A Randomized Open Label Parallel Clinical Study to Evaluate the Safety and Efficacy of Clevira Tablets against Mumps, HPV and Herpes zoster
Received: 08-Feb-2023 / Manuscript No. jcidp-24-127256 / Editor assigned: 11-Feb-2023 / PreQC No. jcidp-24-127256(PQ) / Reviewed: 25-Feb-2023 / QC No. jcidp-24-127256 / Revised: 01-Mar-2023 / Manuscript No. jcidp-24-127256(R) / Published Date: 08-Mar-2023 DOI: 10.4172/2476-213X.1000229
Abstract
Objective: To compare the clinical efficacy of Clevira tablets in Human adult patients, with Mumps, HPV and Herpes zoster. Methods: An Open Label, Balanced, Randomized, Multi-Dose, Two-Treatment, Parallel, Comparative Phase III Clinical trial to determine the Safety and efficacy of Clevira Tablets. 20 Patients were enrolled for two types of treatments received. Treatment 1-Clevira Tablets for Mumps/HPV (n=10) and Treatment 2- Clevira Tablets for Herpes Zoster virus(n=10). Enrolment was based upon the diagnosis of Haematology, Biochemistry, Serology, RT-PCR and Chest X-Ray and inclusion and none of the exclusion criteria and included in the study. Results: All the patients demonstrated safety measures with respect to Blood pressure and Pulse rate. Also, statistically significant (p < 0.0001) improvement showed in temperature from baseline for Treatment 1- (101.89 ± 1.14) and at the end of the study period (97.84 ± 0.83), improvement showed in temperature from baseline for Treatment 2- (101.82 ± 1.15) and at the end of the study period (97.74 ± 0.88). Conclusion: The study demonstrated an expedited clinical cure with normal vital signs & haematological results which validated that Tablet Clevira is safe and efficacious in patients with Mumps/HPV and Herpes zoster virus. The data’s further entrusted that Clevira can be used in infected patients with Mumps/HPV and Herpes zoster, and relieve the signs and symptoms, with a rapid recovery, without any adverse side effects.
Keywords
Clevira; Mumps; Clinical trail; HPV; Herpes zoster virus; RT – PCR
Introduction
Fever is a clinical feature that manifests due to an infection. The infection may be due to a virus, bacteria or other microorganisms. Viral fever is a condition characterized by a temperature above 38.0 to 38.4 (100.4 to 101 F) associated with symptoms like sudden onset of fever, severe headache especially behind the eyes, severe joint and muscle pain, nausea and vomiting & sometime accompanied by body rash persisting for four to seven days after infection. Viral infections include Herpes, Chikungunya, Dengue, Influenza, etc.
In recent days, the usage of many herbal formulations for various illnesses has increased. Clevira is one among them, which is a polyhedral formulation consisting of many ingredients, which has antiviral activity against HSV-1 and HSV-2. Pre-clinical, Clinical and docking studies have also shown its antiviral activity against, fever of viral origin.
Clevira is a Proprietary Ayurvedic Medicine. The individual herbal ingredients used are known to have variety of medicinal properties against fever of viral origin and proven to have effective antipyretic, analgesic, anti-viral and immuno-modulatory properties [1].
Clevira tablet is made out of Carica papaya, Melia azedarach, Andrographis paniculata, Vettivera zizanoides, Tricosanthus dioica, Cyperus rotundus, Zingiber officinale, Piper nigrum, Mollugo cerviana and Tinospora cordifolia, which is a Proprietary Ayurvedic Medicine. The individual herbal drugs used are known to have variety of medicinal values, against fever of viral origin and proven to have effective antipyretic, analgesic, anti-viral and immunity boosting properties [2]. These ingredients were found to have anti-inflammatory, anti-pyretic, antibacterial, anti-microbial, anti-cancer, antihelmintic, larvicidal,hepatoprotective, antidiabetic, antiobesity and hypolipidemic activity [3].
Methodology
Study design and patients
This study was an Open Label, Balanced, Randomized, Multi- Dose, Two-Treatment, Parallel, Comparative Phase III Clinical Trial to determine the safety and efficacy of Clevira tablets.
Totally 20 patients were enrolled for the study and divided into 2 groups as Treatment 1 and Treatment 2. Whereas in Treatment 1 group patients were treated with received Clevira Tablet, twice daily, after food for Mumps / HPV (n=10), In Treatment 2 group patients received Clevira Tablet twice daily, after food for Herpes Zoster (n=10).
Ethical conduct of the study
The study was conducted as per the Ethical guidelines for biomedical research on human participants, ICMR (2017), ICH (Step 5) 'Guidance on Good Clinical Practice. The study was initiated after obtaining proper ethical committee approvals and registered in Clinical trial registry of India (CTRI/2023/02/050004).
Patient information and consent
Patients were asked to read the informed consent document which was followed by a presentation by the trained study personnel. All the queries of the patients were resolved before obtaining their consent. Copy of the informed consent documents (English and vernacular language versions) used for obtaining consent for participation in the study. Patients were under medical supervision throughout their stay in the clinical facility to ensure safety and wellbeing of the patients.
Diagnosis and main criteria for inclusion and exclusion
Patients who met all of the following criteria [4] were considered for enrolment in the study:
Before enrolling in the study Haematology, Biochemistry, Serology, RT-PCR will be done to the patients for Diagnostic purpose/ Conformation of infection
Inclusion Criteria
Patients meeting all of the following criteria were considered for enrolment in the study:
• Either sex between the ages of 18-75 years, with an oral temperature of more than 38.0°C (100.4°F), with or without associated rash, body pain and joint pain, severe headache especially behind the eyes, nausea and vomiting.
• Mild to Influenza and other respiratory viral infections, Common cold and cough, Mumps / HPV and Herpes zoster associated disease as defined by the WHO.
• With Viral fever accompanied by thrombocytopenia with a platelet count between 80,000 /micro litre to100, 000/micro litre, along with stable vitals like pulse and blood pressure.
• Female patients who tested negative for pregnancy (up to two weeks prior to the study).
Exclusion Criteria
• Patients with Dengue haemorrhagic fever grade III and IV
• Patients with platelet count less than 80,000/micro litre.
• Pregnant or lactating women
• Patients who have received blood or blood products
transfusion, during the current illness
• Patients with Thrombocytopenia Purpura (ITP), Leukemia, Hemophilia
• Patients with serum ALT level 3 times higher than the upper limit of the normal range (>165 U/L) and Impaired renal function with serum creatinine > 1.5 mg/dl (males) and > 1.4 mg/dl (females).
• Patients who were hypersensitive, to any of the components of the formulation.
Primary selection of patients: The primary selection was to assess the efficacy of Clevira from day one of enrolment/treatment initiation, soon after the confirmation of illness, which is defined as time taken for clinical recovery. Patient enrolment was confirmed by RT-PCR results with symptoms of Mumps and other respiratory viral infections.
Sample size and treatment
Treatment 1: Totally 12 patients were screened and 10 Patients were enrolled and received Clevira Tablet for Mumps and HPV.
Treatment 2: Totally 11 patients were screened and 10 Patients were enrolled and received Clevira Tablet for Herpes Zoster.
Dosage: A dose of 1 to 2 Clevira tablets, twice a day for 7 to 10 days / 30 days based on the severity of infection.
Data analysis
Analysis sets: The statistical evaluation was performed using Chisquare test or Fisher exact test between the treatment groups. The proportion of patients with Mumps/HPV and Herpes Zoster on Day 10/30 and the percentage of patients receiving rescue therapy during the treatment period were analysis by using Pearson correlation coefficient or Spearman rank correlation. Statistical analysis was performed using the appropriate software.
Safety analysis: A total of 20 patients were dosed successfully recovered from the infections. There were no adverse events and serious adverse events reported during course of the study. The planned safety analyses consisted of descriptive summaries of the data as relevant to the scale of data, e.g., frequency and percent for recovered days, and mean changes from baseline as appropriate. Frequency and percentage of patients were to be provided for each categorical variable by treatment group.
Efficacy and safety assessment
Evaluation schedule: The first visit (Visit 1) is the screening Visit, followed by the second visit (Visit 2) which is a randomization visit/ Study enrolment visit (Day 0). The third visit (Visit 3) is subdivided into two viz., (i) Evaluation visit on Day 1 to 10 (T1); (ii) Evaluation visit for One month (Treatment 2) if required and followed by the final fourth visit (Visit 4) which is an follow up visit after One month, if required. The visit is based upon the patient’s signs and symptoms, which are reduced between the treatment days and based on the Investigator’s decision.
Results
Treatment 1
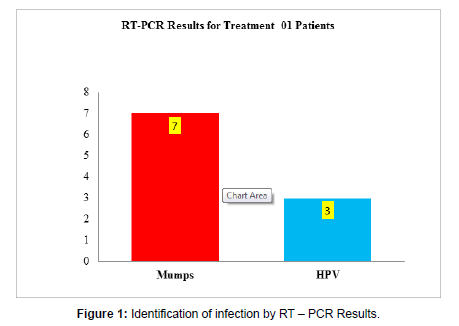
Out of 12 patients 2 were found to be Negative for RT- PCR, out of ten enrolled patients seven were positive for Mumps Virus, three were detected with Human Papilloma Virus (HPV) (Figure 1).
Some common symptoms were observed from the patients with Mumps like, Fever, difficulty in chewing, Cold, Loss of appetite Discomfort in salivary glands. Patients with HPV have symptoms like, Common warts occur on the fingers, nails and back of the hands Genital warts, Flat warts, etc.,
Treatment 2
Out of 11 patients, 1 was found to be Negative for RT- PCR, out of ten enrolled patients all are detected with Herpes zoster virus (Figure 2).
Patients with Herpes zoster have symptoms like, Fever, Burning sensation, Headache, Itching, Rash, Stomach upset, Chills, and Blisters.
Demographic and Other Baseline Characteristics
A total of 20 patients were enrolled into the study and their mean age, height, weight and BMI were recorded (Table 1 and Table 2).
| S .No | Patient Enrolment Number | Gender | Race | Age (years) | Height (cm) | Weight (Kg) | BMI (Kg/m2) |
|---|---|---|---|---|---|---|---|
| 1 | S002-03-001 | F | Asian | 30 | 160.0 | 63.0 | 24.6 |
| 2 | S002-03-002 | M | Asian | 30 | 170.0 | 73.0 | 25.3 |
| 3 | S002-03-003 | F | Asian | 27 | 162.0 | 66.0 | 25.1 |
| 4 | S002-03-004 | F | Asian | 65 | 154.0 | 55.0 | 23.2 |
| 5 | S002-03-005 | M | Asian | 26 | 171.0 | 69.0 | 22.3 |
| 6 | S002-03-006 | F | Asian | 47 | 162.0 | 60.0 | 22.9 |
| 7 | S002-03-007 | M | Asian | 47 | 175.0 | 74.0 | 24.2 |
| 8 | S002-03-008 | M | Asian | 30 | 170.0 | 66.0 | 22.8 |
| 9 | S002-03-009 | M | Asian | 39 | 172.0 | 68.0 | 23.0 |
| 10 | S002-03-010 | F | Asian | 70 | 160.0 | 55.0 | 21.5 |
Table1: Demographic details of patients of Treatment 1.
| S. No | Patient Enrolment Number | Gender | Race | Age (years) | Height (cm) | Weight (Kg) | BMI (Kg/m2) |
|---|---|---|---|---|---|---|---|
| 1 | S002-04-001 | F | Asian | 19 | 145.0 | 52.0 | 24.7 |
| 2 | S002-04-002 | M | Asian | 38 | 175.0 | 77.0 | 25.1 |
| 3 | S002-04-003 | F | Asian | 32 | 164.0 | 66.0 | 24.5 |
| 4 | S002-04-004 | M | Asian | 58 | 162.0 | 69.0 | 26.3 |
| 5 | S002-04-005 | M | Asian | 38 | 174.0 | 68.0 | 22.5 |
| 6 | S002-04-006 | M | Asian | 75 | 167.0 | 70.0 | 25.1 |
| 7 | S002-04-007 | M | Asian | 65 | 173.0 | 64.0 | 21.4 |
| 8 | S002-04-008 | M | Asian | 37 | 174.0 | 72.0 | 23.8 |
| 9 | S002-04-009 | M | Asian | 30 | 169.0 | 68.0 | 23.8 |
| 10 | S002-04-010 | M | Asian | 23 | 165.0 | 63.0 | 23.1 |
Table 2: Demographic detail of patients of Treatment 2.
All patients included in the study were Asians. Table 3 explains the Summarized Demographic details of patients who were enrolled in the study.
| Treatment | Parameters | Mean | SD | Min | Max | CV% |
|---|---|---|---|---|---|---|
| Treatment -1 (Mumps / HPV, N=10) |
Age (years) | 41.10 | 15.91 | 26.00 | 70.00 | 38.70 |
| Height (cm) | 165.60 | 6.83 | 154.00 | 175.00 | 4.13 | |
| Weight (kg) | 64.90 | 6.67 | 55.00 | 74.00 | 10.28 | |
| BMI (kg/m2) | 23.49 | 1.25 | 21.50 | 25.30 | 5.33 | |
| Treatment- 02 (Herpes zoster, N = 10) |
Age (years) | 41.50 | 18.45 | 19.00 | 75.00 | 44.45 |
| Height (cm) | 166.80 | 8.97 | 145.00 | 175.00 | 5.38 | |
| Weight (kg) | 66.90 | 6.59 | 52.00 | 77.00 | 9.85 | |
| BMI (kg/m2) | 24.03 | 1.43 | 21.40 | 26.30 | 5.94 |
Table 3: Summarized Demographic details of patients.
Efficacy evaluation
Statistical analysis of phase III clinical trial of Clevira tablet:
Primary and secondary end point efficacy evaluations were performed for Clevira Tablet. Primary and secondary end point of recovery analysis data from Day 01 to Day10 and safety measure analysis data for the all the patients (Haematology and vital signs) were performed by SAS software.
Haematology parameters
All haematology parameters were found to be normal and within limits, and at the end of the study period of day 30 for Treatment 1 and day 10 for Treatment 2 (Table 4 and Table 5).
Patient Enrolment Number |
Time of evaluation | RBC count (x1012/ µL) |
Packed Cell Volume (%) | Total WB C count (/ µl) |
Lymphocytes (%) | Neutrophils (%) | Eosinophils (%) | Monocyte (%) | Basophils (%) | Platelet count (× 10^9 / L) |
|---|---|---|---|---|---|---|---|---|---|---|
| S002-03-001 | Day 0 | 4.12 | 38.1 | 8475 | 57 | 34 | 3 | 5 | 1 | 162 |
| Day 30 | 4.7 | 40.3 | 7320 | 40 | 54 | 2 | 3 | 1 | 167.9 | |
| S002-03-002 | Day 0 | 4.3 | 42.4 | 9627 | 52 | 42 | 2 | 4 | 0 | 198 |
| Day 30 | 4.62 | 44.1 | 9762 | 36 | 61 | 1 | 2 | 0 | 200 | |
| S002-03-003 | Day 0 | 4.4 | 36.6 | 9128 | 50 | 41 | 5 | 3 | 1 | 200 |
| Day 30 | 5.2 | 38.8 | 8459 | 32 | 63 | 2 | 2 | 1 | 218.4 | |
| S002-03-004 | Day 0 | 4.57 | 37.8 | 9500 | 47 | 44 | 4 | 5 | 0 | 232.5 |
| Day 30 | 5.1 | 39.4 | 8652 | 35 | 60 | 2 | 3 | 0 | 275.3 | |
| S002-03-005 | Day 0 | 4.8 | 41.2 | 9910 | 51 | 42 | 3 | 4 | 0 | 248.6 |
| Day 30 | 5.34 | 43.5 | 9072 | 31 | 66 | 1 | 2 | 0 | 277 | |
| S002-03-006 | Day 0 | 4.6 | 39.4 | 8978 | 48 | 44 | 2 | 5 | 1 | 268 |
| Day 30 | 5.47 | 41.7 | 8950 | 29 | 67 | 1 | 2 | 1 | 282.6 | |
| S002-03-007 | Day 0 | 4.75 | 42.3 | 9320 | 45 | 48 | 4 | 3 | 0 | 246.8 |
| Day 30 | 5.8 | 44.6 | 9015 | 37 | 60 | 2 | 1 | 0 | 292 | |
| S002-03-008 | Day 0 | 4.5 | 43.4 | 8105 | 52 | 40 | 3 | 4 | 1 | 178 |
| Day 30 | 5.6 | 45.2 | 7915 | 32 | 63 | 2 | 2 | 1 | 185.2 | |
| S002-03-009 | Day 0 | 4.2 | 42.7 | 7326 | 47 | 45 | 2 | 5 | 1 | 211 |
| Day 30 | 5.19 | 43.9 | 6876 | 36 | 59 | 1 | 3 | 1 | 268 | |
| S002-03-010 | Day 0 | 4.9 | 40.8 | 7753 | 49 | 48 | 1 | 2 | 0 | 244.3 |
| Day 30 | 5.7 | 42.1 | 6890 | 39 | 58 | 1 | 2 | 0 | 294.3 | |
| Note: Day 0- Screening baseline | ||||||||||
Table 4: Comparison of Hematology parameter between baseline and end line (Day 0 vs Day 30) for Treatment 1.
| Patient Enrolment Number | Time of evaluation | Haematology RBC count (x1012/ µL) |
Packed Cell Volume (%) |
Total WB C count (/ µl) |
Lymphocytes (%) | Neutrophils (%) | Eosinophils (%) | Monocyte (%) | Basophils (%) |
Platelet count (× 10^9 / L) |
|---|---|---|---|---|---|---|---|---|---|---|
| S002-04-001 | Day 0 | 3.8 | 37.3 | 7943 | 47 | 46 | 2 | 5 | 0 | 157 |
| Day 10 | 4.4 | 39.9 | 7725 | 39 | 55 | 2 | 4 | 0 | 172 | |
| S002-04-002 | Day 0 | 4.3 | 42.2 | 8246 | 45 | 47 | 3 | 4 | 1 | 164 |
| Day 10 | 4.7 | 43.6 | 6870 | 37 | 56 | 2 | 4 | 1 | 180 | |
| S002-04-003 | Day 0 | 4.45 | 36.5 | 8167 | 49 | 41 | 3 | 6 | 1 | 158.2 |
| Day 10 | 5.06 | 38.7 | 7803 | 36 | 58 | 1 | 5 | 0 | 182.3 | |
| S002-04-004 | Day 0 | 4.1 | 44.7 | 8100 | 48 | 43 | 4 | 5 | 0 | 163.4 |
| Day 10 | 4.65 | 46.1 | 7710 | 40 | 53 | 2 | 4 | 1 | 184 | |
| S002-04-005 | Day 0 | 4.6 | 43.5 | 7845 | 50 | 40 | 2 | 7 | 1 | 170 |
| Day 10 | 5.2 | 45.4 | 7739 | 37 | 57 | 1 | 5 | 0 | 179 | |
| S002-04-006 | Day 0 | 4.4 | 44.1 | 8273 | 47 | 42 | 5 | 6 | 0 | 168 |
| Day 10 | 4.9 | 46.3 | 6147 | 35 | 57 | 3 | 4 | 1 | 186 | |
| S002-04-007 | Day 0 | 4.7 | 42.6 | 8355 | 51 | 42 | 3 | 4 | 0 | 156 |
| Day 10 | 5.34 | 44.5 | 6072 | 38 | 58 | 2 | 2 | 0 | 188.4 | |
| S002-04-008 | Day 0 | 4.9 | 43.8 | 8040 | 49 | 39 | 4 | 7 | 1 | 176 |
| Day 10 | 5.5 | 45.9 | 7984 | 36 | 57 | 2 | 5 | 0 | 177.1 | |
| S002-04-009 | Day 0 | 5.1 | 45.1 | 7890 | 52 | 36 | 5 | 6 | 1 | 184 |
| Day 10 | 5.67 | 46.2 | 6050 | 39 | 53 | 3 | 4 | 1 | 194 | |
| S002-04-010 | Day 0 | 4.8 | 45.3 | 7758 | 50 | 43 | 2 | 5 | 0 | 180 |
| Day 10 | 5.4 | 46.8 | 6763 | 38 | 58 | 1 | 3 | 0 | 202 | |
| Note: Day 0- Screening baseline | ||||||||||
Table 5: Comparison of Hematology parameter between baseline and end line (Day 0 vs Day 10) for Treatment 2.
Vital signs
During the course of study at Day 1 and Day 30/ Day 10, blood Pressure, radial pulse rate, temperature and wellbeing status were enquired and recorded (Table 6 and Table 7). Paired T-test, Baseline vs End of treatment comparison given in Table 8.
S.No |
Patient Enrolment Number | Treatment.1 | Day 01 | Day 30 | ||||
|---|---|---|---|---|---|---|---|---|
| Blood pressure (mm Hg) |
Radial pulse rate (Per min) | Body temperature (°F) | Blood pressure (mm Hg) | Radial pulse rate (Per min) | Body temperature (°F) | |||
| 01. | S002-03-001 | Mumps | 114/80 | 76 | 100.58 | 120/82 | 72 | 97.7 |
| 02. | S002-03-002 | Mumps | 115/70 | 75 | 101.66 | 123/75 | 68 | 98.78 |
| 03. | S002-03-003 | Mumps | 112/76 | 80 | 101.12 | 120/82 | 71 | 98.42 |
| 04. | S002-03-004 | Mumps | 120/74 | 83 | 102.92 | 123/76 | 70 | 97.52 |
| 05. | S002-03-005 | Mumps | 110/70 | 84 | 101.66 | 118/74 | 68 | 99.14 |
| 06. | S002-03-006 | Mumps | 122/80 | 76 | 103.1 | 125/84 | 72 | 97.7 |
| 07. | S002-03-007 | Mumps | 118/76 | 81 | 102.56 | 124/80 | 70 | 96.44 |
| 08. | S002-03-008 | Mumps | 120/86 | 79 | 100.58 | 122/82 | 69 | 97.88 |
| 09. | S002-03-009 | Mumps | 119/81 | 78 | 100.94 | 120/79 | 72 | 98.06 |
| 10. | S002-03-010 | Mumps | 115/75 | 82 | 103.82 | 119/83 | 70 | 96.8 |
Table 6: Vital signs (Blood pressure, Radial pulse rate, Temperature Day 0 vs Day 30).
| S.No | Patient Enrolment Number | Treatment 02 | Day 1 | Day 10 | ||||
|---|---|---|---|---|---|---|---|---|
| Blood pressure (mm Hg) |
Radial pulse rate (Per min) | Body temperature (°F) | Blood pressure (mm Hg) | Radial pulse rate (Per min) | Body temperature (°F) | |||
| 01. | S002-04-001 | Herpes zoster | 130/85 | 84 | 100.76 | 128/83 | 76 | 98.06 |
| 02. | S002-04-002 | Herpes zoster | 110/81 | 81 | 100.76 | 114/78 | 78 | 98.96 |
| 03. | S002-04-003 | Herpes zoster | 122/80 | 84 | 101.3 | 125/77 | 70 | 99.14 |
| 04. | S002-04-004 | Herpes zoster | 119/83 | 78 | 100.94 | 121/80 | 74 | 97.34 |
| 05. | S002-04-005 | Herpes zoster | 121/84 | 82 | 103.64 | 117/85 | 80 | 96.98 |
| 06. | S002-04-006 | Herpes zoster | 118/82 | 80 | 102.02 | 122/84 | 83 | 97.16 |
| 07. | S002-04-007 | Herpes zoster | 116/79 | 78 | 103.28 | 120/83 | 75 | 96.98 |
| 08. | S002-04-008 | Herpes zoster | 115/90 | 82 | 103.28 | 117/86 | 77 | 96.62 |
| 09. | S002-04-009 | Herpes zoster | 126/84 | 80 | 101.12 | 123/80 | 82 | 98.42 |
| 10. | S002-04-010 | Herpes zoster | 122/81 | 86 | 101.12 | 125/86 | 76 | 97.7 |
Table 7: Vital signs (Blood pressure, Radial pulse rate, Temperature Day 1vs Day 10).
| Difference | Paired T-Test | |||||
|---|---|---|---|---|---|---|
| Treatment 1 | Treatment 2 | |||||
| DF | t Value | Pr> |t| | DF | t Value | Pr> |t| | |
| Systolic Blood pressure | 9 | -5.86 | 0.0002 | 9 | -1.33 | 0.2165 |
| Diastolic Blood pressure | 9 | -2.54 | 0.0315 | 9 | 0.65 | 0.5314 |
| Pulse Rate | 9 | 7.33 | <.0001 | 9 | 2.68 | 0.0252 |
| Temperature | 9 | 7.42 | <.0001 | 9 | 6.83 | <.0001 |
Table 8: Paired t-test (Baseline vs End of treatment comparison).
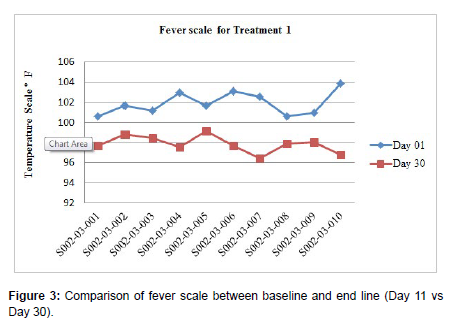
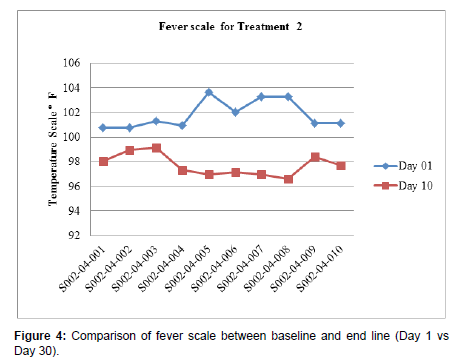
Patients undergoing the treatment with Clevira tablets clearly illustrated the safety aspects with respect to Blood pressure and Pulse rate. Also, statistically significant (p < 0.0001) improvement showed in temperature from baseline (101.89 ± 1.14) to end of the study treatment (97.84 ± 0.83) for Treatment 1 and from baseline (101.82 ± 1.15) to end of the study treatment (97.74 ± 0.88) for Treatment 2 given in Figure 3 and Figure 4 respectively.
Recovery analysis
Mean recovery day (Mean ± SD) of Treatment (Clevira tablet were found to be (20.40 ± 3.20) for Treatment 1 and (8.90 ± 0.74) for Treatment 2 (Table 9). The overall clinical efficacy shows healthy recovery rate found from 20 patients.
| Treatment | N Obs |
Mean | Std Dev | Minimum | Maximum | Coeff of Variation |
|---|---|---|---|---|---|---|
| Treatment 1 – (Clevira Tablet) Day 30 | 10 | 20.40 | 3.20 | 15.00 | 25.00 | 15.71 |
| Treatment 2 – (Clevira Tablet) Day 10 | 10 | 8.90 | 0.74 | 8.00 | 10.00 | 8.29 |
Table 9: Analysis Variables: Recovery Day.
Discussion
The main objectives of this study were to evaluate the efficacy of Clevira Tablets in the treatment in Human Adult Patients with Mumps/ HPV and Herpes zoster infections. Further it is also interesting to evaluate the additional clinical benefits of an approved antiviral herbal drug for SARS-CoV-2 (Covid 19) where Clevira, is found to be effective and safe for respiratory infections [5].
The pharmacological properties of C. papaya, M. azedarach, A. paniculata, V. zizanoides, T. dioica, C. rotundus, Z. officinale, P. nigrum, M. cerviana and T. cordifolia, P. nigrum, M. ceruviana, T. cordifolia plants have concrete approach for developing healthy therapeutic options against respiratory virus infection [6] caused by Mumps/HPV and Herpes zoster.
Availability of potential Phytochemicals of Flavonoids, Alkaloids, Lignan, Coumarinsare targeting the viral infections and arrest the spreading of infections. Flavonoid components of quercetin has significant inhibitory activity against NS2B-NS3 serine protease, particularly against Dengue virus serotype 2 and exerts its antiviral property by preventing viral assembly. Coumarins in T. dioica are effective in inhibiting the viral growth and it provides faster relief in conditions of viral fever in chikungunya and dengue and other respiratory illness [7]. However the present study has shown that there was a significant improvement in the patient’s recovery on day 7 to day 10, who got treated with Clevira tablets along with standard treatment.
From the study results, it is clear that the normalization of body temperature was evident on Day 5 onwards in Clevira treated group, compared to the control group were it was normalized after day 6 and 7. Thus, Clevira is having a good antipyretic activity. It is also evident that Clevira is having a significant improvement (P < 0.001*) in the Arthralgia and Myalgia score on day 3, 4 & 5, suggesting a good analgesic and antipyretic activity of Clevira [8]. All the haematological and biochemical parameters were within the normal range with significant reduction in haematocrit and WBC count, suggesting the anti-Viral property and immune-modulatory capacity of Clevira against Dengue infections and other viral infectious fever conditions [9]. Unaltered Renal and liver function tests suggest the Safety of Clevira tablets at the recommended dose [1].
There was a significant improvement in the quality of life of subjects in Clevira group related to the fatigue, sense of feeling week, dizziness and sense of feeling depressed, compared to that of baseline and control group. The overall response of Clevira group showed remarkable improvement and were completely free from viral symptoms and very good subject compliance was also observed [10]. There were no clinically significant adverse events during the entire study period.
It was evident on Day 20 onwards Recovery percentage was increased in Clevira patients (Mumps and HPV- T1) group, Thus, Clevira is having a good anti-pyretic activity for curing the symptoms of Fever, Difficulty in chewing, Cold, Loss of appetite and Discomfort in salivary glands for Mumps, 7 patients with highly contagious lesions, skin irritation, Rough skin, painful bumps on the fingers, hands were cured, while having Clevira Tablet Treatment for HPV infected patients [11].
There was significant reduction in the mean score of all the clinical symptoms (decrease the fever, itching, skin irritation and blisters) in Treatment 2 which was more prominent in Clevira tablets. The presence of A. paniculata and its active compounds like isoandrographolide might act through cell-differentiation-inducing activity in proliferation of HL-60 cells and it developed antiviral and endodermal activity on affected area [6]. Due to that infected skin recovery was found in Clevira treated patients Day 1 when compared to the cure Day 10, were it was normalized on day 8, Day 9 and Day 10 [12].
Conclusion
This randomized, Phase III, multicentre study has shown that Clevira is clinically effective and safe in Mumps, HPV and Herpes zoster.
The Overall Clinical efficacy shows high recovery percentage was observed in infected patients for Treatment with Clevira tablet. However the Clevira tablet was showed expedited cure clinically on Day 7 to Day 10 showing the marked improvement of cure status.
All haematology lab parameters found to be normal and within limit at the end of the study period (Day 10/30) and All patients in Treatment with Clevira Tablet (N = 20) showed no safety issues with respect to Blood pressure, Pulse rate and Temperature and also high recovery percentage of 50 was observed in infected patients of both Treatment 1&2- Clevira tablets for Mumps, HPV and Herpes zoster. However the Clevira tablets were showed expedited cure clinically on Day 9 & Day 20- 25 showing the marked improvement of cure status.
As there is expedited clinical cure and normal vital signs & haematological results showed that Clevira is safe and efficacious in patients with Mumps, HPV and Herpes zoster. Hence forth, Clevira can be used in infected patients with signs and symptoms of the viral infection and for a rapid recovery without any adverse effects [1].
Conflict of Interest
There is no conflict of Interest.
Acknowledgements
The authors are thankful to the management of apex laboratories private limited, and Mr. S.S. Vanagamudi, Chairman & Managing Director Mr. Vishagan Sulur Vanagamudi, Director & President and Ms. Subashini Vanagamudi, Executive Director, for their continuous encouragement and support in carrying out the study.
References
- Ramesh Kannan S, Sivraman V, Muralikumar VR, Sakthibalan M, Jayashree S, et al. (2019) A Randomized Open Label Parallel Group Clinical Study to Evaluate the Efficacy and Safety of Clevira in Fever of Viral Origin. IJMSIR 4: 556–566.
- R Narayanababu et al. (2022) Evaluation of Efficacy and Safety of Clevira as an Add on Drug in Mild to Moderate COVID-19 Positive Patients: A Randomised Control Trial. JCDR 16: 1-7.
- JV Sabarianandh et al. (2021) Acute and Repeated Dose Toxicity Study of Clevira Syrup – A Polyherbal Formulation. BPJ 14: 459-1467.
- Colvin JM, Muenzer JT, Jaffe DM, Smason A, Deych E, et al. (2012) Detection of viruses in young children with fever without an apparent source. Pediatrics 130: e1455-1462.
- Gonzalez Plaza JJ, Hulak N, Zhumadilov Z, Akilzhanova A. (2016) Fever as an important resource for infectious diseases research. Intractable Rare Dis Res 5: 97-102.
- Xu M, Zuest R, Velumani S, Tukijan F, Toh YX, et al. (2017) A potent neutralizing antibody with therapeutic potential against all four serotypes of dengue virus. NPJ Vaccines 2: 2.
- Ansari RM (2016) Extract of Carica papaya L. leaves: Standardising its use in dengue fever. Indian J Pharmacol 48: 338-339.
- Mohamed Ali-Seyed, KavithaVijayaraghavan (2020) Dengue virus infections and anti-dengue virus activities of Andrographispaniculata. Asian Pac J Trop Med 13: 49-55.
- Kadir SLA, Yaakob H, Zulkifli RM (2013) Potential anti-dengue medicinal plants: A review. J Nat Med 67: 677-689.
- Alche LE, Ferek GA, Meo M, Coto CE, Maier MS (2003) An antiviral meliacarpin from leaves of Meliaazedarach L. Naturforsch C 58: 215- 219.
- Wadhwa S, Singhal S, Rawal S (2014) Bioavailability enhancement by piperine: A review. AJBPS 4: 1.
- Kuruvila A (2002) Herbal formulations as pharmacotherapeutic agents. Indian J Exp Biol 40: 7-11.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Austin A, Abiraamasundari, Esekia Raja Selvan D (2024) A Randomized Open Label Parallel Clinical Study to Evaluate the Safety and Efficacy of Clevira Tablets against Mumps, HPV and Herpes zoster. J Clin Infect Dis Pract 9: 229. DOI: 10.4172/2476-213X.1000229
Copyright: © 2024 Subashini Vanagamudi, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 2017
- [From(publication date): 0-2024 - Nov 24, 2025]
- Breakdown by view type
- HTML page views: 1703
- PDF downloads: 314