A Review of Real-World Use of Ceftazidime-avibactam for Multidrug-Resistant Gram-Negative Bacterial Infections
Received: 17-Dec-2021 / Manuscript No. JIDT-21-50027 / Editor assigned: 20-Dec-2021 / PreQC No. JIDT-21-50027 (PQ) / Reviewed: 31-Dec-2021 / QC No. JIDT-21-50027 / Revised: 31-Dec-2021 / Manuscript No. JIDT-21-50027 (R) / Accepted Date: 02-Jan-2022 / Published Date: 09-Jan-2022 DOI: 10.4172/2332-0877.1000483
Abstract
The incidence of multidrug-resistant-Gram negative bacilli (MDR-GNB) infections is increasing. Ceftazidime-avibactam (CAZ-AVI) is recommended as one of the preferred agents for the treatment of infections caused by carbapenem-resistant Enterobacterales (CRE) or Pseudomonas aeruginosa with Difficult-To-Treat Resistance (DTR-PA). Given the rising threat of infections caused by MDR-GNB, in particular Carbapenem-Resistant (CR) pathogens, it is important to understand the use of CAZ-AVI for the treatment of GNB infections with limited treatment options. Evidence from 28 real-world studies suggest that CAZ-AVI is an effective and well-tolerated alternative to standard of care antibiotics for treating different types of infection caused by MDR-GNB, including CRE and MDR-Pseudomonas spp. Notably, CAZ-AVI is well tolerated even in severely or critically ill patients, patients with multiple comorbidities, or those with bacteremia. These real-life experiences provide valuable insights into the use of CAZ-AVI across diverse types of GNB infections for which limited treatment options exist.
Keywords: Ceftazidime-avibactam ; Multidrug-resistant gramnegative bacilli infections; Carbapenem-resistant Enterobacterales;Pseudomonas aeruginosa; Real-world
Introduction
The incidence of antimicrobial resistance is increasing and continues to be a worldwide threat [1-3]. Antimicrobial resistant pathogens accounted for close to three million infections and caused over 35,000 people to die yearly in the United States (US) between 2012 and 2017 [2]. The selection of an effective antimicrobial treatment for patients infected by resistant pathogens is challenging. The Centers for Disease Control and Prevention (CDC) have identified three groups of antimicrobial resistant Gram-negative pathogens as urgent or serious antibiotic resistance threats that pose particular therapeutic challenges: carbapenem-resistant Enterobacterales (CRE), Pseudomonas aeruginosa with difficult-to-treat resistance [2] (DTR-PA; defined by the Infectious Diseases Society of America [IDSA] as “P. aeruginosa exhibiting non-susceptibility to all of the following: piperacillin-tazobactam, ceftazidime, cefepime, aztreonam, meropenem, imipenem-cilastatin, ciprofloxacin, and levofloxacin”) [4], and extended-spectrum β-lactamase (ESBL)-producing Enterobacterales. These pathogens caused a wide variety of serious infections that are associated with significant morbidity and mortality [2]. In the US, infections due to ESBL-producing Enterobacterales have increased by around 1.5 times in the US between 2012 and 2017 [5]. CRE has been reported to be associated with over 13,000 nosocomial infections and more than 1,000 deaths per year, with Klebsiella pneumoniae carbapenemases (KPCs) being the most common carbapenemases [2]. Multidrug-Resistant Pseudomonas aeruginosa (MDR-PA) accounted for 32, 600 infections in patients hospitalized in the US and caused 2,700 deaths in 2017 [2]. Appropriate treatment against these pathogens is a healthcare priority.
Limited treatment options are available for infections caused by multidrug-resistant gram-negative bacilli (MDR-GNB). Colistin (polymyxin), aminoglycosides, tigecycline, and carbapenems have been widely used to treat such infections [6,7]. However, uses of these agents have important drawbacks. Treatment with colistin or aminoglycosides is associated with toxicity issues; use of these agents was linked to reports of significant nephrotoxicity [8-10]. In addition, treatment with many of these agents are hampered by suboptimal pharmacokinetic/ pharmacodynamic of the drug, resulting in inadequate concentrations in tissues or blood [9-13]. Recent data on polymyxin (including colistin and polymyxin B) suggest that these agents have important limitations [10]. Existing evidence shows that less than half of patients with normal renal function achieve the target colistin steady state concentration and this level of exposure is not adequate to achieve bacterial stasis in pneumonia models [10,14]. Furthermore, studies demonstrate increased mortality for polymyxin compared with other agents [15,16]. Besides these challenges, the rising trends of MDR-GNB, in particular carbapenem-resistant (CR) pathogens further complicate the management of these infections and highlight the need for new antimicrobial agents [17,18].
Recent published IDSA guidelines propose recommendations on the management of MDR-GNB infections, and Include Ceftazidime- Avibactam (CAZ-AVI) as one of the preferred agents for the treatment of infections caused by CRE or DTR-PA [4]. CAZ-AVI, a β-lactam/β-lactamase inhibitor combination, is one of the recently launched antimicrobial agents developed in response to the need for novel agents to tackle the rising incidence of MDR-GNB infections [4,8,19]. CAZ-AVI is approved in the US and Europe for the treatment of complicated Urinary Tract Infections (cUTIs), complicated intra- abdominal infections (cIAIs), hospital-acquired pneumonia (HAP), and ventilator-associated pneumonia (VAP) [20,21] in adult patients. It is also approved in the US for treating cUTIs and cIAIs in pediatric patients aged three months and above [20]. In Europe, it is additionally approved for the treatment of aerobic GNB infections with limited treatment options and its use is expanded to include pediatric patients aged three months and above [21]. CAZ-AVI represents a valuable new treatment option with the potential to treat infections caused by some of the most problematic MDR-GNB pathogens [22-24]. CAZ-AVI exhibits in vitro activity against isolates of Enterobacterales and Pseudomonas aeruginosa that harbor class A, C, and some D β-lactamases, including ESBLs, ampicillin C (AmpC) β-lactamases, KPCs, and oxacillinases (OXA)-48, but not those harboring class B metallo-β-lactamases [22- 24]. CAZ-AVI has limited activity against Acinetobacter spp. [22,25].
Data on the efficacy of CAZ-AVI against CR pathogens in clinical trials are limited. In phase III randomized control trials (RCTs) comparing CAZ-AVI with carbapenem. CAZ-AVI was non-inferior to carbapenem comparators for the treatment of cUTI, cIAI, and HAP/ VAP [26-29]. CAZ-AVI treatment was associated with high response rates at the test-of-cure (TOC) visit in patients with infections caused by ceftazidime-susceptible and resistant Gram-negative pathogens. CAZ- AVI was generally safe and well tolerated, with a profile consistent with that of ceftazidime alone. However, few CR pathogens were included in these trials [26-29]. In an open-label phase III trial (the REPRISE trial) of patients with cUTI and cIAI due to ceftazidime-resistant Gram-negative organisms, a similar proportion of patients in the CAZ- AVI group and best available therapy (mostly carbapenem-containing regimens) group achieved clinical cure at the TOC visit [30]. No new safety concerns were identified for CAZ-AVI. Among the 292 isolates of Enterobacterales recovered from the REPRISE trial, only nine isolates were CRE (six were KPC producers and three were OXA-48 producers) [31]. Real-world experience with CAZ-AVI in treating a variety of infections caused by a number of important MDR-GNB including CR pathogens has accumulated in recent years. Given the rising threat of infections caused by MDR-GNB, in particular CR pathogens, it is important to understand the use of CAZ-AVI for the treatment of GNB infections with limited treatment options.
Literature Review
This article reviews the real-world evidence on the therapeutic effectiveness and safety of CAZ-AVI in adult patients with infections due to aerobic Gram-negative pathogens with limited treatment options, including CRE and MDR Pseudomonas spp. A structured literature search of PubMed and EMBASE databases was conducted for studies published from 2005 through Nov 2020 that evaluated clinical experience of CAZ-AVI in treating adult patients with infections caused by aerobic Gram-negative pathogens with limited alternatives available, including CRE and MDR-Pseudomonasspp. Records were restricted to those in English language. Preclinical studies, clinical trials, reviews, case reports, studies with no relevant results, or studies in pediatric patients were excluded.
Characteristics of included real-world studies on CAZ-AVI
Twenty-eight real-world studies met the selection criteria and were included. These studies described clinical experience of using CAZ-AVI in adult patients for treating infections caused by aerobic GNB with limited treatment options, including CRE and MDR-Pseudomonas spp. Collectively, these studies provided data on the use of CAZ-AVI across diverse types of infection, such as bacteremia, pneumonia, IAI, Skin- Soft Tissue Infection (SSTI), bone and joint infection, Central Nervous System Infection (CNSI), UTI, etc., with limited alternatives available. The characteristics of these studies are presented in Table 1 [32-59].
| Reference (Year) | Study design | Study population/ types of infection | Target pathogens | Treatment(s) | Key effectiveness outcomes | Safety outcomes | Other relevant outcomes | |
|---|---|---|---|---|---|---|---|---|
| Ackley (2020) [32] | Multicenter, retrospective | Patients with infections caused by KPC-producing Enterobacterales (excluded those with localized urinary tract infection and repeat study drug exposures after the first episode) [Mixed infection types] | KPC-producing CRE | CAZ-AVI (n=105) Monotherapy: 39% Types of infection: Bacteremia (42%), respiratory (29%), soft tissue (17%), IAI (11%), and others (1%) | Meropenem- Vaborbactam (n=26) Monotherapy: 85% Types of infection: Bacteremia (35%), respiratory (38%), Soft tissue (8%), IAI (19%), and others (0%) | Clinical success, 30-day mortality, 90-day mortality, 90-day infection recurrence | Treatment-related AEs | Development of resistance in patients with recurrent infection |
| Aitken (2016) [33] | Single center, retrospective | Cancer patients with CRE bloodstream infection Bacteremia | CRE | CAZ-AVI (n=5) Monotherapy: 0% | - | Microbiologic success, clearance of infection, 30-day mortality | - | - |
| †Algwizani (2018) [34] | Single center, retrospective, case series | Patients with infections caused by CR-organisms Types of infection: VAP (2 pts), bacteremia (2 pts), and CNSI (1 pt) | CR-organisms including OXA- 48-producing Kp and CR-PA | CAZ-AVI (n=5) Monotherapy: 60% | - | Microbiological cure, clinical cure | - | - |
| Alraddadi (2019) [35] | Single center, retrospective | Patients with established CRE infections [Mixed infection types] | 74% OXA-48- producing CRE | CAZ-AVI (n=10) Types of infection: Bacteremia (70%), HAP (50%), cUTI (30%), cIAI (30%), and SSTI (20%) | Other agents (n=28) (mostly included colistin and/or carbapenem among others) Monotherapy: 11% Types of infection: Bacteremia (54%), HAP (50%), cUTI (29%), cIAI (18%), and SSTI (11%) | Clinical remission, all- cause mortality | - | - |
| Bassetti (2019) [36] | Multicenter, retrospective | Patients with KPC- Kp gut colonization | KPC-Kp | CAZ-AVI (n=12) Monotherapy: 8% Types of infection: cIAI (42%), HAP (17%), sepsis (17%), surgical wound infection (8%), and others (25%) | Other regimens (n=24) (included 83% tigecycline, 46% colistin, and/or 54% carbapenem among others) Monotherapy: 0% Types of infection: cIAI (17%), HAP (21%), sepsis (42%), surgical wound infection (17%), and others (4%) | Decolonization rate | - | - |
| Caston (2017) [37] | Multicenter, retrospective | Patients with hematologic malignancies who had CPE bacteremia Bacteremia | CPE <61% OXA; 39% KPC> | CAZ-AVI (n=8) Monotherapy: 0% | Other agents (n=23) Monotherapy: 6% | 14-day clinical cure, 30-day crude mortality | - | - |
| Chen (2020) [38] | Single center, retrospective | Lung transplant patients with XDR- GNB infections Types of infection: Pneumonia and/or tracheobronchitis (90%) and cholecystitis and bacteremia (10%) | XDR GNB (90% KPC-Kp) | CAZ-AVI (n=10) Monotherapy: 20% | - | Microbiological cure, 30-day and 90-day survival, infection relapse, time to microbiological cure | Treatment-related AEs | - |
| De la Calle (2019) [39] | Single center, retrospective | Patients with infections caused by CRE Types of infection: Bacteremia (33%), IAI (29%), UTI (25%), pneumonia (21%); osteoarticular/SSTI (17%), device- related meningitis (4%), and catheter- related bacteremia (4%) | OXA-48- producing Enterobacterales | CAZ-AVI (n=23) Monotherapy: 58% | - | 30-day clinical cure, 30-day and 90- day mortality, mortality, 90- day infection recurrence | Treatment-related AEs | - |
| Falcone (2020) [40] | Multicenter, retrospective | Patients with BSI due to KPC-Kp hospitalized in ICU (excluded those with polymicrobial BSIs) Bacteremia | KPC-Kp | CAZ-AVI- containing regimens (n=13) | Colistin-containing regimen (n=61); other regimens (n=17) | Composite endpoint (30- day mortality or nephrotoxicity), 30-day mortality | - | - |
| Guimarães (2019) [41] | Multicenter, prospective, case series | Patients with severe infections caused by KPC-producing Enterobacterales coresistant to carbapenems and polymyxins Types of infection: Bacteremia (41%), UTI (28%), IAI (14%), nosocomial pneumonia (10%), and complicated SSTI (7%) | KPC-producing Enterobacterales | CAZ-AVI (n=29) Monotherapy: 52% | - | Clinical success, 14 and 30- day all-cause mortality | Treatment-related AEs | - |
| Iannaccone (2020) [42] | Single center, retrospective | Patients with BSI caused by KPC-Kp Bacteremia | KPC-Kp | CAZ-AVI (n=23) Monotherapy: 13% | - | Recovered from infection, in- hospital mortality, recurrent infection | - | Development of CAZ-AVI resistance in CAZ- AVI-treated patients |
| †Jorgensen (2019) [43] | Multicenter, retrospective | Patients with MDR- GN infections Types of infection: Respiratory tract (37%), UTI (20%), IAI (19.7%), bacteremia (11%), SSTI (9%), and osteoarticular (7%) | MDR-GN organisms (58% CRE and 31% Pseudomonas spp.) | CAZ-AVI (n=203) Monotherapy: 67% | - | Composite clinical failure, 30-day mortality, 30-day recurrence | Treatment-related AEs | Development of CAZ-AVI resistance during treatment in patients with repeat susceptibility testing (n=61) |
| Jorgensen (2020) [44] | Multicenter, retrospective | Patients with CRE infections Types of infection: Respiratory tract (35%), IAI (21%), UTI (20%), SSTI (6%), osteoarticular 7 (6%), bacteremia (6%), and others (5%) | CRE | CAZ-AVI (n=109) Monotherapy: 60% | - | 30-day all-cause mortality | - | - |
| Katchanov (2018) [45] | Single center, retrospective | Critically ill patients with severe infections due to CRE Types of infection: HAP (4 pts), bacteremia (1 pt), and cIAI (1 pt) | OXA-48 producing Kp | CAZ-AVI (n=5) Monotherapy: 0% | - | In-hospital mortality | - | - |
| King (2017) [46] | Multicenter, retrospective | Severely ill patients with CRE infection Types of infection: Bacteremia (38%), UTI (28%), pneumonia (27%), wound (13%), IAI (7%), and bone/joint (3%) | CRE | CAZ-AVI (n=60) Monotherapy: 55% | - | Microbiological cure, clinical success, in- hospital mortality | Treatment-related AEs | - |
| Krapp (2017) [47] | Single center, retrospective | Patients with infections caused by KPC-Kp. Types of infection: Pneumonia (2 pts), IAI (1 pt), peritonitis (1 pt), perinephric abscess (1 pt), and wound (1pt) | KPC-Kp | CAZ-AVI (n=6) Monotherapy: 33% | - | Clinical cure, infection relapse | - | - |
| Rodríguez- Núñez (2018) [48] | Single center, retrospective | Patients with infections due to MDR-or XDR-PA Types of infection: Hospital-acquired lower respiratory tract infection (5 pts), osteomyelitis (1 pt), meningitis (1 pt) and catheter-related bacteremia (1 pt). | MDR or XDR-PA (including 2 pt with CR MDR PA) | CAZ-AVI (n=8) Monotherapy: 25% | - | Clinical cure, 30- day and 90-day mortality | - | - |
| Santevecchi (2018) [49] | Single center, retrospective, case series | Patients with infections due to MDR-organisms other than Kp Types of infection: Pneumonia (46%), skin and soft tissue (23%), bacteremia (15%), and intra– abdominal (15%) | MDR-organisms other than Kp (most common: MDR-PA) | CAZ-AVI (n=10) Monotherapy: 50% | - | Microbiological cure, clinical success, 30- day in-hospital mortality | Treatment-related AEs | Development of CAZ-AVI resistance in CAZ- AVI-treated patients |
| Shield (2016) [50] | Single center, retrospective | Patients with CRE Types of infection: Pneumonia (32%), bacteremia (27%), IAI (11%), SSTI (11%), pyelonephritis (11%), mediastinitis (3%), subdural empyema/ Ventriculitis (3%), and purulent tracheobronchitis (3%) | CRE (78% KPC- producing Enterobacterales) | CAZ-AVI (n=37) Monotherapy: 70% | - | Microbiologic failure, 30-day clinical success, 30-day survival, 90-day infection recurrence | AKI, treatment discontinuation | Development of CAZ-AVI resistance in CAZ- AVI-treated patients |
| Shields (2017) [51] | Single center, retrospective | Patients with CR-Kp bacteremia [Bacteremia] | CR-Kp (97% KPC-Kp) | CAZ-AVI (n=13) Monotherapy: 62% | Carbapenem + colistin (n=30); carbapenem + aminoglycoside(n=25); Others (n=41) | clinical success, 90-day survival | AKI | - |
| Shields (2018) [52] | Single center, retrospective | Patients with CRE infections Types of infection: Pneumonia (43%), bacteremia (26%), urinary tract infection (10%), intra- abdominal (9%), skin/soft tissue (8%), and mediastinitis, subdural empyema/ ventriculitis and purulent tracheobronchitis (1% each) | 75% KPC- producing Enterobacterales | CAZ-AVI (n=77) Monotherapy: 69% | - | Microbiologic failure, clinical success, 30 and 90-day survival | AKI | Development of CAZ-AVI resistance in CAZ- AVI-treated patients |
| Sousa (2018) [53] | Single center, prospective | Patients with infections caused by OXA-48-producing Enterobacterales Types of infection: Intra-abdominal (28%), pulmonary (26%), urinary (25%), Others (10%) Severe infection (54%) | OXA-48- producing Enterobacterales | CAZ-AVI (n=57) Monotherapy: 81% | - | Microbiological cure, clinical cure, 14 and 30-day mortality, 90-day infection recurrence | Treatment-related AEs | Development of CAZ-AVI resistance in CAZ- AVI-treated patients |
| Mortality, CRP level, FEV1% | Single center, retrospective | Patients with cystic fibrosis with infections due to MDR-GN organisms Types of infection: Pulmonary infection (7 pts) and systemic infection (cepacia syndrome) (1 pt) Patients had moderate-to-severe lung disease | MDR-GN organisms including MDR- PA and MDR- Burkholderia spp. | CAZ-AVI (n=8) Monotherapy: 0% | - | Treatment-related AEs | ||
| Temkin (2017) [55] | Multicenter, retrospective, case series | Patients with infections caused by CR GN organisms Types of infection: Bacteremia (68%), IAI (39%), pneumonia (18%), SSTI (11%), UTI (11%), osteomyelitis (8%), endocarditis (5%), surgical site infection (5%), others (8%) Life-threatening infection (61%) | CRE including KPC-, OXA- 48-producing Enterobacterales and CRPa | CAZ-AVI (n=38) Monotherapy: 34% | - | Microbiological cure, clinical cure, survival to hospital discharge | Treatment-related AEs | - |
| Tsolaki (2020) [56] | Multicenter, retrospective | Critically ill, mechanically ventilated patients with mixed infections caused by CRE [Mixed infection types; subgroup: bacteremia] | CRE (94% KPC-producing Enterobacterales) | CAZ-AVI (n=41) Monotherapy: 22% Types of infection: Bacteremia (54%), VAP (46%), IAI (10%), UTI (5%), CNSI (2%) | BAT (n=36) (86% included colistin among others) Monotherapy: 3% Types of infection: Bacteremia (78%), VAP (19%), IAI (11%), UTI (3%), CNSI (3%) | 10-day microbiological cure, clinical cure, 28-day survival, relapse, Sequential Organ Failure Assessment (SOFA) score on days 4 and 10, | Liver and renal function and coagulation tests | Development of resistance in patients with relapse |
| Tumbarello (2019) [57] | Multicenter, retrospective | Patients with KPC- Kp infections [Mixed infection types; subgroup: bacteremia] | KPC-Kp | CAZ-AVI (n=104) Monotherapy: 21% | Other agents (n=104) Monotherapy: 26% | 30-day mortality, infection relapse | - | - |
| Van Duin (2018) [58] | Multicenter, prospective | Patients with infections caused by KPC–producing Enterobacterales Types of infection (all): Bacteremia (46%), pneumonia (22%), UTI (14%), wound (10%), and others (8%) | KPC-producing Enterobacterales | CAZ-AVI (n=38) Monotherapy: 37% Types of infection: Bacteremia (39%), pneumonia (24%), UTI (16%), wound (16%), and others (5%) | Colistin (n=99) (~60% included tigecycline and/or carbapenem among others) Monotherapy: 6% Types of infection: Bacteremia (48%), pneumonia (21%), UTI (13%), wound (8%), and others (9%) | 30-day adjusted all-cause-hospital mortality; 30-day disposition | - | - |
| Vena (2020) [59] | Multicenter, retrospective | Patients with infections caused by MDR-GNB other than CRE Types of infection: Nosocomial pneumonia (49%), bacteremia (17%), IAI (10%), bone infection (10%), acute bacterial skin and skin structure infection (5%), and other infections (10%) | MDR-GNB other than CRE (89% MDR-PA) | CAZ-AVI (n=37) Monotherapy: 20% | - | Clinical cure, 5-day infection recurrence | MDR-GNB other than CRE (89% MDR-PA) | Development of CAZ-AVI resistance in CAZ- AVI-treated patients |
Abbreviations: AKI: Acute Kidney Injury; BAT: Best Available Therapy; BSI: Blood Stream Infections; CRE: Carbapenem-Resistant Enterobacterales; CR-Kp: Carbapenem-Resistant Klebsiella Pneumonia; CR-PA: Carbapenem-Resistant Pseudomonas Aeruginosa; CAZ-AVI : Ceftazidime-avibactam ; ESBL: Extended- Spectrum ?-Lactamase; XDR:Extensively Drug-Resistant; GNB: Gram-Negative Bacilli; KPC:Klebsiella Pneumoniae Carbapenemase; KPC-Kp: Klebsiella Pneumoniae Carbapenemase-Producing Klebsiella Pneumoniae; MDR-GN: Multidrug-Resistant Gram-Negative; MDR-GNI: Multidrug-Resistant Gram-Negative Infection; MDR-PA: Multidrug-Resistant Pseudomonas Aeruginosa; PA: Pseudomonas Aeruginosa; OXA: Oxacillinase; UTI: Urinary Tract Infection; VAP: Ventilator-Associated Pneumonia; IAI: Intra-Abdominal Infection; CNSI: Central Nervous System Infection; SSTI: Skin-Soft Tissue Infection; cIAI: Complicated Intra-Abdominal Infection, cUTI: Chronic Urinary Tract Infection; ICU: Intensive Care Unit.
Table 1: Characteristics of real-world studies on CAZ-AVI.
All 28 studies described the effects of CAZ-AVI treatment on clinical outcomes and the key findings are summarized according to the type of target pathogens in Table 2 [32-59]. More than half of the studies reported safety of CAZ-AVI treatment and the results are presented in Table 3 [32,38,39,41,43,46,49-56,59]. About one-third of the studies reported findings on development of resistance to CAZ- AVI treatment [32,42,43,49,50,52,53,56,59] and the relevant results are shown in Table 2.
| Reference (Year) | Study population/ Types of Infection | Target pathogens | Treatment(s) | Safety outcomes | Definitions | |
|---|---|---|---|---|---|---|
| King (2017) [46] | Severely ill patients with CRE infection Types of infection: Bacteremia (38%), UTI (28%), pneumonia (27%), wound (13%), IAI (7%), and bone/ joint (3%) | CRE | CAZ-AVI (n=60) Monotherapy: 55% | - | No treatment-related AEs reported | |
| Spoletini (2019) [54] | Patients with cystic fibrosis with infections due to MDR-GN organisms not responding to standard of care antibiotic treatment Types of infection: Pulmonary infection (7 pts) and systemic infection (cepacia syndrome) (1 pt) Patients had moderate-to-severe lung disease | MDR-GN organisms including MDR-PA and MDR-Burkholderia spp. | CAZ-AVI (n=8) | - | No episodes of AKI or elevation in transaminase were observed. Mouth dryness: 1 pt No other AEs were observed | |
| Chen (2020) [38] | Lung transplant patients with XDR- GNB infections Types of infection: Pneumonia and/ or tracheobronchitis (90%), cholecystitis and bacteremia (10%) | XDR GNB (90% KPC-Kp) | CAZ-AVI (n=10) Monotherapy: 20% | - |
|
|
| De la Calle (2019) [39] | Patients with infections caused by CRE Types of infection: Bacteremia (33%), IAI (29%), UTI (25%), pneumonia (21%); osteoarticular/SSSTI (17%), device-related meningitis (4%), and catheter-related bacteremia (4%) | CRE (96% OXA- 48-producing Enterobacterales and 96% ESBL Enterobacterales) | CAZ-AVI (n=23) Monotherapy: 58% | - | AE: 17%
|
|
| Guimarães (2019) [41] | Patients with infections caused by KPC-producing Enterobacterales coresistant to carbapenems and polymyxins Types of infection: Bacteremia (41%), UTI (28.%), IAI (14%), nosocomial pneumonia (10%), and complicated SSTI (7%) | KPC-producing Enterobacterales | CAZ-AVI (n=29) Monotherapy: 52% | - | AEs: 14%
|
|
| Jorgensen (2019) [43] | Patients with MDR-GN infections Types of infection: Respiratory tract (37%), UTI (20%), IAI (19.7%), bacteremia (11%), SSTI (9%), and osteoarticular (7%) | MDR-GN organisms (58% CRE and 31% Pseudomonas spp.) | CAZ-AVI (n=203) Monotherapy: 67% | - | AE: 8%
|
Acute kidney injury (AKI) was evaluated in patients not receiving hemodialysis at the time of CAZ initiation and was defined as a serum creatinine increase of ≥ 0.5 mg/dL or 50% from baseline on 2 consecutive measurements while on CAZ and up to 72 hours after the last dose. |
| Santevecchi (2018) [49] | Patients with infections due to MDR-organisms other than Kp Types of infection: Pneumonia (46%), skin and soft tissue (23%), bacteremia (15%), and intra–abdominal (15%) | MDR-organisms other than Kp (most common: MDR-PA) | CAZ-AVI (n=10) Monotherapy: 50% | - | No treatment-related AEs were reported. | |
| Shield (2016) [50] | Patients with CRE Types of infection: Pneumonia (32%), bacteremia (27%), IAI (11%), SSTI (11%), pyelonephritis (11%), mediastinitis (3%), subdural empyema/ Ventriculitis (3%) and purulent tracheobronchitis (3%) | CRE (78% KPC-producing Enterobacterales) | CAZ-AVI (n=37) Monotherapy: 70% | - | AKI: 3/31 (10%) 1 of 3 (33%) who developed AKI received concomitant colistin)
|
AKI within 7 days of treatment initiation (defined by 1.5X increase in serum creatinine from baseline) Leukopenia (absolute neutrophil count=90 × 109/L) |
| Shields (2018)[52] | Patients with CRE infections [Mixed infection types] | CRE (75% KPC-producing Enterobacterales) | CAZ-AVI (n=61) | - | AKI: 7/61 (11%) Among the 7 patients who developed AKI, 1 (14%) and 2 (29%) pts received concomitant colistin and aminoglycosides, respectively) | AKI; defined by modified KDIGO guidelines as a 1.5X increase in serum creatinine levels from baseline within 7 days of treatment initiation |
| Sousa (2018)[53] | Patients with infections caused by OXA-48- producing Kp Types of infection: Intra-abdominal (28%), pulmonary (26%), urinary (25%), Others (10%) Severe infection (54%) | OXA-48-producing Kp | CAZ-AVI (n=57) Monotherapy: 81% | - | AKI: 2/57 (4%) 1 of 2 patients [50%] who developed AKI was on concomitant IV colistin No other treatment- related AEs were observed. | |
| Temkin (2017) [55] | Patients with infections caused by CR GN organisms Types of infection: Bacteremia (68%), IAI (39%), pneumonia (18%), SSTI (11%), UTI (11%), osteomyelitis (8%), endocarditis (5%), surgical site infection (5%), others (8%) Life-threatening infection (61%) | CR GN organims including KPC-, OXA-48-producing Enterobacterales and CR-PA | CAZ-AVI (n=38) Monotherapy: 34% | - | AE: 16%
|
|
| Vena (2020) [59] | Patients with infections caused by MDR-GNB other than CRE Types of infection: Nosocomial pneumonia (49%), bacteremia (17%), IAI (10%), bone infection (10%), acute bacterial skin and skin structure infection (5%), and other infections (10%) | MDR-GNB other than CRE (89% MDR-PA) | CAZ-AVI (n=37) Monotherapy: 20% | - |
|
|
| Tsolaki (2020) [56] | Critically ill, mechanically ventilated patients with mixed infections caused by CRE [Mixed infection types] | CRE (94% KPC-producing Enterobacterales) | CAZ-AVI (n=41) Monotherapy: 22% Types of infection: Bacteremia (54%), VAP (46%), IAI (10%), UTI (5%), CNSI (2%) | BAT (n=36) (86% included colistin among others) Monotherapy: 3% Types of infection: Bacteremia (78%), VAP (19%), IAI (11%), UTI (3%), CNSI (3%) |
|
|
| Shields (2017)[51] | Patients with CR-Kp bacteremia [Bacteremia] | CR-Kp (97% KPC-Kp) | CAZ-AVI (n=11) Monotherapy: 64% The remaining (4/11; 36%) received combination with aminoglycoside | Carbapenem + colistin (n=23) | ⴕEOT AKI: 2/11 (18%) vs. 13/23 (57%) 1 of 2 (50%) patients who developed AKI received CAZ-AVI with aminoglycosides | Acute kidney injury was defined by KDIGO criteria as a 1.5X increase in serum creatinine from baseline at the end of treatment. |
| Carbapenem + aminoglycoside (n=18) | ⴕEOT AKI: 2/11 (18%) vs. 8/18 (44%) 1 of 2 (50%) patients who developed AKI received CAZ-AVI with aminoglycosides | |||||
| Others (n=33) | ⴕEOT AKI: 2/11 (18%) vs. 6/33 (18%) 1 of 2 (50%) patients who developed AKI received CAZ-AVI with aminoglycosides | |||||
| Ackley (2020) [32] | Patients with infections caused by KPC-producing Enterobacterales (excluded those with localized urinary tract infection and repeat study drug exposures after the first episode) [Mixed infection types] | KPC-producing CRE | CAZ-AVI (n=105) Monotherapy: 39% Types of infection: Bacteremia (42%), respiratory (29%), soft tissue (17%), IAI (11%), and others (1%) | Meropenem- Vaborbactam (n=26) Monotherapy: 85% Types of infection: Bacteremia (35%), respiratory (38%), Soft tissue (8%), IAI (19%), and others (0%) | AE: 34% vs 23% ⴕNephrotoxicity (most frequent AE): 26/89 (29%) vs. 3/21 (14%) Leukopenia: 11% vs. 8% Rash: 4% vs. 4% Neurotoxicity: 1% vs. 0 Among the 26 pts who experienced nephrotoxicity in the CAZ-AVI group, 16 (62%) received combination therapy: 23% received colistin, 15% polymyxin B, 15% tigecycline, 12% fluoroquinolone, and 4% aminoglycoside. In the meropenem- vaborbactam group, one of three (33%)pts who had nephrotoxicity received combination therapy with colistin, the remaining two patients received monotherapy. | Nephrotoxicity was defined using the Acute Kidney Injury Network (AKIN) classification and/ or the initiation of RRT while receiving treatment. Leukopenia=white blood cell count of <4,000 cells/mm3. |
Abbreviations: AKI:Acute Kidney Injury; ALP:Alkaline Phosphatase; ALT:Alanine Aminotransferase; AST:Aspartate Aminotransferase; BAT:Best Available Therapy; BSI:Blood Stream Infections CRE Carbapenem-Resistant Enterobacterales; CR-Kp:Carbapenem-Resistant Klebsiella Pneumoniae; CR-PA:Carbapenem-Resistant Pseudomonas Aeruginosa; CAZ-AVI:Ceftazidime-avibactam ; ESBL:Extended-Spectrum Β-Lactamase, XDR:Extensively Drug-Resistant; GGT:Γ-Glutamyltranspeptadase; GNB:Gram-Negative Bacilli; KPC:Klebsiella Pneumoniae Carbapenemase; KPC-Kp:Klebsiella Pneumoniae Carbapenemase-Producing Klebsiella Pneumoniae; MDR-GN:Multidrug-Resistant Gram-Negative; MDR-GNI:Multidrug-Resistant Gram-Negative Infection; MDR-PA:Multidrug-Resistant Pseudomonas Aeruginosa; PA:Pseudomonas Aeruginosa; OXA:Oxacillinase; RRT:Renal Replacement Therapy; TBIL:Total Bilirubin.
Fifteen studies were single-center studies and the remaining 13 were multi-center studies (Table 1). Twenty-five studies were retrospective and only three were prospective studies. About two-thirds of the studies focused on only CAZ-AVI treatment whereas the remaining one-third also included a comparison group of patients treated with other antimicrobial agents. Six had included more than 100 patients whereas the remaining 22 studies had smaller sample sizes of 5-77 patients. Fourteen studies included severely ill or critically ill patients who were in intensive care unit (ICU), required mechanical ventilation, or had cancer, cystic fibrosis or lung transplant, and had serious or severe infections (predominantly bacteremia or those from respiratory sources) [33,37,38,40-42, 45,46,51,53-57].
Effectiveness of CAZ-AVI for GNB infections with limited treatment options
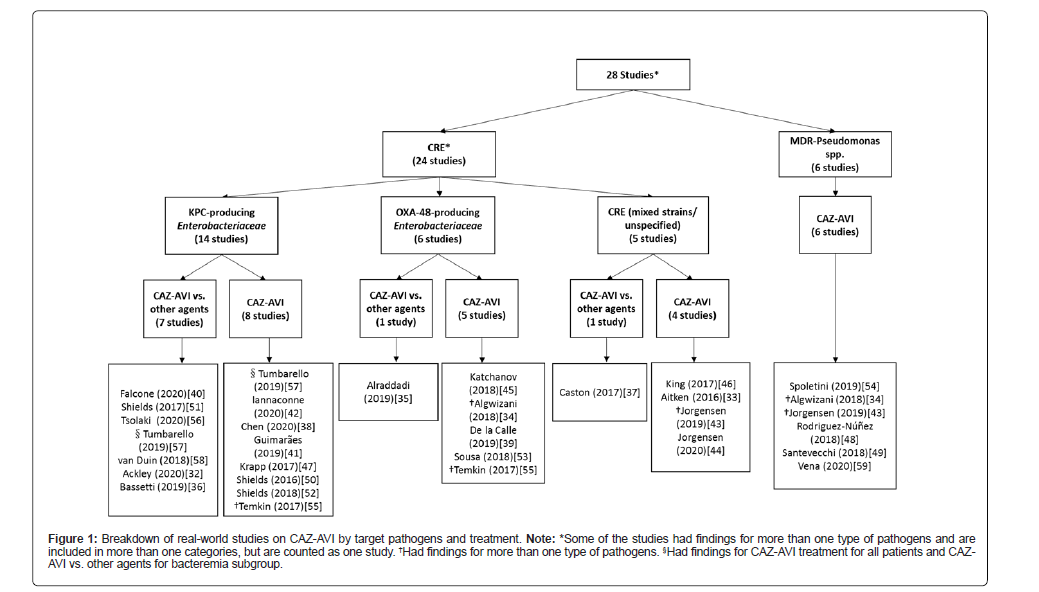
Findings on the effects of CAZ-AVI treatment on key clinical outcomes are presented according to the target pathogens-CRE and MDR- PA in Table 2 [32-59]. Figure 1 shows the breakdown of the included studies by target pathogens and treatment [32-59]. Twenty-four studies described the effects of CAZ-AVI in treating infections due to CRE and six studies in infections due to MDR-PA. Key outcomes reported included microbiological cure or failure, clinical cure/success or failure, mortality or survival, and infection recurrence/relapse among others. The outcomes were reported heterogeneously across studies. For instance, mortality was defined as 14-, 28-, 30-, or 90-day mortality, in-hospital mortality, all-cause mortality, etc. or not specified. Outcome definitions used in the studies (where available) are included in Table 2. Therefore, caution should be exercised when interpreting the results.
| Reference (Year) | Study population/ Types of infection | Treatment(s) | Effectiveness outcomes | Other relevant outcomes | Definitions | |
|---|---|---|---|---|---|---|
| Target pathogen: CRE (24 studies)* | ||||||
| Target pathogen: CRE subtype: Kp carbapenemase (KPC)-producing Enterobacterales | ||||||
| Treatment: CAZ-AVI vs. other agents | ||||||
| Types of infection: bacteremia or mixed infection types (predominantly bacteremia, VAP or cIAIs) | ||||||
| §Tumbarello (2019) [57] | Patients with infections caused by KPC-Kp [Bacteremia] | CAZ-AVI (n=104) Monotherapy: 21% | Others (n=104) Monotherapy: 26% | 30-day mortality: 37% vs. 56% Infection relapse: 10% vs 9% | Relapse was defined as the onset, during the index hospitalization, of a second microbiologically documented KPC- Kp infection in a patient whose original infection had been classified as a clinical cure (with or without microbiological confirmation). | |
| Tsolaki (2020) [56] | Critically ill, mechanically ventilated patients with mixed infections caused by CRE (94% KPC-producing Enterobacterales) [Mixed infection types] | CAZ-AVI (n=41) Monotherapy: 22% Types of infection: Bacteremia (54%), VAP (46%), IAI (10%), UTI (5%), CNSI (2%) | BAT (n=36) (86% included colistin among others)) Monotherapy: 3% Types of infection: Bacteremia (78%), VAP (19%), IAI (11%), UTI (3%), CNSI (3%) | 10-day microbiological cure: 94% vs. 68% Clinical cure: 81% vs. 53% 28-day survival: 85% vs. 61% Relapse: 5% vs. 33% | Development of resistance in patients with relapse: 0/2 (0%)=10/12 (83%)* *developed resistance to colistin, 5 pts received monotherapy with colistin | Interpretation of the susceptibility results was based on EUCAST criteria. |
| Critically ill, mechanically ventilated patients with bacteremia caused by CRE (94% KPC-producing Enterobacterales) Bacteremia | CAZ-AVI (n=22) Monotherapy: 32% | Best available therapy (n=28) Monotherapy: 4% | 10-day microbiological cure: 100% vs. 74% Clinical cure: 82% vs. 54% 28-day survival: 82% vs. 57% Relapse rate: 0% vs. 21% | Development of resistance in patients with relapse: 0/0 (0%)=5/6 (83%)* *developed resistance to colistin | ||
| Bassetti (2019) [36] | Patients with KPC-Kp gut colonization | CAZ-AVI (n=12) Monotherapy: 8% Types of infection: cIAI (42%), HAP (17%), sepsis (17%), surgical wound infection (8%), and others (25%) | Other regimens (n=24) (included 83% tigecycline, 46% colistin, and/or 54% carbapenem among others) Monotherapy: 0% Types of infection: cIAI (17%), HAP (21%), sepsis (42%), surgical wound infection (17%), and others (4%) | Gut decolonization: 92% vs. 0%. | ||
| Treatment: CAZ-AVI vs. other specific agents | ||||||
| Types of infection: bacteremia or mixed infection types (predominantly bacteremia or pneumonia) | ||||||
| Van Duin (2018) [58] | Patients with infections caused by KPC–producing Enterobacterales Types of infection (all): Bacteremia (46%), pneumonia (22%), UTI (14%), wound (10%), and others (8%) | CAZ-AVI (n=38) Monotherapy: 37% Types of infection: Bacteremia (39%), pneumonia (24%), UTI (16%), wound (16%), and others (5%) | Colistin (n=99) (~60% included tigecycline and/or carbapenem among others) Monotherapy: 6% Types of infection: Bacteremia (48%), pneumonia (21%), UTI (13%), wound (8%), and others (9%) | 30-day adjusted all-cause-hospital mortality: 9% vs. 32% | ||
| Falcone (2020) [40] | Patients with BSI due to KPC-Kp hospitalized in ICU (excluded those with polymicrobial BSIs) Bacteremia | CAZ-AVI-containing regimens (n=13) | Colistin-containing regimen (n=61) | Composite endpoint (30-day mortality or nephrotoxicity): 23%vs.69% 30-day mortality: 23%vs.44% | Composite endpoint of mortality or nephrotoxicity (postbaseline increase in serum creatinine > 1.0 mg/dL or adverse events preferred term of renal failure, renal failure acute, or renal impairment). | |
| Other regimens (n = 17) | Composite endpoint (30-day mortality or nephrotoxicity): 23% vs. 47% 30-day mortality: 23% vs. 41% | |||||
| Shields (2017) [51] | Patients with CR-Kp bacteremia (97% are KPC-Kp) [Bacteremia] | CAZ-AVI (n=13) Monotherapy: 62% | Carbapenem + colistin (n=30) | Clinical success: 85% vs. 40% 90-day survival: 92% vs. 63% | Clinical success was defined at 30 days as survival, resolution of signs and symptoms of infection, sterilization of blood cultures within 7 days of treatment initiation, and absence of recurrent infections. | |
| Carbapenem + aminoglycoside (n=25) | Clinical success: 85% vs. 48% 90-day survival: 92% vs. 56% | |||||
| Others (n=41) | Clinical success: 85% vs. 37% 90-day survival: 92% vs. 49% | |||||
| Ackley (2020) [32] | Patients with infections caused by KPC-producing Enterobacterales (excluded those with localized urinary tract infection and repeat study drug exposures after the first episode) [Mixed infection types] | CAZ-AVI (n=105) Monotherapy: 39% Types of infection: Bacteremia (42%), respiratory (29%), soft tissue (17%), IAI (11%), and others (1%) | Meropenem- vaborbactam (n=26) Monotherapy: 85% Types of infection: Bacteremia (35%), respiratory (38%), Soft tissue (8%), IAI (19%), and others (0%) | Clinical success: 62% vs. 69% 30-day mortality: 19% vs. 12% 90-day mortality: 29% vs. 27% 90-day infection recurrence: 14% vs. 12% | Development of resistance in patients with recurrent infection: 3/15 (20%)* vs. 0/3 (0%) *all on CAZ-AVI monotherapy and had respiratory infection (and received RRT) | Clinical success was defined as survival at 30 days, resolution of signs and symptoms of infection, sterilization of blood cultures within 7 days of treatment initiation. Recurrent infections were defined as the same organism at the same site within 90 days of the index infection. Development of resistance per FDA- approved breakpoints for CAZ-AVI and MVB were evaluated in patients with recurrent infection. |
| Treatment: CAZ-AVI | ||||||
| Types of infection: bacteremia | ||||||
| Iannaccone (2020) [42] | Patients with BSI caused by KPC-Kp Bacteremia | CAZ-A (n=23) Monotherapy: 13% | - | Recovered from infection: 74% In-hospital mortality: 26% Recurrent infection: 17% | Development of CAZ- AVI resistance in CAZ- AVI-treated patients: 2/23 (9%)* *Both were on combination therapy with other resistant antimicrobials | |
| Types of infection: mixed infection types (predominantly bacteremia) | ||||||
| §Tumbarello (2019) [57] | Patients with infections caused by KPC-Kp Types of infection: Bacteremia (75%), lower respiratory tract infections (9%), abdominal infections (9%), UTI (4%), others (2%) | CAZ-AVI (n=138) Monotherapy: 21% | - | 30-day mortality: 34% Infection relapse: 9% 30-day mortality by infection types Bacteremia: 37% Lower respiratory tract infections: 30% Abdominal infections: 25% UTI: 17% Others: 33%) | Relapse was defined as the onset, during the index hospitalization, of a second microbiologically documented KPC- Kp infection in a patient whose original infection had been classified as a clinical cure (with or without microbiological confirmation). | |
| †Temkin (2017) [55] | Patients with infections caused by KPC-producing Enterobacterales [Mixed infection types] | CAZ-AVI (n=23) | - | Microbiological cure: 78% Clinical cure: 74% Survival to hospital discharge: 74% | ||
| Guimarães (2019) [41] | Patients with infections caused KPC-producing Enterobacterales coresistant to carbapenems and polymyxins Types of infection: Bacteremia (41%), UTI (28%), IAI (14%), nosocomial pneumonia (10%), and complicated SSTI (7%) | CAZ-AVI (n=29) Monotherapy: 52% | - | Clinical success: 83% 14-day all-cause mortality: 31% 30-day all-cause mortality: 52% Outcomes by infection types
|
Clinical success was classified as improved signs and symptoms from baseline to the end of therapy with defervescence based on information entered in the medical records. Microbiological cure was classified as a negative culture at the same site as basal culture after treatment. | |
| Types of infection: mixed infection types (predominantly from respiratory sources) | ||||||
| Chen (2020) [38] | Lung transplant patients with XDR- GNB infections (90% KPC-Kp) Types of infection: Pneumonia and/ or tracheobronchitis (90%), cholecystitis and bacteremia (10%) | CAZ-AVI (n=10) Monotherapy: 20% | - | Microbiological cure: 90% 30-day survival:100% 90-day survival: 90% Infection relapse: 50% | Relapse was defined as the onset of a second microbiologically documented XDR- GNB infection in a patient whose original infection had been classified as a clinical cure (with or without microbiological confirmation). | |
| Krapp (2017) [47] | Patients with infections caused by KPC-Kp Types of infection: Pneumonia (2 pts), IAI (1 pt), peritonitis (1 pt), perinephric abscess (1 pt), and wound (1pt) | CAZ-AVI (n=6) Monotherapy: 33% | - | Clinical cure: 83% Infection relapse: 33% (among those who achieved clinical cure) | Clinical cure was defined as symptom resolution or significant improvement at completion of antibiotic treatment. | |
| Types of infection: mixed infection types (predominantly bacteremia and pneumonia) | ||||||
| Shields (2018) [52] | Patients with CRE infections (75% KPC-producing Enterobacterales) [Mixed infection types] Types of infection: Pneumonia (43%), bacteremia (26%), urinary tract infection (10%), intra-abdominal (9%), skin/soft tissue (8%), and mediastinitis, subdural empyema/ventriculitis and purulent tracheobronchitis (1% each) | CAZ-AVI (n=77) Monotherapy: 69% | - | Microbiologic failure: 32% Clinical success: 55% 30-day survival: 81% 90-day survival: 69% 90-day infection recurrence: 17% (among those who received clinical success) Clinical success rates by infection types Urinary tract: 88% Bacteremia: 75% Skin/soft tissue: 67% Intra-abdominal: 43% Pneumonia: 36% Others: 33% | Development of CAZ- AVI resistance in CAZ- AVI-treated patients: 8/77 (10%)* *6 on monotherapy, 7 had pneumonia, 1 had intra-abdominal infection. Resistant isolates carried mutant blaKPC- encoding variant KPC-3 enzymes. Receipt of RRT was an independent predictor of the development of CAZ-AVI resistance. | Microbiologic failure was defined as isolation of CRE following ≥7 days of ceftazidime-avibactam treatment. Clinical success was defined as survival and absence of recurrence at 30 days following the onset of infection, resolution of signs and symptoms of infection, and sterilization of site- specific cultures within 7 days of treatment initiation. Recurrences within 90 days of onset were defined by microbiologic failure and concomitant signs of infection. Ceftazidime-avibactam resistance (MIC > 8 mg/L) |
| Shield (2016) [50] | Patients with infections due to CRE (78% KPC-producing Enterobacterales) Types of infection: Pneumonia (32%), bacteremia (27%), IAI (11%), SSTI (11%), pyelonephritis (11%), mediastinitis (3%), subdural empyema/ Ventriculitis (3%) and purulent tracheobronchitis (3%) | CAZ-AV (n=37) Monotherapy: 70% | - | Microbiologic failure: 27% 30-day clinical success: 59% 30-day survival: 76% 90-day infection recurrence: 23% (among those who achieved clincial success) Clinical success rates by infection types Pyelonephritis: 100% Bacteremia: 70% Pneumonia: 50% Skin/soft tissue: 50% Intra-abdominal: 50% Others: 33% | Development of CAZ- AVI resistance in CAZ- AVI-treated patients: 3/37 (8%)* *all on monotherapy, 2 had pneumonia, 1 had intra-abdominal infection. | Microbiologic failure was defined as isolation of CRE following ≥7 days of ceftazidime-avibactam treatment. Clinical success was defined as survival and absence of recurrence at 30 days following the onset of infection, resolution of signs and symptoms of infection, and sterilization of site- specific cultures within 7 days of treatment initiation. Recurrences within 90 days of onset were defined by microbiologic failure and concomitant signs of infection. Ceftazidime-avibactam resistance (MIC > 8 mg/L) |
| Target pathogen: CRE subtype: OXA-48-producing Enterobacterales | ||||||
| Treatment: CAZ-AVI vs. others | ||||||
| Types of infection: mixed infection types (predominantly bacteremia and HAP) | ||||||
| Alraddadi (2019) [35] | Patients with established CRE infections (74% OXA-48- producing CRE) | CAZ-AVI (n=10) Types of infection: Bacteremia (70%), HAP (50%), cUTI (30%), cIAI (30%), SSTI (20%) | Other agents (n=28) (mainly colistin and/ or carbapenem among others) Monotherapy: 11% Types of infection: Bacteremia (54%), HAP (50%), cUTI (29%), cIAI (18%), SSTI (11%) | Clinical remission: 80% vs. 54% 30-day all-cause mortality: 50% vs. 57% | Complete remission is defined as resolution of fever and eradication of bacteria in subsequent cultures. | |
| Target pathogen: CRE subtype: OXA-48-producing Enterobacterales | ||||||
| Treatment: CAZ-AVI | ||||||
| Types of infection: mixed infection types | ||||||
| Sousa (2018) [53] | Patients with infections caused by OXA-48-producing Enterobacterales Types of infection: Intra-abdominal (28%), pulmonary (26%), urinary (25%), Others (10%), Severe infection (54%) | CAZ-AVI (n=57) Monotherapy: 81% | - | Microbiological cure: 65% Clinical cure: 77% 14-day all-cause mortality: 14% 30-day all-cause mortality: 22% 90-day infection recurrence: 10% | Development of CAZ- AVI resistance in CAZ- AVI-treated patients: 0/57 (0%) | Clinical cure was defined as resolution of signs and symptoms of infection (assessed according to vital signs, the course of the SOFA score and laboratory data) within 7 days of treatment initiation. Microbiological cure was defined as sterilization of site- specific cultures and/ or blood cultures after treatment ending and/ or within 7 days after treatment initiation. Recurrence within 90 days of onset was defined as microbiological failure and concomitant signs of infection. Microbiological failure was defined as isolation of CPE from a sample obtained from the same source of infection and/or blood cultures following ≥7 days of ceftazidime/ avibactam treatment initiation. A disc diffusion zone diameter of <=21 mm was interpreted as resistance (equivalent to MIC>8/4 mg/L for ceftazidime/ avibactam). |
| De la Calle (2019) [39] | Patients with infections caused by CRE (96% OXA-48-producing Enterobacterales) Types of infection: Bacteremia (33%), IAI (29%), UTI (25%), pneumonia (21%); osteoarticular/SSSTI (17%), device-related meningitis (4%), and catheter-related bacteremia (4%) | CAZ-AVI (n=23) Monotherapy: 58% | - | 30-day clinical cure: 63% 30-day mortality: 8% 90-day mortality: 21% 90-day infection recurrence: 35% Outcomes by infection types
|
Clinical cure was defined as the survival, resolution of symptoms and signs of infection, and absence of recurrence within 30 days following the onset of treatment with ceftazidime-avibactam , with negative infection site cultures in those patients in whom control samples were obtained. Recurrence of infection was defined as the appearance of signs and symptoms of infection in the same or different location with positive cultures for OXA-48 CPE within 90 days of the end of treatment with ceftazidime-avibactam | |
| †Algwizani (2018) [34] | Patients with infections caused by OXA-48- producing Kp Types of infection: bacteremia (1 pts), CNSI (1 pt) | CAZ-AVI (n=2) Monotherapy: 0% | - | Microbiological cure: 100% Clinical cure: 100% | ||
| Types of infection: mixed infection types (predominantly pneumonia or bacteremia) | ||||||
| Katchanov (2018) [45] | Critically ill patients with severe infections due to OXA-48- producing Kp Types of infection: HAP (4 pts), bacteremia (1 pt), and cIAI (1 pt) | CAZ-AVI (n=5) Monotherapy: 0% | - | In-hospital mortality: 100% | ||
| †Temkin (2017) [55] | Patients with infections caused by OXA-48-producing Enterobacterales [Mixed infection types] | CAZ-AVI (n=13) | - | Microbiological cure: 46% Clinical cure: 62% Survival to hospital discharge: 38% | ||
| CRE (mixed strains/mechanisms of carbapenem resistance not specified) | ||||||
| Treatment: CAZ-AVI vs. other agents | ||||||
| Types of infection: bacteremia | ||||||
| Caston (2017) [37] | Patients with hematologic malignancies who had CPE bacteremia Bacteremia | CAZ-AVI (n=8) Monotherapy: 0% | Other agents (n=23) Monotherapy: 6% | 14-day clinical cure: 86% vs. 35% 30-day crude mortality: 25% vs. 52% | i) crude mortality at 30 days from the day the blood cultures were taken, and ii) clinical cure (resolution of all signs and symptoms of infection) at 14 days after the onset of antibiotic treatment. | |
| Treatment: CAZ-AVI | ||||||
| Types of infection: mixed infection types (most common – infections from respiratory sources) | ||||||
| †Jorgensen (2019) [43] | Patients with MDR- GNIs caused by CRE Types of infection: Respiratory tract (33%), UTI (20%), IAI (22%), bacteremia (9%), SSTI (9%), and osteoarticular (6%)<CRE; no mention of specific CRE genes> | CAZ-AVI (n=117) Monotherapy: 62% | Composite clinical failure: 29% 30-day mortality: 16% 30-day recurrence: 6% | Development of CAZ-AVI resistance during treatment in patients with repeat susceptibility testing: 0% | Composite clinical failure was defined as a composite of all- cause 30-day mortality, microbiological failure, and/or failure to resolve or improve signs and symptoms of infections during CAZ therapy. | |
| Jorgensen (2020) [44] | Patients with CRE infections Types of infection: Respiratory tract (35%), IAI (21%), UTI (20%), SSTI (6%), osteoarticular 7 (6%), bacteremia (6%), and others (5%) | CAZ-AVI (n=109) Monotherapy: 60% | - | 30-day all-cause mortality: 17% 30-day all-cause mortality by infection types: Pneumonia: 24% IAI: 9% UTI: 5% | 30-day all-cause mortality, measured from infection onset. | |
| Types of infection: mixed infection types (predominantly bacteremia and pneumonia) | ||||||
| King (2017)[46] | Severely ill patients with CRE infection Types of infection: Bacteremia (38%), UTI (28%), pneumonia (27%), wound (13%), IAI (7%), and bone/ joint (3%) | CAZ-AVI (n=60) Monotherapy: 55% | - | Microbiological cure: 53% Clinical success: 65% In-hospital mortality:32% Outcomes by infection types
|
Microbiologic cure, defined as a negative culture at the end of therapy, and clinical success, defined as improved signs and symptoms from baseline to the end of therapy with defervescence. | |
| Types of infection: bacteremia | ||||||
| Aitken (2016) [33] | Cancer patients with CRE bloodstream infection Bacteremia | CAZ-AVI (n=5) Monotherapy: 0% | Microbiologic cure: 80% Clearance of infection: 100% Mortality: 40% | microbiologic cure (ie, failure to isolate the bacteria in subsequent blood cultures after at least one negative blood culture) | ||
| Target pathogen: MDR-Pseudomonas spp. (six studies)* | ||||||
| Treatment: CAV-AZI | ||||||
| Types of infection: mixed infection types (predominantly infection from respiratory sources) | ||||||
| †Jorgensen (2019) [43] | Patients with MDR- GNIs caused by Pseudomonas spp. Types of infection: Respiratory tract (60%), UTI (11%), IAI (5%), bacteremia (5%), SSTI (10%), and osteoarticular (10%) <MDR-Pseudomonas spp., data on mechanisms of resistance not available> | CAZ-AVI (n=63) Monotherapy: 68% | - | Composite clinical failure: 30% 30-day mortality: 18% 30-day recurrence: 6% | Development of CAZ-AVI resistance during treatment in patients with repeat susceptibility testing: 0% | Composite clinical failure was defined as a composite of all- cause 30-day mortality, microbiological failure, and/or failure to resolve or improve signs and symptoms of infections during CAZ therapy. |
| Vena (2020) [59] | Patients with infections due to MDR-PA (including strains resistant to carbapenem) Types of infection: Nosocomial pneumonia (55%), bacteremia (15%), IAI (6%), bone infection (9%), acute bacterial skin and skin structure infection (6%), and other infections (9%) | CAZ-AVI (n=33) | - | Clinical cure: 88% Recurrent infection: 3% | Development of CAZ- AVI resistance in CAZ- AVI-treated patients: 0/33 (0%) | Cure, patients had complete resolution of clinical signs and symptoms related to the infection and/or infection cleared with no positive cultures reported at the end of ceftazidime-avibactam therapy MIC values of ceftazidime-avibactam were determined by E-test (bioMérieux, Marcy l’Etoile, France) and interpreted according to the current European Committee on Antimicrobial Susceptibility Testing (EUCAST) clinical breakpoints [37]. EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 8.1, M.. 2018. Available online: http:// www.eucast.org/ clinical_breakpoints/ (accessed on 22 May 2019). |
| †Spoletini (2019)[54] | Patients with cystic fibrosis with infections due to MDR-PA not responding to standard of care antibiotic treatment Types of infection: Pulmonary infection (5 pts) and systemic infection (cepacia syndrome) (1 pt)<100% MDR-PA, 33% Burkholderia spp.> | CAZ-AVI (n=6) Monotherapy: 0% | - | Mortality: 17% | ||
| Santevecchi (2018) [49] | Patients with infections caused by MDR-PA. Types of infection: VAP (3 pts), SSTI (3 pts), and IAI (1 pt) <MDR-PA, data on mechanisms of resistance not available> | CAZ-AVI (n=6) Monotherapy: 33% | - | Microbiological cure: 100% Clinical success: 83% 30-day in-hospital mortality: 17% | Development of CAZ- AVI resistance in CAZ- AVI-treated patients: 1/6 (17%)* *patient had VAP, were on combination therapy, and had fluctuating renal function during treatment, requiring multiple dose adjustments throughout the course of therapy | Microbiological cure was defined as clearance of site- specific cultures following initiation of ceftazidime/avibactam. Clinical success was defined as resolution of all signs and symptoms of infection and survival at completion of ceftazidime/avibactam therapy. The FDA MIC breakpoint for ceftazidime/avibactam susceptibility for PA is ≤ 8/4 mg/L [13]. Ref: Avycaz (ceftazidime and avibactam). Irvine (CA): Allergan USA, Inc.; 2017. Package insert. |
| †Algwizani (2018) [34] | Patients with infections caused by CR-PA Types of infection: VAP (2 pts) and bacteremia (1 pt) | CAZ-AVI (n=3) Monotherapy: 100% | - | Microbiological cure: 100% Clinical cure: 100% | ||
| Rodríguez-Núñez (2018) [48] | Patients with infections due to MDR or XDR- PA (including 2 pt with CR MDR PA) Types of infection: Hospital-acquired lower respiratory tract infection (5 pts), osteomyelitis (1 pt), meningitis (1 pt) and catheter-related bacteremia (1 pt). | CAZ-AVI (n=8) Monotherapy: 25% | - | Clinical cure: 50% 30-day mortality: 13% 90-day mortality: 38% | Clinical cure was considered as survival, resolution of symptoms and signs of infection, and absence of relapse at 30 days following the end of treatment with CAZ/ AVI. | |
Table 2: Effectiveness of CAZ-AVI for GNB infections with limited treatment options.
CRE
A total of 14 studies described clinical experience with CAZ- AVI in treating infections predominantly caused by KPC-producing Enterobacterales and six studies in infections mainly due to OXA-48- producing Enterobacterales(Figure 1). Five studies described the experience in treating infections caused by a mixture of CRE strains (including KPC- , OXA-48-producing Enterobacterales, and/or New Delhi metallo-β- lactamase [NDM]-producing CRE) or CRE with unspecified mechanism of carbapenem resistance (Figure 1).
KPC-producing Enterobacterales
Fourteen studies involving a total of 924 patients described the effects of CAZ-AVI treatment against a variety of infections predominantly caused by KPC-producing Enterobacterales, including bacteremia, pneumonia, cIAIs, SSTIs, CNSIs, bone infections, and wound infections, etc., (Table 2). Six studies compared CAZ-AVI with other agents and seven studies focused only on CAZ-AVI treatment. Another study examined only CAZ-AVI treatment for the total study population and compared CAZ-AVI vs. other agents for the bacteremia subgroup (Figure 1). CAZ-AVI monotherapy was used in 52%-70% of patients in four studies whereas CAZ-AVI in combination with other antimicrobial agents was predominantly used in the remaining studies (Table 2).
Among studies that examined the effects of CAZ-AVI vs. standard of care antibiotics in treating a range of infections caused by KPC-producers (predominantly bacteremia, pneumonia, or cIAIs) [36,56,57], CAZ-AVI treatment exhibited more favorable outcomes compared with other agents. CAZ-AVI treatment was associated with higher microbiological cure (94%-100%), decolonization (92%) and clinical cure (81%-82%) rates than other agents (68%-74%, 0%, and 53%-54%, respectively). Higher survival rates (63%-85%) and lower infection relapse (0%-10%) rates were also noted in the CAZ-AVI group compared with the group containing other agents (44%-61% and 9%-33%, respectively) (Table 2). Notably, the better outcomes observed with CAZ-AVI treatment compared with other agents were noted even in critically ill patients requiring mechanical ventilation who were treated for a variety of infections, including those with bacteremia [56]. CAZ-AVI treatment was found to be associated with higher clinical success (85%), 30-day survival (77%-91%) and 90-day survival rates (92%) than colistin or aminoglycoside-containing regimens (40%-48%, 56%- 68%, and 56%-63%, respectively) [40,51,58] in patients infected by KPC- producers, including those with bacteremia hospitalized in ICU and those treated for a variety of infections (46% of whom had bacteremia) [40,58]. In another study comparing CAZ-AVI with meropenem-vaborbactam (another beta-lactam combination agent) in patients with infections due to KPC-producing Enterobacterales (71% of whom had bacteremia or pneumonia), similar rates of clinical success (62% vs. 69%), survival (90- day survival: 71% vs. 73%), and 90-day infection recurrence (14% vs. 12%) were noted in both treatment groups [32].
Among the eight studies describing clinical experience with CAZ- AVI in treating a variety of infections (predominantly bacteremia and/ or pneumonia), seven reported microbiological cure in 68%-90% of the patients, clinical success in 55%-83%, and 30-day survival rate of 66%- 100% and in-hospital survival rate of 74% in patients infected by KPC- producing Enterobacterales[38,42,47,50,52,55, 57] (Table 2). Although the remaining study on patients infected by KPC-producing Enterobacteralescoresistant to carbapenems and polymyxins reported a relatively lower 30- day survival rate (48%), a high rate of clinical success (83%) was observed even though patients were severely ill (59% at the ICU and 48% had moderate-to-severe renal impairment at the time of diagnosis), had serious infections (51% had bacteremia or pneumonia), and received CAZ-AVI on a compassionate basis [41]. Six of the studies assessed infection recurrence in patients infected by KPC producers [38,42,47,50,52,57] (Table 2). Of these, two studies reported relapse rate of 9%-17% [42,57]. Shield and colleagues reported a 90-day recurrence rate of 17%-24% among patients who achieved clinical success [50,52]. The median time to recurrence ranged from 38 to 74 days. Relapse rates appeared to be relatively higher in two small studies [38,47]. Krapp et. al described two of five patients who achieved clinical cure (40%) had relapsed within 21 days of completing CAZ/AVI treatment [47]. Most of the patients in the study had multiple comorbidities, including significant renal impairment, diabetes mellitus, and Human Immunodeficiency Virus (HIV), etc. In the study by Chen et al. which included patients who underwent lung transplant, five patients (5/10; 50%) had relapse of infections in the respiratory tract [38]. A high proportion of patients included in this study were noted to have airway complications (7/10; 70%) and infections from respiratory sources (9/10; 90%).
Four studies examined the effects of CAZ-AVI treatment according to the types of infection [41,50,52,57]. Clinical success rates were found to be lowest for pneumonia (36%-50%), and higher for bacteremia (70%- 75%) and UTIs (88%) [41,50,52]. Thirty-day survival rates were lower for bacteremia (63%) and respiratory infections (70%), and highest for UTIs (83%) [57].
OXA-48-producing Enterobacterales
Six studies involving a total of 138 patients reported clinical experience of CAZ-AVI against diverse types of infection, such as bacteremia, pneumonia, cUTIs, cIAIs, SSTIs, CNSIs, endocarditis, bone and joint infections, and surgical site infections, etc., in patients predominantly infected by OXA-48-producing Enterobacterales (Table 2). Of these, one study compared CAZ-AVI vs. other antimicrobial agents and the remaining five examined only CAZ-AVI treatment (Figure 1). CAZ-AVI monotherapy was used in 58%-81% of patients in two studies whereas CAZ-AVI in combination with other agents was the predominant treatment in three other studies (Table 2).
Figure 1: Breakdown of real-world studies on CAZ-AVI by target pathogens and treatment. Note: *Some of the studies had findings for more than one type of pathogens and are included in more than one categories, but are counted as one study. †Had findings for more than one type of pathogens. § Had findings for CAZ-AVI treatment for all patients and CAZAVI vs. other agents for bacteremia subgroup.
Alraddadi et al. examined the effects of CAZ-AVI vs. standard of care antibiotics (mainly colistin and/or carbapenem among others) in patients with infections caused by OXA-48-producers (predominantly bacteremia and HAP among others) [35] (Table 2). The CAZ-AVI group was associated with a higher clinical remission rate and similar 30-day all-cause mortality compared with the group receiving other agents (80% vs. 54% and 50% vs. 57%, respectively), despite having a higher proportion of patients with bacteremia (70% vs. 54%).
Three studies examining the effects of CAZ-AVI treatment against a range of infections (including bacteremia, CNSIs, IAIs, UTIs, pneumonia, bone and joint infections, SSTIs among others) reported microbiological cure in 65%-100% of patients, clinical cure in 63%-100% of patients, and 30-day survival rate of 78%-92% in patients infected by OXA-48-producing Enterobacterales[34,39,53] (Table 2). However, in another study where CAZ-AVI was administered to critically ill patients with severe infections due to MDR Klebsiella Pneumoniae (Kp) harboring both OXA-48 and a CTX-M-14 type ESBL, in-hospital deaths occurred in all five patients (4 of whom had HAP) [45] (Table 2). In the remaining study which had a high proportion of patients with life-threatening infections (mostly bacteremia) treated with CAZ-AVI on a compassionate basis, 47% of patients infected by OXA-producers achieved microbiological cure, 62% had clinical cure, and 38% survived until discharged [55] (Table 2). These rates were observed to be lower compared with patients infected by KPC-producers (78%, 74%, and 74%, respectively). Although OXA-48 does not hydrolyze ceftazidime, most OXA-48-producing isolates are resistant to ceftazidime due to ESBL coproduction [60]. However, both OXA-48 and ESBL enzymes are inhibited by avibactam [61], the lower success and survival rates observed in patients infected by OXA-48 producers are unexpected, and more studies are warranted [55].
De la Calle and colleagues examined the effects of CAZ-AVI treatment according to the types of infection [39] (Table 2). Clinical cure rates were found to be lowest for pneumonia and IAIs (40% and 43%, respectively), and higher for bacteremia and UTIs (63% and 83%, respectively). Ninety-day survival rates were lowest for IAIs (71%), bacteremia and pneumonia (75% each), and higher for UTIs (83%). Two studies assessed infection recurrence in patients treated with CAZ- AVI [39,53] (Table 2). Sousa et al. observed recurrence in six patients (10%) within 41 days (median) after the end of treatment, while De la Calle et al. reported a recurrence rate of 35% within 19 days (median) after completing CAZ-AVI treatment.
CRE (mixed strains/mechanisms of carbapenem resistance not specified)
Five studies involving a total of 322 patients described the effects of CAZ-AVI treatment against a variety of CRE infections, including bacteremia, respiratory tract infections, pneumonia, UTIs, wound infections, IAIs, bone and joint infections, SSTIs, among others (Table 2). Of these, two included patients infected by different CRE strains (including KPC-, OXA-48-producers, and/or NDM-producers) whereas the remaining three studies did not describe the mechanism of carbapenem resistance. One study examined CAZ-AVI vs. other antimicrobial agents and the remaining four evaluated only CAZ-AVI treatment (Figure 1). CAZ-AVI monotherapy was used in 55%-62% of patients in three studies whereas the remaining two studies used CAZ- AVI in combination with other agents in all patients (Table 2).
Caston and colleagues examined the effects of CAZ-AVI vs. standard of care antibiotics in patients with hematologic malignancies who presented with bacteremia caused by CRE (61% OXA-48 producer and 39% KPC-producers) [37] (Table 2). Notably, CAZ-AVI treatment was associated with higher rates of clinical cure (86%) and 30-day survival (75%) compared with other agents (86% vs. 38% and 75% vs. 48%, respectively).
Four studies describing the effects of CAZ-AVI in treating a variety of infections due to CRE (primarily bacteremia and/or infections from respiratory sources) [33,43,44,46] (Table 2). Microbiological cure and clinical success were achieved in 53%-80% and 65%-71% of patients, respectively, in-hospital deaths were noted in 32% of patients, and 30- day survival ranged from 83%-84% across the studies.
Two studies examined the effects of CAZ-AVI treatment according to the types of infection [44,46] (Table 2). King et al. found that clinical success rates were lowest for pneumonia (56%) and higher for bacteremia, wound infections, and UTIs (61%, 63%, and 88%, respectively) [46]. In-hospital survival rates were lowest for pneumonia (44%) and higher for bacteremia, wound infections, and UTIs (61%, 75%, and 88%). Similarly, Jorgensen et al. reported lowest 30-day survival in patients with pneumonia (76%) and highest survival in those with IAIs and UTIs (91% and 95%, respectively) [44]. One study examined recurrence in patients with CRE infections and recorded a 30-day recurrence rate of 6% [43] (Table 2).
MDR-Pseudomonas spp
Six studies involving a total of 119 patients described clinical experience with CAZ-AVI in treating a variety of infections caused by MDR-Pseudomonas spp., including pulmonary infections, VAP, hospital-acquired respiratory tract infection, bacteremia, IAIs, SSTIs, bone and joint infections, and CNSIs, etc. (Table 2). None of the studies described the mechanism of drug resistance and only three reported patients infected by CR-PA being included in their studies. CAZ-AVI in combination with other antimicrobial agents was used in 67%-100% of patients in three studies (Table 2) whereas CAZ-AVI monotherapy was the predominant treatment in two other studies.
Among the studies describing the effects of CAZ-AVI treatment against a range of infections due to MDR-Pseudomonas spp. (most of which were respiratory infections), outcomes were generally favorable in five of the studies [34,43,49,54,59], even in patients with cystic fibrosis who had pulmonary and/or systemic infections (cepacia syndrome) and moderate-to-severe lung disease [54] (Table 2). Microbiological cure and clinical cure/success were achieved in 100% and 83%-100% of patients, respectively, and 30-day survival ranged from 82–83% across the studies. The remaining study on a series of eight patients, however, reported a relatively lower clinical cure rate (4/8; 50%) but similar 30-day survival rate (7/8; 87.5%) [48] (Table 2). Patients had serious infections due to MDR and Extensively Drug-Resistant (XDR)-PA and received CAZ-AVI on a compassionate basis. Five of eight patients had respiratory infections and only one of whom achieved clinical cure.
Two studies examined infection recurrence in patients infected by MDR-Pseudomonas spp. [43,59] (Table 2). Jorgensen et al. reported a 30-day recurrence rate of 6% [43], while Vena et al. reported only one patient (3%) with bacteremia who experienced recurrent infection five days (median) after completing CAZ-AVI treatment [59].
To summarize, results from the real-world studies suggest that CAZ-AVI may be effective in treating different types of infections caused by GNB with limited treatment options, including CRE and MDR-Pseudomonas spp.
Safety of CAZ-AVI for GNB infections with limited treatment options
Fifteen studies involving a total of 829 patients documented the safety of using CAZ-AVI-based regimens in treating a variety of GNB infections with limited treatment options and the results are summarized in Table 3 [32,38,39,41,43,46,49-56,59]. Of these, three studies compared CAZ-AVI with other antimicrobial agents and the remaining 12 focused only on CAZ-AVI treatment.
| Reference (Year) | Study population/ Types of Infection | Target pathogens | Treatment(s) | Safety outcomes | Definitions | |
|---|---|---|---|---|---|---|
| King (2017) [46] | Severely ill patients with CRE infection Types of infection: Bacteremia (38%), UTI (28%), pneumonia (27%), wound (13%), IAI (7%), and bone/ joint (3%) | CRE | CAZ-AVI (n=60) Monotherapy: 55% | - | No treatment-related AEs reported | |
| Spoletini (2019) [54] | Patients with cystic fibrosis with infections due to MDR-GN organisms not responding to standard of care antibiotic treatment Types of infection: Pulmonary infection (7 pts) and systemic infection (cepacia syndrome) (1 pt) Patients had moderate-to-severe lung disease | MDR-GN organisms including MDR-PA and MDR-Burkholderia spp. | CAZ-AVI (n=8) | - | No episodes of AKI or elevation in transaminase were observed. Mouth dryness: 1 pt No other AEs were observed | |
| Chen (2020) [38] | Lung transplant patients with XDR- GNB infections Types of infection: Pneumonia and/ or tracheobronchitis (90%), cholecystitis and bacteremia (10%) | XDR GNB (90% KPC-Kp) | CAZ-AVI (n=10) Monotherapy: 20% | - |
|
|
| De la Calle (2019) [39] | Patients with infections caused by CRE Types of infection: Bacteremia (33%), IAI (29%), UTI (25%), pneumonia (21%); osteoarticular/SSSTI (17%), device-related meningitis (4%), and catheter-related bacteremia (4%) | CRE (96% OXA- 48-producing Enterobacterales and 96% ESBL Enterobacterales) | CAZ-AVI (n=23) Monotherapy: 58% | - | AE: 17%
|
|
| Guimarães (2019) [41] | Patients with infections caused by KPC-producing Enterobacterales coresistant to carbapenems and polymyxins Types of infection: Bacteremia (41%), UTI (28.%), IAI (14%), nosocomial pneumonia (10%), and complicated SSTI (7%) | KPC-producing Enterobacterales | CAZ-AVI (n=29) Monotherapy: 52% | - | AEs: 14%
|
|
| Jorgensen (2019) [43] | Patients with MDR-GN infections Types of infection: Respiratory tract (37%), UTI (20%), IAI (19.7%), bacteremia (11%), SSTI (9%), and osteoarticular (7%) | MDR-GN organisms (58% CRE and 31% Pseudomonas spp.) | CAZ-AVI (n=203) Monotherapy: 67% | - | AE: 8%
|
Acute kidney injury (AKI) was evaluated in patients not receiving hemodialysis at the time of CAZ initiation and was defined as a serum creatinine increase of ≥ 0.5 mg/dL or 50% from baseline on 2 consecutive measurements while on CAZ and up to 72 hours after the last dose. |
| Santevecchi (2018) [49] | Patients with infections due to MDR-organisms other than Kp Types of infection: Pneumonia (46%), skin and soft tissue (23%), bacteremia (15%), and intra–abdominal (15%) | MDR-organisms other than Kp (most common: MDR-PA) | CAZ-AVI (n=10) Monotherapy: 50% | - | No treatment-related AEs were reported. | |
| Shield (2016) [50] | Patients with CRE Types of infection: Pneumonia (32%), bacteremia (27%), IAI (11%), SSTI (11%), pyelonephritis (11%), mediastinitis (3%), subdural empyema/ Ventriculitis (3%) and purulent tracheobronchitis (3%) | CRE (78% KPC-producing Enterobacterales) | CAZ-AVI (n=37) Monotherapy: 70% | - | AKI: 3/31 (10%) 1 of 3 (33%) who developed AKI received concomitant colistin)
|
AKI within 7 days of treatment initiation (defined by 1.5X increase in serum creatinine from baseline) Leukopenia (absolute neutrophil count=90 × 109/L) |
| Shields (2018)[52] | Patients with CRE infections [Mixed infection types] | CRE (75% KPC-producing Enterobacterales) | CAZ-AVI (n=61) | - | AKI: 7/61 (11%) Among the 7 patients who developed AKI, 1 (14%) and 2 (29%) pts received concomitant colistin and aminoglycosides, respectively) | AKI; defined by modified KDIGO guidelines as a 1.5X increase in serum creatinine levels from baseline within 7 days of treatment initiation |
| Sousa (2018)[53] | Patients with infections caused by OXA-48- producing Kp Types of infection: Intra-abdominal (28%), pulmonary (26%), urinary (25%), Others (10%) Severe infection (54%) | OXA-48-producing Kp | CAZ-AVI (n=57) Monotherapy: 81% | - | AKI: 2/57 (4%) 1 of 2 patients [50%] who developed AKI was on concomitant IV colistin No other treatment- related AEs were observed. | |
| Temkin (2017) [55] | Patients with infections caused by CR GN organisms Types of infection: Bacteremia (68%), IAI (39%), pneumonia (18%), SSTI (11%), UTI (11%), osteomyelitis (8%), endocarditis (5%), surgical site infection (5%), others (8%) Life-threatening infection (61%) | CR GN organims including KPC-, OXA-48-producing Enterobacterales and CR-PA | CAZ-AVI (n=38) Monotherapy: 34% | - | AE: 16%
|
|
| Vena (2020) [59] | Patients with infections caused by MDR-GNB other than CRE Types of infection: Nosocomial pneumonia (49%), bacteremia (17%), IAI (10%), bone infection (10%), acute bacterial skin and skin structure infection (5%), and other infections (10%) | MDR-GNB other than CRE (89% MDR-PA) | CAZ-AVI (n=37) Monotherapy: 20% | - |
|
|
| Tsolaki (2020) [56] | Critically ill, mechanically ventilated patients with mixed infections caused by CRE [Mixed infection types] | CRE (94% KPC-producing Enterobacterales) | CAZ-AVI (n=41) Monotherapy: 22% Types of infection: Bacteremia (54%), VAP (46%), IAI (10%), UTI (5%), CNSI (2%) | BAT (n=36) (86% included colistin among others) Monotherapy: 3% Types of infection: Bacteremia (78%), VAP (19%), IAI (11%), UTI (3%), CNSI (3%) |
|
|
| Shields (2017)[51] | Patients with CR-Kp bacteremia [Bacteremia] | CR-Kp (97% KPC-Kp) | CAZ-AVI (n=11) Monotherapy: 64% The remaining (4/11; 36%) received combination with aminoglycoside | Carbapenem + colistin (n=23) | ⴕEOT AKI: 2/11 (18%) vs. 13/23 (57%) 1 of 2 (50%) patients who developed AKI received CAZ-AVI with aminoglycosides | Acute kidney injury was defined by KDIGO criteria as a 1.5X increase in serum creatinine from baseline at the end of treatment. |
| Carbapenem + aminoglycoside (n=18) | ⴕEOT AKI: 2/11 (18%) vs. 8/18 (44%) 1 of 2 (50%) patients who developed AKI received CAZ-AVI with aminoglycosides | |||||
| Others (n=33) | ⴕEOT AKI: 2/11 (18%) vs. 6/33 (18%) 1 of 2 (50%) patients who developed AKI received CAZ-AVI with aminoglycosides | |||||
| Ackley (2020) [32] | Patients with infections caused by KPC-producing Enterobacterales (excluded those with localized urinary tract infection and repeat study drug exposures after the first episode) [Mixed infection types] | KPC-producing CRE | CAZ-AVI (n=105) Monotherapy: 39% Types of infection: Bacteremia (42%), respiratory (29%), soft tissue (17%), IAI (11%), and others (1%) | Meropenem- Vaborbactam (n=26) Monotherapy: 85% Types of infection: Bacteremia (35%), respiratory (38%), Soft tissue (8%), IAI (19%), and others (0%) | AE: 34% vs 23% ⴕNephrotoxicity (most frequent AE): 26/89 (29%) vs. 3/21 (14%) Leukopenia: 11% vs.` 8% Rash: 4% vs. 4% Neurotoxicity: 1% vs. 0 Among the 26 pts who experienced nephrotoxicity in the CAZ-AVI group, 16 (62%) received combination therapy: 23% received colistin, 15% polymyxin B, 15% tigecycline, 12% fluoroquinolone, and 4% aminoglycoside. In the meropenem- vaborbactam group, one of three (33%)pts who had nephrotoxicity received combination therapy with colistin, the remaining two patients received monotherapy. | Nephrotoxicity was defined using the Acute Kidney Injury Network (AKIN) classification and/ or the initiation of RRT while receiving treatment. Leukopenia=white blood cell count of <4,000 cells/mm3. |
Abbreviations: AKI:Acute Kidney Injury; ALP:Alkaline Phosphatase; ALT:Alanine Aminotransferase; AST:Aspartate Aminotransferase; BAT:Best Available Therapy; BSI:Blood Stream Infections CRE Carbapenem-Resistant Enterobacterales; CR-Kp:Carbapenem-Resistant Klebsiella Pneumoniae; CR-PA:Carbapenem-Resistant Pseudomonas Aeruginosa; CAZ-AVI:Ceftazidime-avibactam ; ESBL:Extended-Spectrum Β-Lactamase, XDR:Extensively Drug-Resistant; GGT:Γ-Glutamyltranspeptadase; GNB:Gram-Negative Bacilli; KPC:Klebsiella Pneumoniae Carbapenemase; KPC-Kp:Klebsiella Pneumoniae Carbapenemase-Producing Klebsiella Pneumoniae; MDR-GN:Multidrug-Resistant Gram-Negative; MDR-GNI:Multidrug-Resistant Gram-Negative Infection; MDR-PA:Multidrug-Resistant Pseudomonas Aeruginosa; PA:Pseudomonas Aeruginosa; OXA:Oxacillinase; RRT:Renal Replacement Therapy; TBIL:Total Bilirubin.
Table 3: Safety of CAZ-AVI for GNB infections with limited treatment options.
Overall, the incidence of adverse events (AEs) was largely comparable between patients treated with CAZ-AVI and those treated with other agents [32,51,56], except for some differences in renal AEs noted in two of the studies [32,51] (Table 3). In the study by Shield et al. which examined CAZ-AVI vs. colistin-or aminoglycoside-based regimens, Acute Kidney Injury (AKI; defined as 1.5 X increase in serum creatinine from baseline) was reported in only 2 of 11 (18%) patients in the CAZ-AVI group at the end of treatment (EOT), one of whom received concomitant aminoglycosides. However, more patients who received colistin-or aminoglycoside-based treatment developed AKI (13/23; 57% and 8/18; 44%, respectively) [51]. In another study comparing CAZ-AVI vs. meropenem-vaborbactam, nephrotoxicity (defined using the Acute Kidney Injury Network (AKIN) classification and/or the initiation of renal replacement therapy [RRT] while receiving treatment) was documented in 29% (26/89) and 14% (3/21) of the patients, respectively [32]. More patients in the former group received concomitant nephrotoxic agents. Of the 26 patients who had nephrotoxicity in the CAZ-AVI group, 62% received combination therapy, including 23% colistin, 15% polymyxin B, and 4% aminoglycoside among others. Of the three patients who had nephrotoxicity In the meropenem-vaborbactam group, one (33%) received concomitant colistin [32].
Among the 12 studies that examined only CAZ-AVI treatment, three reported no treatment-related AEs [46,49,59] whereas the remaining studies documented occurrence of AEs during CAZ- AVI treatment [38,39,41,43,50, 52-55]. Only one study had reported discontinuation of CAZ-AVI treatment in one patient who developed leukopenia [50]. Most of the reported AEs occurred in less than 5% of the patients, with only a few others reported in less than 15% in some studies: AKI was reported in 4%-11% of patients in four studies [43,50,52,53] neurological symptoms were documented in 9% of patients in one study [39], diarrhea in 2–7% of patients in four studies [39,41,43,55]. Among patients who experienced AKI, the proportion of patients who received concomitant nephrotoxic agents (colistin or aminoglycosides) was noted to range from 33% to 90% [43,50,52,53].
In summary, CAZ-AVI appears to be well-tolerated in the real-world setting in adult patients with infections caused by aerobic MDR-GNB, including CRE and MDR-Pseudomonas spp. Although there were few reports of renal events in patients who received CAZ-AVI treatment, many of whom received concomitant nephrotoxic agents, and the incidence of renal events was lower than those who received standard of care antibiotic treatment. The safety of CAZ-AVI was examined in pediatric patients aged three months to below 18 years [62-64]. Results from these pediatric studies demonstrate that the safety profile of CAZ- AVI was consistent with those from studies in adult patients and no new safety issues were observed.
Development of CAZ-AVI resistance in CAZ-AVI-treated patients diagnosed with GNB infections with limited treatment options
Nine observational studies involving a total of 621 patients assessed emergence of resistance to CAZ-AVI treatment for infections caused by a variety of resistant GNB with limited alternatives available [,42,43,49, 50,52,53,56,59]. The results are presented in Table 2. Of these studies, six evaluated post-treatment CAZ-AVI resistance in CRE infections (predominantly caused by KPC-producing Enterobacterales or OXA-48-producing Enterobacterales) [32; 42; 50; 52; 53; 56], two in infections due to MDR-Pseudomonasspp. [49; 59], and one in infections caused by MDR-GNB (CRE or Pseudomonas spp.) [43].
CRE
Two studies examined resistance development in patients who received CAZ-AVI-based regimens vs. those who received other antimicrobials for CRE infections caused by KPC-producing Enterobacterales [32,56]. In the study by Tsolaki et al. which included critically ill, mechanically ventilated patients with life-threatening infections (65% of whom had bacteremia), emergence of CAZ-AVI resistance was not observed in the CAZ-AVI group; however, ten of twelve (83%) patients in the group receiving other antimicrobials
(mainly colistin among others) who had relapse infection developed resistance to colistin within 30 days of treatment [56]. A secondary analysis in patients with bacteremia revealed similar results [56]. In another study involving patients with infections due to KPC-producing Enterobacterales (71% of whom had bacteremia or pneumonia), three of 15 (20%) patients in the CAZ-AVI group who had recurrent infection developed resistance to CAZ-AVI within 90 days of the index infection, whereas development of resistance to meropenem- vaborbactam treatment was not observed [32]. All three patients who developed CAZ-AVI resistance had received CAZ-AVI monotherapy and RRT, and had infections from respiratory sources. Data regarding the mechanisms responsible for CAZ-AVI resistance were not available in this study [32].
In three other studies examining the development of resistance to CAZ-AVI treatment in infections caused by KPC-producing Enterobacterales, CAZ-AVI resistance emerged in 8%-10% of the patients following 7-31 days of CAZ-AVI treatment [42,50,52]. Among the 13 patients who developed CAZ-AVI-resistant bacterial isolates in the three studies, 11 had received CAZ-AVI monotherapy and two had received CAZ-AVI in combination with other antimicrobials. In terms of In terms of the type of infections, nine had pneumonia, two had bacteremia, and two others had IAI [42,50,52]. One of the studies investigated the mechanisms of CAZ-AVI resistance and found mutant blaKPC-3 in resistant strains [52]. Additionally, receipt of RRT was found to be a predictor of the development of CAZ-AVI resistance in the study [52]. Two other studies by Sousa et al. and Jorgensen et al. examined development of CAZ-AVI resistance in infections caused by OXA-48-producing Enterobacterales and CRE (mechanism of carbapenem resistance not specified), respectively and did not detect any CAZ-AVI resistant strains during the study period [43,53].
MDR-Pseudomonas spp.
Three studies evaluated emergence of resistance to CAZ-AVI treatment for infections caused by MDR-Pseudomonas spp. with limited alternatives available (mechanisms of multi-drug resistance not specified) [43, 49,59]. In the study by Santevecchi et al. which included patients with infections due to MDR-PA, one of six patients developed resistance to CAZ-AVI within 50 days of treatment [49]. The patient had received CAZ-AVI in combination with other antimicrobials and had VAP. This patient had fluctuating renal function and required multiple dose adjustments during the course of treatment [49]. No CAZ-AVI resistant strains were detected in two other studies involving patients with infections due to MDR-PA (including CR-PA) or MDR- Pseudomonas spp. [43,59].
To summarize, although limited data exist especially for infections caused by OXA-producing Enterobacterales and CR-PA, CAZ-AVI resistance has been reported to appear in infections caused by KPC- producing Enterobacterales or MDR-PA within 7-50 days of CAZ-AVI treatment. In infections caused by KPC-producing Enterobacterales, development of resistance appears to be more common in patients treated with monotherapy, and those who had respiratory infection and RRT.
Role of CAZ-AVI for GNB infections with limited treatment options
Our review of CAZ-AVI in real-world studies suggests that CAZ- AVI is an effective and well-tolerated alternative to standard of care antibiotics for treating different types of infection caused by MDR- GNB, including CRE and MDR-Pseudomonas spp. Notably CAZ-AVI is well tolerated in the real-world setting even in severely or critically ill patients, patients with multiple comorbidities, or those with bacteremia. These real-life experiences provide valuable insights into the use of CAZ-AVI across diverse types of GNB infections for which limited treatment options exist.
Results from the included studies from the real-world setting suggest that CAZ-AVI may be effective for treating infections caused by MDR-GNB, including CRE and MDR-Pseudomonas spp. [32- 59]. CAZ-AVI exhibited favorable microbiological cure, clinical success, and survival rates in patients who had infections due to KPC- producing Enterobacterales, OXA-48-producing Enterobacterales, CRE with unspecified mechanism of carbapenem resistance, or MDR- Pseudomonas spp. When compared with standard of care antibiotic treatment, CAZ-AVI treatment exhibited higher rates of microbiological cure and clinical success in treating CRE infections [35-37,40,51,56- 58]. When compared with another beta-lactam combination agent, meropenem-vaborbactam, CAZ-AVI exhibited similar rates of clinical success and survival in treating KPC infections [32]. However, it should be noted that many of these comparative real-world studies have small sample sizes and the results may be subject to selection bias due to the observational nature of these studies. Hence, the results are not completely conclusive. As with standard of care antibiotic treatment [65-67], treatment outcomes were noted to vary by infection sites among patients with CRE infections who received CAZ-AVI treatment [39,41,44,46,50,52,57], possibly owing to the underlying disease burden. Notably, favorable outcomes were observed even in patients with bacteremia [37,42,51,57], including those who were critically ill [40; 56]. However, less favorable outcomes were noted in a few studies involving severely ill patients or patients with life-threatening or severe infections [41,45,55]. A study on patients infected by KPC- producing Enterobacterales coresistant to carbapenems and polymyxins reported a relatively lower 30-day survival rate (48%), but high rate of clinical success (83%). Patients were severely ill, had serious infections (51% had bacteremia or pneumonia), and received CAZ-AVI on a compassionate basis [41]. In another study which had a high proportion of patients with life-threatening infections (mostly bacteremia) treated with CAZ-AVI on a compassionate basis, 47% of patients infected by OXA-producers achieved microbiological cure, 62% had clinical cure, and 38% survived until discharged [55]. In another study where CAZ- AVI was administered to critically ill patients with severe infections due to MDR Kp harboring both OXA-48 and a CTX-M-14 type ESBL, in- hospital deaths occurred in all five patients (4 of whom had HAP) [45]. It should be noted that CAZ-AVI was used on a compassionate basis in two of the studies. In addition, mortality is only partially related to the infection process and may be affected by the severity of the clinical conditions. Taken together, evidence from real-world studies and RCTs suggest that CAZ-AVI is a valuable treatment option for infections mediated by CRE or MDR-PA.
Results from the observational studies revealed CAZ-AVI is generally well-tolerated in the real-world setting in patients who had MDR-GNB infections due to CRE or MDR-Pseudomonas spp., including severely or critically ill patients, patients with multiple comorbidities, or those with bacteremia [32,38,39,41,43,46,49-56,59]. The reported AEs were within the expected profile for CAZ-AVI, with no new safety concerns [20,21]. Treatment with colistin or aminoglycosides has been reported to be associated with nephrotoxicity issues [8-10]. A prospective randomized trial comparing colistin vs. meropenem for the treatment of VAP was terminated after interim analysis due to excessive nephrotoxicity in the colistin group [68]. In the real-world studies reviewed, although there were few reports of renal events in patients who received CAZ-AVI treatment, many of whom received concomitant nephrotoxic agents and there were no reports of CAZ-AVI discontinuation due to these events [32,43,50-53]. Notably, patients who received CAZ-AVI treatment had lower incidence of renal events compared with those who received standard of care treatment containing colistin or aminoglycosides [51]. In a study comparing CAZ-AVI vs. meropenem-vaborbactam, CAZ- AVI was associated with a slightly higher incidence of nephrotoxicity (defined using the AKIN classification and/or the initiation of RRT while receiving treatment) than meropenem-vaborbactam in treating KPC infections [32]. It should be noted, however, that more patients in the former group received concomitant nephrotoxic agents. The observation is likely due to the effects of concomitant nephrotoxic agents [32].
Limited data from the real-world studies revealed emergence of CAZ-AVI resistance following CAZ-AVI exposure. CAZ-AVI resistance has been reported to appear in 8%-10% of patients infected by KPC-producing Enterobacterales following 7-31 days of treatment [42,50,52]. Another study reported one of six patients with infections due to MDR-PA developed resistance to CAZ-AVI within 50 days of treatment [49]. One study evaluated the mechanisms for CAZ-AVI resistance and identified point mutation in blaKPC-3 [52]. Notably, development of resistance appears to be more common in patients treated with monotherapy, and those who had respiratory infection and RRT, suggesting these factors may be related to the onset of CAZ- AVI resistance [32,42,49,50,52]. Further studies are needed to evaluate emergence of CAZ-AVI resistance in a variety of resistant GNB with limited treatment options and to identify factors related to its onset.
There are a few important limitations to this review. First, the majorities of the studies have a retrospective, single-center design, and have not properly characterized the mechanisms of carbapenem resistance. Although the review included 28 studies, many of them have small sample size, and data were heterogeneous. Most of the studies that reported treatment outcomes by infection sites were limited by the small sample size at each site. Next, in studies that examined only CAZ-AVI, the interpretation is limited by the lack of a control group. Although the interpretation of CAZ-AVI’s effectiveness and safety with respect to other agents is limited by small sample sizes and the observational nature of the studies, evidence from the real-world studies reviewed provide important insights to the clinical utility of CAZ-AVI as an effective and well-tolerated alternative to standard of care antibiotics. A large-scale retrospective study including patients from 22 hospitals in Italy was recently published [69]. This real-world study examined 577 adult patients who received CAZ-AVI treatment for infections caused by KPC-Kp. Of these, 68% had bacteremia while the remaining had cUTIs, lower respiratory infections, IAIs, or other infections. More than half of the patients received CAZ-AVI treatment within 48 hours from the start of infection; most of whom had bacteremia. The overall mortality rate at 30 days after the start of infection was 25% and 3.5% of patients developed resistance to CAZ-AVI. The mortality rate in patients treated with CAZ-AVI monotherapy was similar as those who received CAZ-AVI in combination with other antimicrobial agents. These findings are consistent with previous reports [32,38,40,42,50- 52,55-58,70]. Interestingly, the study found that extending the duration of CAZ-AVI infusion to at least three hours may possibly improve survival of patients with KPC-Kp infections although more research is needed to confirm this [69].
Conclusion
This review provides valuable insights into the utility of CAZ-AVI across diverse types of infection with limited alternatives available. Collectively, data from RCTs and real-world studies support the use of CAZ-AVI in adult patients with infections caused by aerobic GNB with limited treatment options, including CRE and MDR-Pseudomonas spp. CAZ-AVI is a useful addition to the limited antimicrobial armamentarium against MDR-GNB infections, offering an effective and well-tolerated alternative to standard of care antibiotic treatment.
The treatment of MDR-GNB infections remains challenging, especially given the rising trends of CR strains. Rational use of CAZ- AVI would be crucial to ensure its longevity in the armamentarium. Treatment decisions should be guided by the characteristics of the pathogen(s) (e.g. mechanism of resistance), unique characteristics of the patient (e.g. site of infection, presence of comorbidities, prior antibiotic treatment, drug allergies, etc.), and antimicrobial properties (e.g. effectiveness and safety profile) to slow down the spread of antimicrobial resistance.
Declaration of Completing Interest
Ana C. Gales has received consulting fees from Cristália and InfectoPharm. She has served on the advisory boards and speaker’s bureaus for Eurofarma, Merck Sharp & Dohme, Pfizer, United Medical, and Zambon. She has received grants from Eurofarma which was awarded to her institution, and support from Merck Sharp & Dohme and Pfizer for attending meetings and/or travel. Luis Fernando Aranha Carmargo declared no potential conflict of interest. Gabriel Trova Cuba has received support from Pfizer for the present manuscript and has received consulting fees from Eurofarma. He has also received honoraria from Merck Sharp & Dohme, Pfizer, Sanofi, and Eurofarma, and support from Merck Sharp & Dohme for attending meetings and/ or travel. Felipe Francisco Tuon has received support from Pfizer for the present manuscript and has received grants from Merck Sharp & Dohme, Abbott, Teva, and Beckman Coulter. He has also received honoraria from Merck Sharp & Dohme, Teva, and Abbott. Hui Hwa Choo is employed by Tech Observer Asia Pacific Pte Ltd., which received funding from Pfizer for providing medical writing support. Maria Lavinea Novis de Figueiredo, Alvaro Quintana, and Paurus Mehelli Irani are employees of Pfizer. Alvaro Quintana owns stocks in Pfizer.
Acknowledgments
Medical writing support was provided by Hui Hwa Choo at Tech Observer Asia Pacific Pte Ltd., Singapore and was funded by Pfizer.
Funding
This work was supported by Pfizer.
References
- World Health Organization (2019) Global antimicrobial resistance surveillance system (GLASS) report: early implementation.
- Antibiotic resistance threats in the United States (2019).
- Peleg AY, Hooper DC (2010) Hospital-acquired infections due to gram-negative bacteria. N Engl J Med 362:1804-1813.
[Crossref] [Google Scholar] [PubMed]
- Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, et al. (2020) Infectious diseases society of america antimicrobial resistant treatment guidance: gram-negative bacterial infections. Clin Infect Dis.
- Jernigan JA, Hatfield KM, Wolford H, Nelson RE, Olubajo B, et al. (2020) Multidrug-resistant bacterial infections in U.S. hospitalized patients. N Engl J Med 382:1309-1319.
[Crossref] [Google Scholar] [PubMed]
- Falagas ME, Lourida P, Poulikakos P, Rafailidis PI, Tansarli GS (2014) Antibiotic treatment of infections due to carbapenem-resistant: systematic evaluation of the available evidence. Antimicrob Agents Chemother 58:654.
[Crossref] [Google Scholar] [PubMed]
- Bassetti M, Peghin M, Vena A, Giacobbe DR (2019) Treatment of infections due to MDR gram-negative bacteria. Front Med. 6:74.
[Crossref] [Google Scholar] [PubMed]
- Giacobbe DR, Mikulska M, Viscoli C (2018) Recent advances in the pharmacological management of infections due to multidrug-resistant Gram-negative bacteria. Expert Rev Clin Pharmacol 11:1219-1236.
[Crossref] [Google Scholar] [PubMed]
- Karaiskos I, Lagou S, Pontikis K, Rapti V, Poulakou G (2019) The “old” and the “new” antibiotics for MDR gram-negative pathogens: for whom, when, and how. Front Public Health 7:151.
[Crossref] [Google Scholar] [PubMed]
- Satlin MJ, Lewis JS, Weinstein MP, Patel J, Humphries RM, et al. (2020) Clinical and laboratory standards institute and European committee on antimicrobial susceptibility testing position statements on polymyxin B and colistin clinical breakpoints. Clin Infect Dis. 71:e523-e529.
[Crossref] [Google Scholar] [PubMed]
- Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, et al. (2011) Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically Ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 55:3284-3294.
[Crossref] [Google Scholar] [PubMed]
- Giamarellou H, Poulakou G (2011) Pharmacokinetic and pharmacodynamic evaluation of tigecycline. Expert Opin Drug Metab Toxicol 7:1459-1470.
[Crossref] [Google Scholar] [PubMed]
- Panidis D, Markantonis SL, Boutzouka E, Karatzas S, Baltopoulos G (2005) Penetration of gentamicin into the alveolar lining fluid of critically Ill patients with ventilator-associated pneumonia. Chest 128:545-552.
[Crossref] [Google Scholar] [PubMed]
- Nation RL, Garonzik SM, Thamlikitkul V, Giamarellos-Bourboulis EJ, Forrest A, et al. (2012) Dosing guidance for intravenous colistin in critically-ill patients. Clin Infect Dis 64:565-571.
[Crossref] [Google Scholar] [PubMed]
- Motsch J, Murta de Oliveira C, Stus V, Koksal I, Lyulko O, et al. (2020) Restore-IMI 1: A multicenter, randomized, double-blind trial comparing efficacy and safety of imipenem/relebactam vs colistin plus imipenem in patients with imipenem-nonsusceptible bacterial infections. Clin Infect Dis 70:1799-17808.
- Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G, Mathers AJ, Bassetti M, et al. (2018) Effect and safety of meropenem-vaborbactam versus best-available therapy in patients with carbapenem-resistant enterobacteriaceae infections: the TANGO II randomized clinical trial. Infect Dis Ther 7:439-455.
[Crossref] [Google Scholar] [PubMed]
- Kadri SS, Adjemian J, Lai YL, Spaulding AB, Ricotta E, et al. (2018) Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis 67:1803-1814.
[Crossref] [Google Scholar] [PubMed]
- Giamarellou H (2016) Epidemiology of infections caused by polymyxin-resistant pathogens. Int J Antimicrob Agents 48:614-1621.
[Crossref] [Google Scholar] [PubMed]
- Pogue JM, Bonomo RA, Kaye KS (2018) Ceftazidime/Avibactam, Meropenem/Vaborbactam, or both? clinical and formulary considerations. Clin Infect Dis 68:519-524.
[Crossref] [Google Scholar] [PubMed]
- Allergan. Allergan, Prescribing Information, Food and Drug Administration, Ceftazidime avibactam.
- Pfizer. Pfizer, Summary of Product Characteristics, European Medicines Agency, Ceftazidime avibactam (Zavicefta).
- Castanheira M, Mills JC, Costello SE, Jones RN, Sader HS (2015) Ceftazidime-avibactam activity tested against enterobacteriase isolates from U.S. hospitals (2011 to 2013) and characterization of ß-lactamase-producing strains. Antimicrob Agents Chemother 59:3509-3517.
[Crossref] [Google Scholar] [PubMed]
- Aktas Z, Kayacan C, Oncul O. (2012) In vitro activity of avibactam (NXL104) in combination with ß-lactams against Gram-negative bacteria, including OXA-48 ß-lactamase-producing Klebsiella pneumoniae. Int J Antimicrob Agents 39:86-89.
[Crossref] [Google Scholar] [PubMed]
- Lob S, Kazmierczak K, Stone G, Sahm DF (2019) In vitro activity of ceftazidime–avibactam and comparator agents against pseudomonas aeruginosa from ICU and non-ICU wards collected in latin America and globally as part of the ATLAS surveillance program 2016-2017. Open Forum Infect Dis 6:S310-S.
- Rossi F, Cury AP, Franco MRG, Testa R, Nichols WW (2017) The in vitro activity of ceftazidime-avibactam against 417 Gram-negative bacilli collected in 2014 and 2015 at a teaching hospital in São Paulo, Brazil. Braz J Infect Dis 21:569-573.
[Crossref] [Google Scholar] [PubMed]
- Wagenlehner FM, Sobel JD, Newell P, Armstrong J, and Huang X, et al. (2016) Ceftazidime-avibactam versus doripenem for the treatment of complicated urinary tract infections, including acute pyelonephritis: RECAPTURE, a phase 3 randomized trial program. Clin Infect Dis. 63:754-762.
[Crossref] [Google Scholar] [PubMed]
- Mazuski JE, Gasink LB, Armstrong J, Broadhurst H, Stone GG, et al. (2013) Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis 62:1380-1389.
[Crossref] [Google Scholar] [PubMed]
- Qin X, Tran BG, Kim MJ, Wang L, Nguyen DA, et al. (2017) A randomised, double-blind, phase 3 study comparing the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem for complicated intra-abdominal infections in hospitalised adults in Asia. Int J Antimicrob Agents 49:579-588.
[Crossref] [Google Scholar] [PubMed]
- Torres A, Zhong N, Pachl J, Timsit JF, Kollef M, et al. (2018) Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): a randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect Dis 18:285-295.
[Crossref] [Google Scholar] [PubMed]
- Carmeli Y, Armstrong J, Laud PJ, Newell P, Stone G, et al. (2016) Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): A randomised, pathogen-directed, phase 3 study. Lancet Infect Dis 16:661-673.
[Crossref] [Google Scholar] [PubMed]
- Mendes RE, Castanheira M, Woosley LN, Stone GG, Bradford PA, et al. (2019) Characterization of ß-lactamase content of ceftazidime-resistant pathogens recovered during the pathogen-directed phase 3 REPRISE trials for ceftazidime-avibactam: correlation of efficacy against ß-lactamase producers. Antimicrob Agents Chemother. 63:e02655-e02718.
[Crossref] [Google Scholar] [PubMed]
- Ackley R, Roshdy D, Meredith J, Minor S, Anderson WE, et al. (2020) Meropenem-Vaborbactam versus Ceftazidime-Avibactam for treatment of Carbapenem-resistant enterobacteriaceae Infections. Antimicrob Agents Chemother. 64:e02313-e02319.
[Crossref] [Google Scholar] [PubMed]
- Aitken SL, Tarrand JJ, Deshpande LM, Tverdek FP, Jones AL, et al. (2016) High rates of non-susceptibility to Ceftazidime-avibactam and identification of New Delhi metallo-beta-lactamase production in enterobacteriaceae bloodstream infections at a major cancer center. Clin Infect Dis 63:954-958.
[Crossref] [Google Scholar] [PubMed]
- Algwizani A, Alzunitan M, Alharbi A, Alsaedy A, Aljohani S, et al. (2018) Experience with ceftazidime-avibactam treatment in a tertiary care center in Saudi Arabia. J Infect Public Health 11:793-795.
[Crossref] [Google Scholar] [PubMed]
- Alraddadi BM, Saeedi M, Qutub M, Alshukairi A, Hassanien A, et al. (2019) Efficacy of ceftazidime-avibactam in the treatment of infections due to Carbapenem-resistant Enterobacteriaceae. BMC Infect Dis 19:772.
[Crossref] [Google Scholar] [PubMed]
- Bassetti M, Carannante N, Pallotto C, Righi E, Di Caprio G, et al. (2019) KPC-producing Klebsiella pneumoniae gut decolonisation following ceftazidime/avibactam-based combination therapy: A retrospective observational study. J Glob Antimicrob Resist 17:109-111.
[Crossref] [Google Scholar] [PubMed]
- Caston JJ, Lacort-Peralta I, Martin-Davila P, Loeches B, Tabares S, et al. (2017) Clinical efficacy of ceftazidime/avibactam versus other active agents for the treatment of bacteremia due to carbapenemase-producing Enterobacteriaceae in hematologic patients. Int J Infect Dis 59:118-123.
[Crossref] [Google Scholar] [PubMed]
- Chen W, Sun L, Guo L, Cao B, Liu Y, et al. (2020) Clinical outcomes of ceftazidime-avibactam in lung transplant recipients with infections caused by extensively drug-resistant gram-negative bacilli. Ann Transl Med 8:39.
[Crossref] [Google Scholar] [PubMed]
- De la Calle C, Rodriguez O, Morata L, Marco F, Cardozo C, et al. (2019) Clinical characteristics and prognosis of infections caused by OXA-48 carbapenemase-producing Enterobacteriaceae in patients treated with ceftazidime-avibactam. Int J Antimicrob Agents 53:520-524.
[Crossref] [Google Scholar] [PubMed]
- Falcone M, Bassetti M, Tiseo G, Giordano C, Nencini E, et al. (2020) Time to appropriate antibiotic therapy is a predictor of outcome in patients with bloodstream infection caused by KPC-producing klebsiella pneumoniae. Crit Care 24:29.
[Crossref] [Google Scholar] [PubMed]
- Guimaraes T, Nouer SA, Martins RCR, Perdigao Neto LV, Martins W, et al. (2019) Ceftazidime-Avibactam as salvage therapy for infections caused by enterobacteriales coresistant to carbapenems and polymyxins. Antimicrob Agents Chemother. 63:e00528-19.
[Crossref] [Google Scholar] [PubMed]
- Iannaccone M, Boattini M, Bianco G, Corcione S, Cavallo R, et al. (2020) Ceftazidime-avibactam susceptible to resistant KPC-producing enterobacterales bloodstream infections: An observational study. J Chemother 32:160-162.
[Crossref] [Google Scholar] [PubMed]
- Jorgensen SCJ, Trinh TD, Zasowski EJ, Lagnf AM, Bhatia S, et al. (2019) Real-world experience with Ceftazidime-Avibactam for multidrug-resistant gram-negative bacterial infections. Open Forum Infect Dis. 6:ofz522.
[Crossref] [Google Scholar] [PubMed]
- Jorgensen SCJ, Trinh TD, Zasowski EJ, Lagnf AM, Bhatia S, et al. (2020) Evaluation of the INCREMENT-CPE, Iitt bacteremia and qPitt scores in patients with carbapenem-resistant enterobacteriaceae infections treated with Ceftazidime-Avibactam. Infect Dis Ther 9:291-304.
[Crossref] [Google Scholar] [PubMed]
- Katchanov J, Asar L, Klupp EM, Both A, Rothe C, et al. (2018) Carbapenem-resistant gram-negative pathogens in a German university medical center: prevalence, clinical implications and the role of novel beta-lactam/beta-lactamase inhibitor combinations. PLoS One 13:e0195757.
[Crossref] [Google Scholar] [PubMed]
- King M, Heil E, Kuriakose S, Bias T, Huang V, et al. (2017) Multicenter study of outcomes with Ceftazidime-Avibactam in patients with Carbapenem-resistant enterobacteriaceae infections. Antimicrob Agents Chemother 61:1-4.
[Crossref] [Google Scholar] [PubMed]
- Krapp F, Grant JL, Sutton SH, Ozer EA, Barr VO (2017) Treating complicated carbapenem-resistant enterobacteriaceae infections with ceftazidime/avibactam: A retrospective study with molecular strain characterisation. Int J Antimicrob Agents 49:770-773.
[Crossref] [Google Scholar] [PubMed]
- Rodriguez-Nunez O, Ripa M, Morata L, de la Calle C, Cardozo C, et al. (2018) Evaluation of ceftazidime/avibactam for serious infections due to multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa. J Glob Antimicrob Resist 15:136-139.
[Crossref] [Google Scholar] [PubMed]
- Santevecchi BA, Smith TT, MacVane SH (2018) Clinical experience with ceftazidime/avibactam for treatment of antibiotic-resistant organisms other than Klebsiella pneumoniae. Int J Antimicrob Agents 51:629-635.
[Crossref] [Google Scholar] [PubMed]
- Shields RK, Potoski BA, Haidar G, Hao B, Doi Y, et al. (2016) Clinical outcomes, drug toxicity, and emergence of Ceftazidime-Avibactam resistance among patients treated for Carbapenem-resistant enterobacteriaceae infections. Clin Infect Dis 63:1615-1618.
[Crossref] [Google Scholar] [PubMed]
- Shields RK, Nguyen MH, Chen L, Press EG, Potoski BA, et al. (2017) Ceftazidime-Avibactam is superior to other treatment regimens against Carbapenem-resistant klebsiella pneumoniae Bacteremia. Antimicrob Agents Chemother 61:e00883-17.
[Crossref] [Google Scholar] [PubMed]
- Shields RK, Nguyen MH, Chen L, Press EG, Kreiswirth BN, et al. (2018) Pneumonia and renal replacement therapy are risk factors for Ceftazidime-Avibactam treatment failures and resistance among patients with Carbapenem-resistant enterobacteriaceae infections. Antimicrob Agents Chemother 62:e02497-17.
[Crossref] [Google Scholar] [PubMed]
- Sousa A, Perez-Rodriguez MT, Soto A, Rodriguez L, Perez-Landeiro A, et al. (2018) Effectiveness of ceftazidime/avibactam as salvage therapy for treatment of infections due to OXA-48 carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 73:3170-3175.
[Crossref] [Google Scholar] [PubMed]
- Spoletini G, Etherington C, Shaw N, Clifton IJ, Denton M, et al. (2019) Use of ceftazidime/avibactam for the treatment of MDR pseudomonas aeruginosa and Burkholderia cepacia complex infections in cystic fibrosis: a case series. J Antimicrob Chemother 74:1425-1429.
[Crossref] [Google Scholar] [PubMed]
- Temkin E, Torre-Cisneros J, Beovic B, Benito N, Giannella M, et al. (2017) Ceftazidime-Avibactam as salvage therapy for infections caused by Carbapenem-Resistant organisms. Antimicrob Agents Chemother 61:e01964-16.
[Crossref] [Google Scholar] [PubMed]
- Tsolaki V, Mantzarlis K, Mpakalis A, Malli E, Tsimpoukas F, et al. (2020) Ceftazidime-Avibactam to treat life-threatening infections by Carbapenem-resistant pathogens in critically Ill mechanically ventilated patients. Antimicrob Agents Chemother 64:e02320-19.
[Crossref] [Google Scholar] [PubMed]
- Tumbarello M, Trecarichi EM, Corona A, De Rosa FG, Bassetti M, et al. (2019) Efficacy of Ceftazidime-Avibactam salvage therapy in patients with infections caused by Klebsiella pneumoniae Carbapenemase-producing K. pneumoniae. Clin Infect Dis 68:355-364.
[Crossref] [Google Scholar] [PubMed]
- Van Duin D, Lok JJ, Earley M, Cober E, Richter SS, et al. (2018) Colistin versus Ceftazidime-Avibactam in the treatment of infections due to Carbapenem-resistant enterobacteriaceae. Clin Infect Dis 66:163-171.
[Crossref] [Google Scholar] [PubMed]
- Vena A, Giacobbe DR, Castaldo N, Cattelan A, Mussini C, et al. (2020) Clinical experience with Ceftazidime-Avibactam for the treatment of infections due to multidrug-resistant gram-negative bacteria other than Carbapenem-resistant enterobacterales. Antibiotics 9:71.
[Crossref] [Google Scholar] [PubMed]
- Poirel L, Potron A, Nordmann P. (2012) OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother 67:1597-1606.
[Crossref] [Google Scholar] [PubMed]
- Lagacé-Wiens P, Walkty A, Karlowsky JA (2014) Ceftazidime-avibactam: an evidence-based review of its pharmacology and potential use in the treatment of Gram-negative bacterial infections. Core Evid 9:13-25.
[Crossref] [Google Scholar] [PubMed]
- Bradley JS, Roilides E, Broadhurst H, Cheng K, Huang LM, et al. (2019) Safety and efficacy of Ceftazidime-Avibactam in the treatment of children =3 Months to <18 years with complicated urinary tract infection: results from a phase 2 randomized, controlled trial. Pediatr Infect Dis J 38:920-928.
[Crossref] [Google Scholar] [PubMed]
- Bradley JS, Broadhurst H, Cheng K, Mendez M, Newell P, et al. (2019) Safety and efficacy of Ceftazidime-Avibactam plus Metronidazole in the treatment of children =3 months to <18 Years with complicated intra-abdominal infection: results from a phase 2, randomized, controlled trial. Pediatr Infect Dis J 38:816-824.
[Crossref] [Google Scholar] [PubMed]
- Bradley JS, Armstrong J, Arrieta A, Bishai R, Das S, et al. (2016) Phase I study assessing the pharmacokinetic profile, safety, and tolerability of a single dose of Ceftazidime-Avibactam in hospitalized pediatric patients. Antimicrob Agents Chemother 60:6252.
[Crossref] [Google Scholar] [PubMed]
- Alexander EL, Loutit J, Tumbarello M, Wunderink R, Felton T, et al. (2017) Carbapenem-resistant enterobacteriaceae infections: results from a retrospective series and implications for the design of prospective clinical trials. Open Forum Infect Dis 4:ofx063.
[Crossref] [Google Scholar] [PubMed]
- Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, et al. (2015) Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 70:2133-2143.
[Crossref] [Google Scholar] [PubMed]
- De Maio Carrilho CMD, de Oliveira LM, Gaudereto J, Perozin JS, Urbano MR, et al. (2016) A prospective study of treatment of carbapenem-resistant Enterobacteriaceae infections and risk factors associated with outcome. BMC Infectious Diseases 16:629.
- Cisneros JM, Rosso-Fernández CM, Roca-Oporto C, De Pascale G, and Jiménez-Jorge S, et al. (2019) Colistin versus meropenem in the empirical treatment of ventilator-associated pneumonia (Magic Bullet study): an investigator-driven, open-label, randomized, noninferiority controlled trial. Critical Care 23:383.
[Crossref] [Google Scholar] [PubMed]
- Tumbarello M, Raffaelli F, Giannella M, Mantengoli E, Mularoni A, et al. (2021) Ceftazidime-avibactam use for KPC-Kp infections: a retrospective observational multicenter study. Clin Infect Dis 73:1664-1676.
[Crossref] [Google Scholar] [PubMed]
- Onorato L, Di Caprio G, Signoriello S, Coppola N (2019) Efficacy of ceftazidime/avibactam in monotherapy or combination therapy against carbapenem-resistant Gram-negative bacteria: a meta-analysis. Int J Antimicrob Agents 54:735-740.
[Crossref] [Google Scholar] [PubMed]
Citation: Gales AC, Carmargo LFA, Cuba GT, Tuon FF, Choo HH, et al. (2022) A Review of Real-World Use of Ceftazidime-Avibactam for Multidrug-Resistant Gram- Negative Bacterial Infections. J Infect Dis Ther 10:483 DOI: 10.4172/2332-0877.1000483
Copyright: © 2022 Gales AC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3236
- [From(publication date): 0-2022 - Apr 29, 2025]
- Breakdown by view type
- HTML page views: 2544
- PDF downloads: 692