDA's initial thoughts on technical considerations related to AM processes as well as recommendations for testing and the characterization of devices that contain at least one AM fabrication step [6]. The revolution in medication production methods has only just begun.
References
- Anderson SE, Meade BJ (2014) Potential health effects associated with dermal exposure to occupational chemicals. Environ Health Insights 8: 51–62.
- Azandjeme CS, Bouchard M, Fayomi B, Djrolo F, Houinato D (2013) Growing of diabetes in sub-saharan Africa: contribution of pesticides? Curr Diabetes Rev 9: 437–449.
- Beard JD, Umbach DM, Hoppin JA (2014) Pesticide exposure and depressionamong male private pesticide applicators in the agricultural health study. EHP 122: 984–991.
- Bulut S, Erdogus SF, Konuk M, Cemek M (2010) The organochlorine pesticide residues in the drinking waters of Afyonkarahisar, Turkey. Ekoloji Dergisi 19: 24– 31.
- Covaci A, Tutudaki M, Tsatsakis AM, Schepens P (2002) Hair analysis: another approach for the assessment of human exposure to selected persistent organochlorine pollutants. Chemosphere 46: 413–418.
- Coxall M (2014) Ethical Eating: A Complete Guide to Sustainable Food Kindle Edition. Cornelio Books.
- Dennis LK, Lynch CF, Sandler DP, Alavanja MC (2010) Pesticide use and cutaneous melanoma in pesticide applicators in the agricultural heath study. Environ Health Perspect 118: 812– 817.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Padsala PV, Asodariya HA, Shah YS, Ranpariya D, Panchal H (2022) A Review on 3D Oral Printed Tablets: A Breakthrough Tool for Individualized Therapy According To Each Patient's Individual Circumstances with Different Techniques and Polymers Used For Formulation. Int J Res Dev Pharm L Sci, 8: 145.
Copyright: © 2022 Padsala PV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 897
- [From(publication date): 0-2022 - Dec 22, 2024]
- Breakdown by view type
- HTML page views: 716
- PDF downloads: 181