A Randomized Study Assessing the Effect of Nattokinase-Meriva Formula on Low Grade Inflammation and Blood Viscosity in Adults with Increased Visceral Adiposity
Received: 16-Dec-2021 / Accepted Date: 30-Dec-2021 / Published Date: 06-Jan-2022
Abstract
Background: Chronic systemic inflammation in obesity spurs from local immune responses generated from visceral adipose body compartment and this entails a wide range of inflammation-mediating cytokines and adipometrics plasticity. On the other hand, while obesity is a leading risk factor for type 2 diabetes, its prevalence is significantly in the elderly. As a matter of fact, it has been shown that the age-associated increase in adipose tissue inflammation represents a different entity from what observed in obesity. In both contexts, a silent low-grade inflammation of adipose tissue (AT), mainly visceral AT (VAT) in which M1 are the main contributors, is a common pathophysiological feature behind insulin resistance and type 2 diabetes (T2D).
Aim of the study: The aim of this study was to characterize the profile of a broad range of pro-inflammatory cytokines, their related gene expression and blood viscosity in individuals with general mild-moderate overweight and in non-obese subjects with increased VAT.
Study design: This was a cross-sectional investigational research including 66 mildly overweight patients (body mass index (BMI) ≤ 29) and also 31 non-overweight subjects (BMI ≤ 22) with impending metabolic syndrome, classified as normal-weight obese (NWO). Subjects were supplemented with 1cp two times a day of J2622/G (Modulase, Named ltd, Italy), a mixture of nattokinase, high-absorption curcumin (Meriva), Bromelin, papain, and mirrha.
Results: As compared to healthy control, overweight status was associated with significantly elevated levels of IL- 6, TNF-α, IL-1β, MCP-1, IL-4, IL13, IL-8 and hsCRP (p<0.05). Treatment with J2622/G enabled a significant decreased of all the above inflammatory markers (p<0.01) whereas it did not affect the values of those markers which were within normal limits at baseline. Cytokines significantly correlated with adipometrics, irrespective of the mildly overweight or NWO subgroups. Gene expressions related to those abnormal cytokines were found to be significantly up regulated (p<0.05) and were reverted to healthy control gene expression levels at the end of the treatment period (p<0.05). There was no correlation between blood viscosity and antrometrics, nor the former were different from healthy control. However, the treatment with J2622/G already after 1 months treatment yielded a statistically significant decrease of blood viscosity (p<0.05). Adiponectin was found to be decreased only in the 25-29 BMI cohort and this was increased at the end of nutraceutical treatment (p<0.05).
Conclusion: We confirmed that either mild over-weight and NWO subjects harbor a relentless low-grade inflammation which may pave the way to silent progression of cardiovascular and metabolic diseases. A nattokinasemeriva formulation proved to yield a therapeutic and likely preventative efficacy for its robust anti-inflammatory and microcirculatory properties.
Keywords
Nattokinase; Curcumin; Bromelin; Cytokines; Blood viscosity; Adipokines
Introduction
Overweight condition as well as overt obesity is one the most expanding medical health threat worldwide [1]. Such excess weight, at different degree, is frequently associated to a number of comorbidities represented by metabolic syndrome, insulin resistance, diabetes, cardiovascular diseases and atherosclerosis and other health disorders linked to chronic inflammatory process [2-4]. As a matter of fact, overweight and obesity show the typical feature of low-grade inflammation where the fat tissue, and namely, visceral adipose tissue (VAT), being the largest endocrine gland in the body, plays the main role in releasing a wide array of inflammatory mediators (cytokines, hormones, acute-phase proteins, chemokimes, Retinol-binding protein 4, growth factors) [5,6]. This seems to originate from infiltrating activated macrophages population (M1-polarized macrophages) and, at minor extent, also from adipocytes. As a matter of fact, it has been observed that in obesity macrophages costituite up to 40% of all VAT cells, thus representing the most abundant cellular population [7].
On the other hand, more than 15 years ago it was defined the new concept of metabolically obese normal-weight (NWO) subjects which has been further confirmed in recent times [8]. These individuals may indeed present some characteristics of metabolic syndrome while still maintaining a BMI ≤ 25. However, these subjects, although to a lesser extent, share with overweight subjects an increase VAT. This is now well recognised to represent a multifunctional endocrine organ secreting several proteins and peptides [9], which, if dis regulated by chemo attractant cytokine, get released and trigger VAT infiltration of M1 macrophages. This phenomenon significantly contributes to a low grade inflammation of VAT and brings about detrimental systemic morbidities [10,11]. In such situations, it occurs a constant dyrsruption of cytokine anti-inflammatory/pro-inflammatory cytokines balance leading to a preponderance of the M1 macrophage activation [12]. On their turn, the activation of pro-inflammatory TH1 and M1 macrophages have been reported to produce IFN-γ, TNF-α, and IL- 12 [13]. Although the VAT secretion of pro-inflammatory adipokines by hypertrophied adipocytes is a common feature in obese subjects, data show that such phenomena are already creepingly building up in overweight non-obese status [14], whereas the secretion of antiinflammatory adipokines seems to be suppressed [15]. Moreover, other adipose tissue depots such as the ones within the liver, heart or skeletal muscle, may also contribute to the production of inflammatory mediators in the absence of obesity. In all such setting, the levels of interferon-γ (IFN-γ), IL-4, IL-5, and IL-13 elevated in MetS [16]. As a matter of fact, IFN-γ and granulocyte macrophage colony-stimulating factor (GM-CSF) exhibit pro-M1-properties [17].
Although there are no clear-cut diagnostic criteria defining low-grade inflammation as yet, a common feature is represented by a significant, albeit modest, over-expression of systemic markers of inflammation such as pro-inflammatory cytokines, chemokines, acute-phase proteins, that is plasminogen activator inhibitor 1 (PAI-1), hsCRP, soluble adhesion molecules and prothrombotic molecules [18]. These may fuel the gradual development of chronic inflammation, insulin resistance and atherosclerosis via a metabolic dysregulation associated with obesity where the pro-inflammatory leptin, secreted by white adipose tissue, as opposed to anti-inflammatory and insulin-sensitising adiponectin, plays a key role [19]. Nattokinase, a Bacillus fermented soybean functional food, has a long established scientific support for its cardio protective, anti-inflammatory and rheological properties as a whole [20-22]. It is also well-known how the known poor bioavailability of curcumin has been greatly improved by a proper phytosomal complex-binding (Meriva) which is still given credit by most recent papers dealing with fat diet- or diabetes-related inflammation [23,24].
The aim of this study was to characterize the profile of a broad range of pro-inflammatory cytokines and the effect of a nattokinase-meriva nutraceutical intervention in individuals with general mild-moderate overweight or also normal weight but with increased VAT. Moreover, haemorrheological variables were also monitored. Finally, an in vitro experimental was planned to test this compound on pre-adipocytes as a prove of concept at a cellular level.
Materials and Methods
Patients
Main general recruitment criteria were, age range (35-66), mildmoderate overweight (BMI ≤ 28), any gender.
Exclusion criteria were serum glucose ≥6.93 mmol/L, moderate-severe dyslipidemia (total cholesterol (T-chol) >260mg/dl and/or triglycerides (TG) >250 mg/dl), a history of cardiovascular or cerebral events, digestive functional disorder (on the basis of the classification of Rome III), weight loss >3kg in the past 3 months, cancer, haematological, infectious or inflammatory disease, kidney or liver disease, pregnancy, substance abuse, consistent alcohol consumption, thromboembolism, treatment with blood thinning drugs, hypertension (diastolic blood pressure >90 mmHg or systolic blood pressure >140 mmHg) or receiving drug treatment for diabetes, dyslipidemia and overt hormonal dysfunction-related secondary obesity. Overall 101 subjects were included into the study. However, 5 were lost at follow up after 4 weeks for personal family reasons, 3 moved to another region, 1 had a car accident unrelated to treatment, 2 started indulging on heavy drinking, 1 had to start a pharmacological treatment for a severe dermatology infection following an accidental leg trauma during gardening. Alltogether, 89 subjects completed the investigation and were considered for study evaluation (Table 1).
| Parameter | Overweight (61) | Normal Weight Obese (28) | p value |
|---|---|---|---|
| Age (years) | 51.2 ± 9.7 | 46.7 ± 10.3 | ns |
| BMI | 27.2 ± 3.2 | 23.2 ± 2.2 | ns |
| Gender (male/female) | 25/36 | 9/20 | ns |
| Light Alcohol use (≤ 4 drinks/ week) | 13/4 | 8/1 | ns |
| Smoking (m/f) | 14/6 | 8/3 | ns |
| Waist-to-hip Ratio (cm) (m/f) | 0.88 ± 0.8/0.84 ± 0.1 | 0.84 ± 0.13/ 0.80 ± 0.08 | ns |
| FAMILY HISTORY (m/f) | |||
| Diabetes | 8/21 | 8/16 | ns |
| Obesity | 6/10 | 7/12 | ns |
| Cardiovascular Disease | 4/7 | 6/9 | ns |
| Ischemic Stroke | 3/2 | 2/1 | ns |
Table 1: Investigation and study evaluation of 89 subjects.
A typical DEXA scan of a NWO is shown in Figure 1 where a normal weight male shows an unsuspected value of VAT over 2 times the upper normal limit.
A separate group of 20 age- and gender-matched healthy controls with BMI ≤ 23, was used as a reference.
Written informed consent was obtained from each subject before entry into the study. The study was approved by the independent ethics committee and was carried out in accordance with the Declaration of Helsinki, the International Conference on Harmonization, and Good Clinical Practice guidelines.
Study design
Subjects were supplemented with 1cp two times a day of J2622/G (Modulase, Named LLC, Italy), a mixture of nattokinase, a patented high-absorption curcumin (Meriva), Bromelin, papain and myrrha (Table 2). All unused compounds were retrieved for inventory. Treatment compliance was assessed by counting the number of product doses returned at the time of specified clinic visits. One full day dose-skipping or 2 non-consecutive 1 capsule-skipping was not considered as a study withdrawal criterion.
| Ingredients | Dosage/activity |
|---|---|
| Fermented soya extract NSK-SD® | 100mg (2000FU nattokinase activity) |
| Meriva® Curcuma phospholipids complex | 200mg |
| Bromelin | 500mg (1250 Gelatin Digestion Units) |
| Papain | 400mg (total proteolytic activity: 40000 TU) |
| Myrrha | 200mg (standardized furanodienes content: ≥6mg) |
| Piperin | 4mg |
| Vitamin C | 48mg |
Table 2: Composition breakdown of the total dosage per day (2cps) of J2622/I.
Anthropometric measurements and dietary monitoring
BMI was calculated by dividing the weight by the square of height. Waist circumference was measured midway between the iliac crest and the lowest rib and recorded to the nearest 0.5 cm. Hip circumference was measured at the maximum level and recorded similarly. Nutritional status was assessed using anthropometric measurements at start of the study at the entry, at 30, 60 and 90 days afterwards. A 3-day weighedfood record of 2 weekdays and 1 weekend day was performed during the first and the last week of the study. The participants were asked to avoid alcohol and caffeine intake on the previous day of blood collection, and also to maintain their sleep habits during the previous night.
Body composition
Body composition (fat free mass (FFM), fat mass (FM) and gynoid and android fat distribution) was calculated each time by dual-energy X-ray absorptiometry (DXA) through a Lunar Prodigy DEXA (GE Medical Systems). The coefficients of variation resulted to be 0.93% and 0.61% for whole FM and FFM, respectively. VAT volume was assessed by applying a correction factor (0.95 g/cm3).
Blood tests
After an overnight fasting individual were asked to rest in an isolated and quiet room for 5 min. Then, systolic (SBP) and diastolic (DBP) blood pressure was measured on the right arm, after the participant had been resting for at least 5 minutes, with a standardized sphygmomanometer and blood was withdrawn from the antecubital vein and split in duplicate handling. A total of 10mls of venous blood was drawn from the antecubital vein from most subjects or dorsum of the hand in a few subjects with difficult veins access and in each case with minimum stasis by applying a tourniquet. One part was put into dextrose K3 EDTA anti-coagulant citrate dextrose solution containing 0.8% citric acid, 2.2% trisodium citrate, and 2% for haematological tests. Glucose, cholesterol, HDL-C, triglycerides and hsCRP were measured using commercial kits (Synchron LXT 20 analyzer, Beckman-Coulter, UK) with all assays performed by using Biochip array technology to perform simultaneous quantitative detection of multiple analytes from a single patient sample containing an array of discrete test regions of immobilized antibodies specific to different cytokines and growth factors. The kit and device were each time handled with appropriate calibrators and quality controls (Roche for Roche assays; and Wako standard). Plasma adiponectin was determined by double antibody radioimmunoassay (Linco Research, St. Charles, MO, USA). The coefficient of intra- and inter-assay variation was found to be always <9%.
Cytokine measurement
From separate tube the serum was immediately centrifuged at 2000 g for 15 min at 4°C and 300 μl aliquots of the supernatant, put into Eppendorf tubes, were frozen in liquid nitrogen and stored in at -80°C until batch analysis. In any case, the whole process was carried out within 30min of blood collection. Cytokines were measured using the Bio-Plex Pro human cytokine Th1/Th2 immunoassay 96-well kit (Bio Rad, Germany), containing coupled magnetic beads and related antibodies. This multiplex kit assays IL-8, IL-4, IL-5, IL-18, IL-13, IL- 1β, IL1α, GM-CSF, MCP-1, IFNγ and TNF-α by using the commercially available Invitrogen Human Ultrasensitive Cytokine Magnetic 10-Plex Panel (Life Technologies, Carlsbad, CA, USA). Assays were read using a Luminex 200 Analyser (Luminex Corporation, Austin, TX, USA) with commercially available calibrators (Life Technologies, Carlsbad, CA, USA) and an in-house quality control. Assay sensitivity is shown in Table 3 all samples were measured in duplicate and the average of the 2 values was used for final data analysis. Samples that did not yield an intra- and inter-assay coefficient of variation < 8% were reanalyzed and all values were averaged for data analysis. Samples with undetectable concentrations were assigned a value corresponding to the lower limit of detection of the assay before intervention, 1 month and 3 months after intervention were determined by using Quantikine high-sensitivity immunoassay kits (R&D Systems, Minneapolis, MN, USA).
| Biomarker | Calibration range (pg/mL) | Sensitivity (pg/mL) |
|---|---|---|
| IL-6 | 0-900 | 8,1 |
| TNF-α | 0-1600 | 3.8 |
| IL-1β | 0-500 | 1.8 |
| IFNγ | 0-1500 | 3.7 |
| MCP-1 | 0-1500 | 11.6 |
| IL-4 | 0-4000 | 5.9 |
| IL-8 | 0-3000 | 7.3 |
| IL-1α | 0-500 | 1.0 |
| IL-18 | 0-1500 | 1.0 |
| GM-CSF | 0-500 | 1.0 |
| hs-CRP | 0-300 | 0.05 |
Table 3: Cytokine assay sensitivity.
Cytokines gene expression
This was conducted only for cytokines found to be significantly different in the serum when compared between patients and controls (twenty healthy hospital staff and laboratory co-researchers). Blood sampling from healthy controls was at one time point only. PBMC were isolated from whole venous blood (10 ml) collected into heparinated tubes and diluted with an equal volume of PBS. Cells separation was carried out by density gradient centrifugation (Ficoll-Paque method). For each sample, two l5-ml centrifuge tubes were used to layer 7 ml of diluted blood onto an equal volume of Ficoll-Hypaque. The suspension was centrifuged for 30 min at 450g and 20℃. The mononuclear cell layer was manually removed with pipetting, washed twice with PBS, and centrifuged for 10 min at 10℃ and 275g after each wash and stored at -80. Total RNA was extracted using the RNeasy kit (Qiagen, Crawley, West Sussex, UK), as described by the manufacturer. Two micrograms of total RNA were used for cDNA synthesis and for subsequent gene expression analysis in Real Time PCR.
Gene expression analysis
The concentration and purity of the recovered RNA was assessed by ultraviolet absorbance at 260 and 280 nm and its integrity by Agarose gel electrophoresis. TaqMan Gene expression assays (ABI Prism 7900 HT, Applied Bio systems) was employed to measure the quantification of gene expression. Expression of cytokine mRNA for each sample was then expressed as an arbitrary ratio of the quantity of mRNA to that of b-actin. The sequence of primers were designed for an annealing temperature of 60 using Primer 3 software except from β-actin primers (a housekeeping gene) annealing at 60. The ratio of sample to standard products remained constant up to 39 cycles. The primers were used for relative quantification of targeted gene expression as follows. For TNF-a: forward primer, 5'-GCCACCACGCTCTTCTGT-3'; reverse primer, 5'-•GGCTACGGGCTTGTCACTC-3'. For MCP-1: forward primer, 5’-TTCTCAAA CTGAAGCTCGC-3’; reverse primer, 5'-AAGCTAGGGGAAAATAAGTT IL- 6: forward primer, 5'- GTATGAACAGCGATGATGCAC-3'; reverse primer, 5'- GAAACGGAACTCCAGAAGACC-3'; for IL-4 forward primer,AGCAGTTCCACAGGCACAAG-3' reverse primer, 5'-CTGGTTGGCTTCCTTCACAG-3'; IL-13: forward primer, GTACTGTGCAGCCCTGGAAT-3' reverse primer, 5'- TTTACAAACTGGGCCACCTC-3'; for IL-8: 5’-CATACGAATTCCATGGGCAAGCTTGAATCTAAATTA-3’ reverse primer: 5’-CATATGGATCCGCTAGTCTTCGTTTTGAACAG; for IL-1β: forward primer GACCTGTTCTTTGAGGCT GAC and reverse primer TTCATCTCGAAGCCTGCAGTG; For β-actin: forward primer: 5'-TCCCTGGAGAAGAGCTACGA-3'; reverse primer, 5'-ATCTGCTGGAAGGTGGACAG-3'. Reactions were optimized to a final volume of 25 μl, IX reaction buffer, 0.2 mM of each deoxynucleotide, 2.5 mM MgClz2 0.15ItM of each primer, 1:100,000 SYBR Green I (Molecular Probes, Leiden, Netherlands) and 0.4 U of HotStart DNA Taq Polimerase. For each target primer set, a validation experiment was carried out to validate PCR efficiencies range of 90- 100% and equal to the efficiencies of the reference gene. Each sample was measured in duplicate and while normalizing each target gene to reference gene expression. Agarose gel electrophoresis was performed to confirm that there were present single-product amplifications without primer dimers.
Whole blood viscosity
Whole blood viscosity (WBV) of each blood sample was determined immediately after collecting the blood sample in a Rheostress-1 double cone viscometer (HAAKE MassTechnik, Karlsruhe, Germany), with a cone angle of 11 at 310 K and equipped with a cp-40 spindle. The analysis was performed over the shear rate range of 1-1000 s-1 C but specifically set at 5, 50, 150, 300 s-1 under a computer monitored program and the coefficient of variation was below 3%.
Adverse events
For safety assessments electrocardiograms, vital signs and biochemical tests (hemoglobin, hematocrit, platelet, blood urea nitrogen, glomerular filtration rate, Creatinine, total protein, and liver tests) were monitored and recorded throughout the trial.
Statistics
All tests were performed by SAS for Windows version 6.9.4 (SAS Institute, Cary, NC). Results are presented with means ± standard deviations (SD) for continuous variables. The analysis of covariance (ANCOVA) test was used to assess the changed values from baseline to 4 months. No separation into groups based on physical activity was performed, given the overall lack of any significant workload among participants. Differences between study participants were assessed by t-tests following Gaussian distribution, and Chi2-tests (gender and smoking) and the statistical significance were accepted for p values < 0.05. In case cytokine values were no detectable then an arbitrary level of 0 was recorded.
The standards and controls used in all the biochemical assays were periodically reviewed as for quality assurance before proceeding in analyzing the samples.
Result and Discussion
No adverse effect connected to the treatment employed was reported by participants and no significant change occurred in the routine blood chemistry (data not shown).
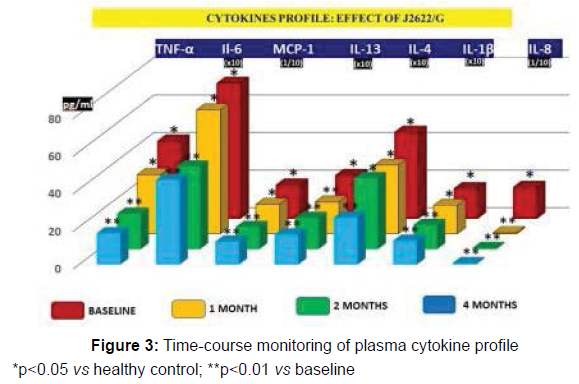
Plasma Cytokine profile analysis. As compared to healthy control, IL-6, TNF-α, IL-1β, MCP-1, IL-4, IL-13, IL-8 and hsCRP showed a statistically significant increase (p<0.05). This equally applied to either mildly overweight and to NWO subgroups without any significant difference between the two. Treatment with J2622/G enabled a significant decrease of all the above inflammatory markers (p<0.01 vs baseline), also when analysed splitting the mildly overweight and NWO subgroups (data not shown). On the other hand, the nutraceutical treatment did not affect the values of the markers which were within normal limits at baseline (Table 4).
| Biomarker | At entry | End of study | Statistics |
|---|---|---|---|
| IL-6 (pg/ml) Control 1.5 ± 0.3 |
2.3±0.1* | 1.6±0.2 | < 0.01 |
| TNF-α (pg/ml) Control 14.4 ± 5.3 |
41.3±9.1* | 16.7±4.1 | < 0.01 |
| IL-1β (pg/ml) Control 2.2 ± 0.3 |
4.6±0.3* | 2.5±0.2 | < 0.01 |
| IFNγ (pg/ml) Control 3.1 ± 0.4 |
2.4 ± 0.3 | 2,2 ± 0.2 | ns |
| MCP-1 (pg/ml) Control 112.2 ± 7.3 |
179.1 ± 22.1* | 122.2 ± 0.1 | < 0.01 |
| IL-4 (pg/ml) Control 3.8 ± 0.5 |
7.2 ± 1.6* | 4.5 ± 0.9 | < 0.01 |
| IL-8 (pg/ml) Control 72.2 ± 24.3 |
188.1 ± 85.1* | 74.1 ± 34.16 | < 0.01 |
| IL-1α (pg/ml) Control 1.2 ± 0.2 |
1.4 ± 0.1 | 1.3 ± 0.6 | ns |
| IL-18 (pg/ml) Control 2465 ± 26.7 |
276.8 ± 65.7§ | 258.9 ± 44.4§ | ns |
| hs-CRP (mg/L) Control 0.4±0.1 |
0.9 ± 0.1* | 0.5 ±0.2 | < 0.01 |
| GM-CSF(pg/ml) Control 3.3 ± 0.3 |
3.5 ± 0.2 | 3.4 ± 0.4 | ns |
| IL-13 (pg/ml) Control 1.21 ± 0.4 |
1.67 ± 0.2* | 1.27 ± 0.1 | < 0.01 |
Table 4: Serum Cytokines and hs-CRP level.
§ Interleukin-18 showed a wide dispersion of values with no statistically significant variation as a whole. However, when this parameter was calculated in the albeit statistically-limited cluster of patients with higher BMI quartile and some of them having also an untreated borderline hypertension, it appeared a trend increased value which normalized at the end of the study period (data not shown).
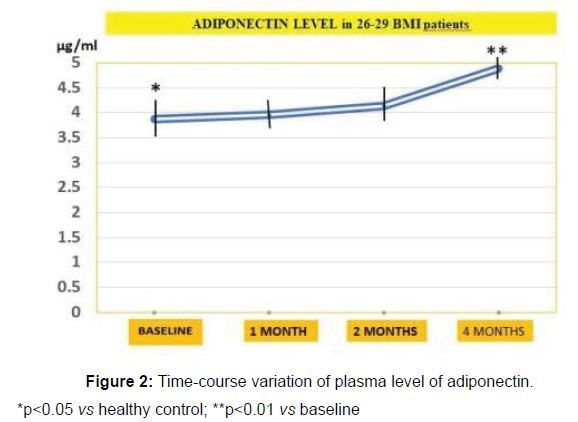
The levels of adiponectin was found decreased in 26-29 BMI cohort (p<0.05 vs healthy controls) and increased significantly in the intervention group (3.88 μg/ml vs 5.65 μg/ml, p<0.01, Figure 2).
*p<0.05 vs healthy control; **p<0.01 vs baseline
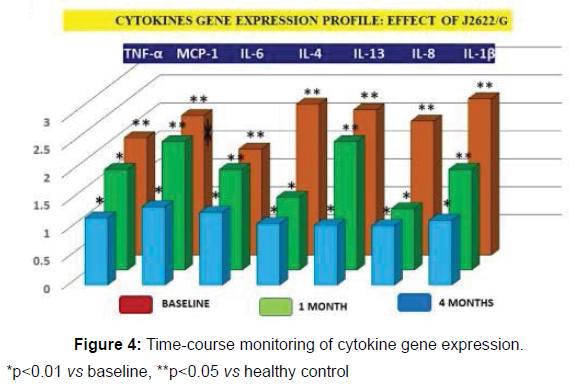
Cytokine gene expression analysis
The gene expressions of abnormal cytokine equally showed a significant elevation (p<0.05 vs control) and the time-course variations due to J2622/G treatments either for cytokines and gene expression is shown in Figures 3 and 4, respectively.
*p<0.05 vs healthy control; **p<0.01 vs baseline
*p<0.01 vs baseline, **p<0.05 vs healthy control
Whole blood viscosity
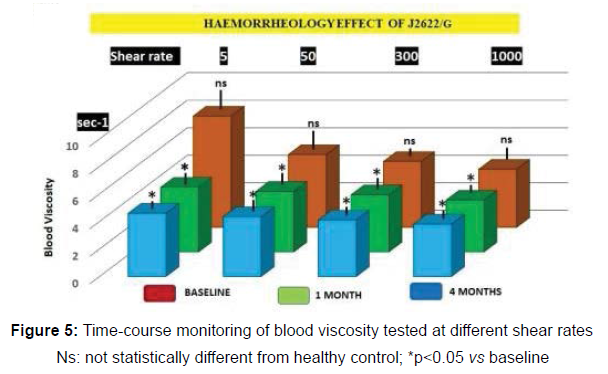
Blood pressure parameters showed no significant differences during the study period and no correlation with relative blood viscosity in the subjects under study. There was no correlation between blood viscosity and anthrometrics, nor was the former different from healthy control at baseline. However, already after 1 month the treatment with J2622/I it appeared a statistically significant improvement of the haemorrheological parameter at all different shear testing which was maintained at the same extent throughout the study period (p<0.05, Figure 5).
Ns: not statistically different from healthy control; *p<0.05 vs baseline
Conclusion
The concept of inflammation, i.e. the common or parallel signalling pathways shared by aging process per sè and chronic inflammatory changes has gained a wide recognition [25]. Such often silent parenchymal and gut microbiota abnormality [26-34] has a traceable hallmark in elevated peripheral levels of inflammatory cytokines and acute phase reaction proteins. The age- but also dietary/life style-associated Insulin resistance thus represents a detrimental turning point in low-grade inflammation in adipose tissue and worsened by associated sarcopenia [35]. While brown adipose tissue (BAT) has a thermogenetic function, the white adipose tissue and namely the visceral adipose tissue (VAT), has one third of capillary network as compared to BAT and potentially accumulating a great deal of inflammatory mediators, namely IL-6, IL-8, MCP-1 and GM-CSF. This happens because high fat/calorie diet triggers the infiltration of VAT by leukocytes and related oxidativeinflammatory phenomena [36]. In this study we purpously investigated the cytokine profile and related gene expression in subject with mildoverweight in an effort to unveil the existence of early low grade inflammation. To further strengthen this working hypothesis, we also included NWO known for their subtle inflammatory phenotype [6-8]. Our study confirmed that a wide array of tested inflammatory plasma cytokines and related genes were significantly elevated as compared to healthy control. Based this rationale we proved the significant beneficial effect of J2622/G formulation in curbing till normalization all abnormal cytokines together with downregulating nutrigenomic effect along the 4-month study period. The planned absence of obese individuals in our study group is probably explaining the lack of significant correlation between cytokines and BMI, as reported elsewhere [37]. Although, to the best of our knowledge, this is the first comprehensive interventional clinical study employing such three main ingredients mixture (i.e. nattokinase, high-bioavailable curcumin and bromelin), each one of them is backed up by a sound literature [38-42]. This holds particularly true for nattokinase but also bromelain and curcumin. The latter has also conflicting negative literature data [43] probably owing to its poor absorption and this had indeed prompted us to use a likely more successful Meriva (a patented high-bioavailability phospholipidscomplexed curcumin).
Our study population didn’t show at plasma level an higher leptin level and/or lower adiponectin as expected in overt obese and more metabolically compromised patients who have large, mature, proinflammatory cytokines-rich and insulin-insensitive adipocytes. In such patients the level of anti-inflammatory adiponectin is negatively correlated with BMI and VAT. However, in the higher BMI patients (26-29 BMI), we could already track down the onset of adiponectin deficiency which positively responded to J2622/G anti-inflammatory treatment. In this low grade inflammatory milieu associated to overweight and expansion of VAT, haemorrheological parameters play a further crucial homeostatic role. Indeed, VAT has less capillary density than brown fat and the increased adipocyte size is a further cellular metabolism hindrance leading to chronic hypoxia. The detrimental synergy between these two factors, on its turn, would perpetuate oxidative-inflammatory phenomena and increased secretion of leptin towards a systemic metabolic dysregulation at the base of chronic inflammation- and dyslipidemia-related disease mechanisms [44,45]. High blood viscosity is known to be a relevant pathophysiological risk factor in overall cerebrovascular and cardiovascular diseases by altering tissue perfusion and being associated to systemic inflammation. This data is confirmed up to the threathening point that, under relentless progression of low-grade inflammation and subtle fat ischemia even brown fat tissue may be turned into a “white fat” phenotype [46] overall contributing to metabolic derangement. The normal blood viscosity observed in our study population can be advocated for by the mildmoderate or NWO status. Nonetheless, when administered J2622/G compound, all of them showed a statistically significant reduction of blood viscosity already starting after one month and this equally applied no matter the shear rate we conducted the test. Vessels haemodynamics follow the Newtonian law: Q= AP 7l.r4/81fl, where Q = total flow, AP= pressure gradient, r = vessel radius, 1= tube length and rj= blood viscosity. However, this formula depends particularly in small arterioles and capillaries by other fundamental variables When analyzing the beneficial rheological effect of J2622/G one has to consider that at lower shear rates, the increased erythrocytes aggregation due to the elevated fibrinogen, CRP, IL-6 or other 'acute-phase' proteins concentration whereas at higher shear rates, higher plasma viscosity and hematocrit are playing a major role. The phenomena have been clearly identified as pathophysiological factors in patients with T2D, hypertension, advanced metabolic syndrome, atherosclerosis and early morning stroke [47-49] and, interestingly, Nattokinase benefit has been proven in experimental model of thrombotic brain injury [50,51].
Finally, these data hold a further interest to beneficially affect some of the current detrimental lifestyle habits. Indeed, especially with the pandemic-related expansion of so called “smart working”, the uninterrupted sitting has greatly increased. It has been shown that such attitude may significantly increase fibrinogen together with reduction of plasma volume while raising hemoglobin and hematocrit [52]. While short bouts of exercise may partly counteract these pro coagulant phenomena, one can envisage that the treatment we proposed here may work as an additional tool.
References
- Williams EP, Mesidor M, Winters K, Dubbert PM, Wyatt SB (2015) Overweight and Obesity: Prevalence, Consequences, and Causes of a Growing Public Health Problem. Curr Obes Rep 4:363-370.
- Catanzaro R, Cuffari B, Italia A, Marotta F (2016) Exploring the metabolic syndrome: Nonalcoholic fatty pancreas disease. World J Gastroenterol 22:7660-7675.
- Catanzaro R, Sciuto M, He F, Singh B, Marotta F (2020) Non-alcoholic fatty liver disease: correlation with hyperuricemia in a European Mediterranean population. Acta Clin Belg 19:1-6.
- Marotta F, Marcellino M, Solimene U, Cuffari B, Yadav H, et al. (2017) A 2-year Double-Blind RCT Follow-up Study with Fermented Papaya Preparation (FPP) Modulating Key Markers in Middle-Age Subjects with Clustered Neurodegenerative Disease-Risk Factors. Clin Pharmacol Biopharm 6:170.
- Saltiel AR, Olefsky JM (2017) Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest 127:1-4.
- Karczewski J, Śledzińska E, Baturo A, Jończyk I, Maleszko A, et al. (2018) Obesity and inflammation. Eur Cytokine Netw 29:83-94.
- Kang YE, Kim JM, Joung KH, Lee JH, You BR, et al. (2016) The Roles of Adipokines, Proinflammatory Cytokines, and Adipose Tissue Macrophages in Obesity-Associated Insulin Resistance in Modest Obesity and Early Metabolic Dysfunction. PLoS One.; 11:e0154003.
- Ding C, Chan Z, Magkos F (2016) Lean, but not healthy: the 'metabolically obese, normal-weight' phenotype. Curr Opin Clin Nutr Metab Care 19:408-417.
- De Lorenzo A, Del Gobbo V, Premrov MG, Bigioni M, Galvano F, et al. (2007) Normal-weight obese syndrome: early inflammation? Am J Clin Nutr 85:40-45.
- Sahakyan KR, Somers VK, Rodriguez-Escudero JP, Hodge DO, Carter RE, et al. (2015) Normal-Weight Central Obesity: Implications for Total and Cardiovascular Mortality. Ann Intern Med 163:827-835.
- Upadhyay J, Farr O, Perakakis N, Ghaly W, Mantzoros C (2018) Obesity as a Disease. Med Clin North Am 102:13-33.
- Mouton AJ, Li X, Hall ME, Hall JE (2020) Obesity, Hypertension, and Cardiac Dysfunction: Novel Roles of Immunometabolism in Macrophage Activation and Inflammation. Circ Res 126:789-806.
- Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, et al. (2018) Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol 233:6425-6440.
- Tareen SHK, Kutmon M, de Kok TM, Mariman ECM, van Baak MA, et al. (2020) Stratifying cellular metabolism during weight loss: an interplay of metabolism, metabolic flexibility and inflammation. Sci Rep 10:1651.
- Fasshauer M, Blüher M (2015) Adipokines in health and disease. Trends Pharmacol Sci 36:461-470.
- Pilatz A, Hudemann C, Wolf J, Halefeld I, Paradowska-Dogan A, et al. (2017) Metabolic syndrome and the seminal cytokine network in morbidly obese males. Andrology 5:23-30.
- Jaguin M, Houlbert N, Fardel O, Lecureur V (2013) Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell Immunol 281:51-61.
- Minihane AM, Vinoy S, Russell WR, Baka A, Roche HM, et al. (2015) Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr 114:999-1012.
- Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N (2014) Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract 105:141-50.
- Wu H, Wang Y, Zhang Y, Xu F, Chen J, et al. (2020) Breaking the vicious loop between inflammation, oxidative stress and coagulation, a novel anti-thrombus insight of nattokinase by inhibiting LPS-induced inflammation and oxidative stress. Redox Biol 32:101500.
- Chen H, McGowan EM, Ren N, Lal S, Nassif N, et al. (2018) Nattokinase: A Promising Alternative in Prevention and Treatment of Cardiovascular Diseases. Biomark Insights 13:1177271918785130.
- Gopikrishna T, Suresh Kumar HK, Perumal K, Elangovan E (2021) Impact of Bacillus in fermented soybean foods on human health. Ann Microbiol 71:30.
- Hatamipour M, Jamialahmadi T, Ramezani M, Tabassi SAS, Simental-MendÃa LE, et al. (2021) Protective Effects of Curcumin Phytosomes Against High-Fat Diet-Induced Atherosclerosis. Adv Exp Med Biol 1308:37-44.
- Mazzolani F, Togni S, Giacomelli L, Eggenhoffner R, Franceschi F (2018) Oral administration of a curcumin-phospholipid formulation (Meriva®) for treatment of chronic diabetic macular edema: a pilot study. Eur Rev Med Pharmacol Sci 22:3617-3625.
- Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A (2018) Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 14:576-590.
- Catanzaro R, Cuffari B, Italia A, Marotta F (2016) Exploring the metabolic syndrome: Nonalcoholic fatty pancreas disease. World J Gastroenterol 22:7660-7675.
- Catanzaro R, Selvaggio F, Sciuto M, Zanoli L, Yazdani A, et al. (2021) Triglycerides to high-density lipoprotein cholesterol ratio for diagnosing nonalcoholic fatty liver disease. Minerva Gastroenterol (Torino).
- Nagpal R, Kumar M, Yadav AK, Hemalatha R, Yadav H, et al. (2016) Gut microbiota in health and disease: an overview focused on metabolic inflammation. Benef Microbes 7:181-94.
- Marotta F, Mao GS, Liu T, Chui DH, Lorenzetti A, et al. (2006) Anti-inflammatory and neuroprotective effect of a phytoestrogen compound on rat microglia. Ann N Y Acad Sci 1089:276-281.
- Vaiserman AM, Koliada AK, Marotta F (2017) Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Res Rev 35:36-45.
- Nagpal R, Mainali R, Ahmadi S, Wang S, Singh R, et al. (2018) Gut microbiome and aging: Physiological and mechanistic insights. Nutr Healthy Aging 4:267-285.
- Shen X, Miao J, Wan Q, Wang S, Li M, et al. (2018) Possible correlation between gut microbiota and immunity among healthy middle-aged and elderly people in southwest China. Gut Pathog 10:4-8.
- Prasad C, Imrhan V, Marotta F, Juma S, Vijayagopal P (2014) Lifestyle and Advanced Glycation End Products (AGEs) Burden: Its Relevance to Healthy Aging. Aging Dis 5:212-217.
- Jain S, Marotta F, Catanzaro R, Yadav H (2012) Immune system and gut flora interactions are important episodes in metabolic diseases. J Gastrointestin Liver Dis 21:347-348.
- Banerjee A, Marotta F, Sriramulu S, Chabria Y, Hari S, et al. (2021) Beyond Physical Exercise: The Role of Nutrition, Gut Microbiota and Nutraceutical Supplementation in Reducing Age-Related Sarcopenia. Curr Aging Sci 14:94-104.
- Muñoz MF, Argüelles S, Marotta F, Barbagallo M, Cano M, et al. (2020) Effect of Age and Lipoperoxidation in Rat and Human Adipose Tissue-Derived Stem Cells. Oxid Med Cell Longev 2020:6473279.
- Himmerich H, Fulda S, Linseisen J, Seiler H, Wolfram G, et al. (2006) TNF-alpha, soluble TNF receptor and interleukin-6 plasma levels in the general population. Eur Cytokine Netw 17:196-201.
- Nagata C, Wada K, Tamura T, Konishi K, Goto Y, et al. (2017) Dietary soy and natto intake and cardiovascular disease mortality in Japanese adults: the Takayama study. Am J Clin Nutr 105:426-431.
- Wu H, Wang Y, Zhang Y, Xu F, Chen J, et al. (2020) Breaking the vicious loop between inflammation, oxidative stress and coagulation, a novel anti-thrombus insight of nattokinase by inhibiting LPS-induced inflammation and oxidative stress. Redox Biol 32:101500.
- Onken JE, Greer PK, Calingaert B, Hale LP (2008) Bromelain treatment decreases secretion of pro-inflammatory cytokines and chemokines by colon biopsies in vitro. Clin Immunol 126:345-352.
- Akbari M, Lankarani KB, Tabrizi R, Ghayour-Mobarhan M, Peymani P, et al. (2019) The effects of curcumin on weight loss among patients with metabolic Syndrome and Related Disorders: A systematic review and meta-analysis of randomized controlled Trials. Front Pharmacol 10:649.
- Panahi Y, Hosseini MS, Khalili N, Naimi E, Simental-MendÃa LE, et al. (2016) Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A post-hoc analysis of a randomized controlled trial. Biomed Pharmacother 82:578-582.
- Vors C, Couillard C, Paradis ME, Gigleux I, Marin J, et al. (2018) Supplementation with Resveratrol and Curcumin Does Not Affect the Inflammatory Response to a High-Fat Meal in Older Adults with Abdominal Obesity: A Randomized, Placebo-Controlled Crossover Trial. J Nutr 148:379-388.
- Jothimani G, Prasad SV, Marotta F, Pathak S (2019) Targeting Wnt Signaling through Small molecules in Governing Stem Cell Fate and Diseases. Endocr Metab Immune Disord Drug Targets 19:233-246.
- Yazdani A, Yazdani A, Bowman TA, Marotta F, Cooke JP, et al. (2018) Arachidonic acid as a target for treating hypertriglyceridemia reproduced by a causal network analysis and an intervention study. Metabolomics 14:78.
- Shimizu I, Walsh K (2015) The Whitening of Brown Fat and Its Implications for Weight Management in Obesity. Curr Obes Rep 4:224-229.
- Zhang G, Zhang T, Sun X, Tang F, Lin H, et al. (2020) Whole blood viscosity is an independent early predictor for metabolic syndrome and its components in men: A prospective cohort study in Northern Chinese population. Clin Hemorheol Microcirc.
- Gyawali P, Lillicrap TP, Tomari S, Bivard A, Holliday E, et al. (2021) Whole blood viscosity is associated with baseline cerebral perfusion in acute ischemic stroke. Neurol Sci.
- Brar SK, Perveen S, Chaudhry MR, AlBabtain S, Amreen S, et al. (2021) Erythropoietin-Induced Hypertension: A Review of Pathogenesis, Treatment, and Role of Blood Viscosity. Cureus 13:e12804.
- Jang JY, Kim TS, Cai J, Kim J, Kim Y, et al. (2013) Nattokinase improves blood flow by inhibiting platelet aggregation and thrombus formation. Lab Animal Res 29:221-225.
- Ji H, Yu L, Liu K, Yu Z, Zhang Q, et al. (2014) Mechanisms of Nattokinase in protection of cerebral ischemia. Eur J Pharmacol 745:144-151.
- Howard BJ, Fraser SF, Sethi P, Cerin E, Hamilton MT, et al. (2013) Impact on hemostatic parameters of interrupting sitting with intermittent activity. Med Sci Sports Exerc 45:1285-1291.
Citation: Rastmanesh R, Pathak S, Celep G, Banerjee A, Kumar N, et al. (2021) A Randomized Study Assessing the Effect of Nattokinase-Meriva Formula on Low Grade Inflammation and Blood Viscosity in Adults with Increased Visceral Adiposity. Clin Pharmacol Biopharm, 10: 242.
Copyright: © 2021 Rastmanesh R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1999
- [From(publication date): 0-2021 - Feb 07, 2025]
- Breakdown by view type
- HTML page views: 1606
- PDF downloads: 393