Research Article Open Access
A Randomized, Double-Blind, Placebo-Controlled Study of the Analgesic Efficacy of Intravenous Acetaminophen in Ambulatory Surgery
Visit for more related articles at Journal of Pain & Relief
Abstract
Purpose: We investigated whether intraoperative intravenous acetaminophen has the potential to reduce pain after ambulatory surgery and reduce time to discharge from the post anaesthesia care unit and hospital.
Methods: We tested this hypothesis by conducting a prospective randomized, double-blind clinical trial in patients undergoing ambulatory surgery. A total of 145 patients were randomized to pre and postoperative placebo (50), intravenous (IV) operative and postoperative oral acetaminophen (49), and pre and postoperative oral acetaminophen (48).
Results: The primary end point; visual analogue scale mean pain intensity over 24 hours after completion of surgery, was not significantly different between the 3 groups, control group 2.0 (1.6), mean (SD), (IV) acetaminophen group 2.1 (1.9) and oral acetaminophen group 2.1 (1.6); (p=0.93). Time to fitness for discharge from the postoperative care unit (p=0.77) and time to fitness for discharge from hospital (p=0.27) also did not vary significantly between the three groups.
Conclusion: The addition of intraoperative IV acetaminophen to a standard analgesia regimen in patients undergoing ambulatory surgery did not significantly improve pain control or discharge times after surgery compared with pre and postoperative oral acetaminophen or placebo.
| Keywords |
| Acetaminophen; Intravenous; Ambulatory anesthesia; Acute pain |
| Introduction |
| Recent advances in anaesthesia and surgery, along with efforts to optimize healthcare cost-efficiency have led to an ever increasing number of surgical procedures being performed on an ambulatory basis. Estimates in the US [1] place the proportion of ambulatory surgery at 70-80% of all surgeries performed. |
| Opioids are among the most widely used analgesics. Opioids have known adverse effects, most notably nausea, vomiting, respiratory depression, pruritis and urinary retention. These may limit recovery and fitness for discharge after ambulatory surgery. Consequently, multimodal, opioid sparing analgesic regimens are widely used. |
| Oral Acetaminophen is frequently used for mild to moderate pain. IV acetaminophen has the potential to reduce mild to moderate pain associated with ambulatory surgery and reduce time to discharge from the post anaesthesia care unit and hospital. Analgesic efficacy of acetaminophen is said to best equate [2] with peak cerebrospinal fluid levels which are reliably achieved approximately 15 minutes after completion of IV infusion. |
| We conducted a prospective randomized, double-blind clinical trial in patients undergoing ambulatory surgery. Eligible consenting patients were randomly allocated to one of three groups. In addition to usual analgesia, patients received pre and postoperative placebo, or pre and postoperative oral acetaminophen. Mean pain intensity by visual analogue scale (VAS) measured over 24 hours was the primary outcome measure. |
| Methods |
| Study population |
| The study protocol was approved by the Ethics and Research Committee of The Alfred Hospital on 6th December 2005. All patients gave written informed consent which described the nature of the trial procedure and hypothesis in detail. Patients were enrolled between February 2006 and July 2007. |
| Subjects were eligible if they provided informed consent and were aged 18-60 years, underwent surgery under general anaesthesia with an expectation of discharge within 24 hours, and agreed to an analgesia plan comprising any one or a combination of opioids and local anaesthetic infiltration of wound as well as the study medication (acetaminophen or placebo). |
| Exclusion criteria included a past or current history of persistent pain syndrome greater than 3 months, significant liver or renal disease, drug or alcohol abuse, continuing requirement for post operative nonsteroidal anti-inflammatory medication, concurrent treatment with anti-epileptic or antidepressant medication including gabapentin or pregabalin allergy to acetaminophen or mannitol, history more than one week of continuous opioid therapy up to the time of surgery. Patients were excluded postoperatively if there was a need for reoperation within 24 hours of initial surgery. |
| Procedures |
| The study was a prospective, randomized, double-blind clinical trial. Patients were randomly assigned by computer generated randomization to receive one of three treatments: (i) pre and postoperative oral placebo and intraoperative intravenous placebo (ii) preoperative placebo, intraoperative intravenous and postoperative oral acetaminophen, or (iii) pre and postoperative oral acetaminophen with intravenous operative placebo. Patients, researchers, carers and clinicians were all blinded to treatment group allocation. |
| After enrolment, patients were randomized to receive a preoperative oral medication immediately before they were called to theatre for their surgery. Blinding of treatment allocation was controlled in the following manner. In the group receiving pre and postoperative placebo, and IV and postoperative oral acetaminophen, the preoperative tablet was a placebo, and in the group receiving pre and postoperative oral acetaminophen the preoperative tablet was 1 gram of acetaminophen. |
| During surgery, anaesthetists and staff in theatre were instructed to administer 100 ml infusion of study drug after induction of anaesthesia. In the pre and postoperative placebo group and the oral pre and postoperative acetaminophen group the infusion consisted of placebo (Normal Saline) while in the IV and postoperative acetaminophen group the infusion consisted of 1 gram of IV acetaminophen. Anaesthetists and surgeons were instructed to infiltrate the wound with 2 mg/kg ropivacaine if desired. |
| All patients received a general anaesthetic with spontaneous ventilation using a laryngeal mask airway. No muscle relaxants were used. Induction of anesthesia was with 1-2 milligram per kilogram of intravenous propofol and maintenance of anaesthesia was with either sevoflurane or isoflurane in oxygen and air. Nitrous oxide was not used. Anaesthetists were also instructed to administer IV opioid as required to titrate analgesia with the aim of achieving analgesic control in their patient on emergence from surgery. IV opioids consisted of either morphine alone or fentanyl followed by morphine as is usual practice in our institution. We used a conversion of 10 milligram IV morphine for every 100 micrograms of IV fentanyl [3]. |
| After completion of surgery patients were managed in PACU with additional IV opioid analgesia with morphine administered as required for control of pain. Administration was guided by a standardized analgesic protocol consisting of a loading dose followed by titrated doses of morphine according to pain intensity and further stratified according to age and co-morbidity (see appendix 1). Patients who experienced an incomplete analgesic response after two cycles of this protocol, defined as a VAS pain rating of greater than 4 cm were allowed to receive IV Ketorolac 10 mg. |
| Fitness for discharge from PACU was defined by standard criteria, including a VAS pain rating of less than 4 cm (see appendix 2). Upon leaving PACU patients were prescribed immediate release oral oxycodone 5-10 mg every 3-4 hours as required and the study medication (acetaminophen 1 gram or placebo) taken strictly orally every 6 hours. Patients were instructed that the oxycodone can treat severe intensity pain and the acetaminophen can treat mild to moderate intensity pain. Patients were advised to take the acetaminophen or placebo every 6 hours and also advised to take both the oxycodone and the acetaminophen or placebo together for severe pain as the two medications can work synergistically. Once discharged to the post operative ward, patients were required to satisfy discharge criteria to indicate fitness for discharge from hospital (see appendix 3). |
| The primary outcome measure was mean VAS pain intensity rating over 24 hours. VAS has the attributes of being a simple, reliable and efficient method for quickly assessing pain intensity [4]. VAS pain was measured immediately after emergence from anaesthesia, and at 30 min, 2h, 4 h, 12 h and 24 h after anaesthesia. Patients rated their pain intensity by placing a mark on a 10 cm scale. Secondary outcome measures include VAS pain intensity on waking from surgery, time to readiness for discharge from PACU, time to readiness from discharge from hospital, opioid requirement from commencement of surgery until discharge from PACU, opioid requirement after discharge from hospital for 24 hours, and side effects attributable to analgesia. |
| We also used the 40 item quality of recovery score (QoR-40), a validated assessment tool for evaluation of the early postoperative health status of patients [5] as a secondary end point. The Score evaluates physical comfort, emotional state, psychological support, physical independence, and pain. |
| Statistical analysis |
| Our sample size calculation was based on detecting a difference in pain intensity ratings of greater than or equal to 33% between any two of the three treatment groups. Assuming a control group measure of 2.0 +/- (1.0), an alpha error of 5% and beta error of 20%, we required 48 subjects per group. Outcome data were first assessed for normality and homogeneity of variance using the Kolmogorov-Smirnov and Levine’s test. Opioid requirement from commencement of surgery till discharge from PACU was parametric and post discharge opioid requirement was non-parametrically distributed. Mean VAS scores were then compared using analysis of variance. The secondary end points which were evaluated using analysis of variance include time to readiness for discharge from PACU, 24 hour opioid requirement, and Quality of Recovery score. Time to readiness for discharge from hospital and post operative opioid requirement were evaluated by the Kruskal-Wallis test owing to non-parametric distribution of data. |
| Results |
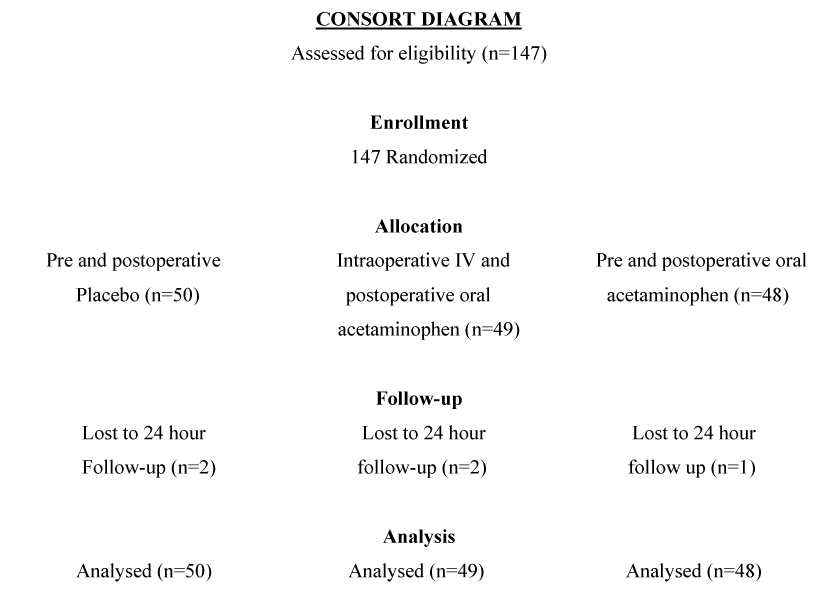
| There were 147 patients recruited to the trial; 142 patients were able to provide complete data while 5 patients (2 in the pre and post operative placebo group, 2 in the IV and postoperative acetaminophen group, and 1 in the oral pre and post operative acetaminophen group) could not be contacted at 24 hours and so were unable to provide complete data. All other data were included in the final analysis (Figure 1). Baseline patient and clinical characteristics were similar in the three groups (Table 1). The most common surgeries were orthopaedic and plastic surgery. |
| Mean 24 hour pain intensity ratings were 2.0 (1.6) in the pre and postoperative placebo group, 2.1 (1.9) in the IV operative and postoperative oral acetaminophen group, and 2.0 (1.6) in the pre and postoperative oral acetaminophen group (p= 0.93). Pain intensity on waking from anaesthesia did not vary significantly among the three groups (p=0.94) (Table 2). Time to fitness for discharge from PACU was 53 (30) min in the pre and postoperative placebo group, 49 (33) min in the IV operative and postoperative oral acetaminophen group and 49 (34) min in the pre and postoperative oral acetaminophen group (p=0.26) (Table 3). Median time to fitness for discharge from hospital were 160([130-220]) min in the pre and postoperative placebo Group, 150([118-185]) min in the IV operative and postoperative oral acetaminophen group, and 165([114-246]) min in the pre and postoperative acetaminophen group (Table 3). |
| Opioid requirement from commencement of surgery until discharge from PACU, calculated as intravenous morphine equivalents were 13.1(6.7) mg in the pre and post operative placebo group, 12.7 (6.3) mg in the IV operative and postoperative acetaminophen group, and 13.6 (7.8) mg in the pre and postoperative oral acetaminophen group (p=0.53) (refer table 4). Post discharge median oxycodone requirement was 0 ([0-5]) mg in the pre and postoperative placebo group, 5([0-14]) mg in the IV operative and postoperative oral acetaminophen group, and 0 ([0-5]) mg in the pre and postoperative oral acetaminophen group (p=0.29). The need for IV nonsteroidal antiinflammatory in PACU as a rescue therapy was also non-significant with 4 cases (3%) in the pre and postoperative placebo group, 10 cases (15 %) in the IV operative and postoperative oral acetaminophen group, and 6 cases (4%) in the pre and postoperative oral acetaminophen group (p=0.10). Infiltration with Ropivacaine wound infiltration occurred in 18 cases (38%) in the pre and postoperative placebo group, 16 cases (33%) in the IV operative and postoperative oral acetaminophen group, and 13 cases (26%) in the pre and postoperative oral acetaminophen group (p=0.39). Mean Quality of Recovery scores were 16.0 (1.3) in the pre and postoperative placebo group, 15.9 (1.9) in the IV operative and postoperative oral acetaminophen group, and 16.3 (1.3) in the pre and postoperative oral acetaminophen group (p=0.65). |
| There were no recorded instances of respiratory depression or clinical jaundice among the trial participants and instances of postoperative nausea and vomiting were similar among the three groups: 7 (15%) in the pre and postoperative placebo group, 6 (13%) in the IV and postoperative oral acetaminophen group, and 9 (18%) in the pre and postoperative oral acetaminophen group (p=0.75). Sedation scores did not differ between the 3 groups (p=0.99). |
| Discussion |
| We conducted a prospective, double-blind, randomized controlled trial comparing the effect of a dose of intraoperative IV acetaminophen with postoperative oral acetaminophen, pre and postoperative oral acetaminophen, and pre and post operative placebo in ambulatory surgery patients. Our results showed no difference between the three patient groups in their average pain intensity ratings in the first 24 hours post surgery and on waking from surgery, and in readiness for PACU and hospital discharge. |
| The patients recruited to this trial were predominantly male, ASA classification 1 and 2, and underwent mostly orthopaedic and plastic surgical procedures. This is a typical representation of mixed ambulatory surgical practice. The anaesthetic technique and drugs for analgesia (including local anaesthetic infiltration where appropriate) reflect common clinical practice. Instructions given to patients on the postoperative use of oxycodone and acetaminophen/placebo reflected common advice given by anaesthetists. Patients were asked to self administer acetaminophen or placebo strictly every 6 hours and only use oxycodone if necessary to reflect realistic practice since the patients spent part of the 24 hour period after surgery at home rather than in hospital where medications can be given according to strict regimens by nursing staff. |
| The form of IV acetaminophen used in this trial is superior to propacetamol as it has comparable analgesic efficacy with a reduced incidence of allergic phenomena [6]. The dose was suitable, with a recent study finding no increase in effect at doses above 15 mg/kg [7]. Giving IV acetaminophen during surgery allows for CSF levels to peak in PACU and be sustained into the post-operative period. Peak CSF levels of acetaminophen most closely correlate with analgesic effect [7]. Studies in dental surgical patients after 1gm IV acetaminophen have shown a rapid onset of analgesia of 5 to 8 minutes with a peak clinical effect measured at approximately 1-2 hours [8]. |
| The analgesic efficacy of IV and oral acetaminophen has been summarised by two recent meta-analyses of available randomized controlled trials [9,10]. Both meta-analyses showed that IV acetaminophen reduced morphine requirement by approximately 20% when compared to placebo, but did not reduce the incidence of morphine related side effects, such as sedation and postoperative nausea and vomiting. There was also no difference in pain scores measured post-operatively. Both analyses, however, included trials using other routes of administration for acetaminophen such as oral and studied patient populations undergoing major surgery, where morphine requirement was prolonged for several days and measured using patient controlled analgesia infusion [11-17]. We did not show a difference in perioperative or post discharge opioid requirement in a mixed ambulatory surgery setting. |
| A 2002 review [18] concluded that more studies were needed to test the evidence for any difference in analgesic efficacy of acetaminophen given by different routes. A study in dental surgical patients showed more rapid onset of analgesia with IV acetaminophen in comparison to oral acetaminophen but did not demonstrate a clinically significant benefit [8] while a study in orthopaedic surgical patients [19] found lower pain intensity scores with the IV form of acetaminophen. |
| Moller et al [8] did show a benefit of IV acetaminophen over placebo in dental practice, but our study did not show any difference in analgesia between all three groups, including a placebo group. Mean pain scores on waking across all three groups were not significantly different but also very low making it potentially more difficult to show a benefit when using an analgesic to treat something that is generally less painful. The same can be said of one of our secondary end points, with average time to discharge from PACU across all three groups being less than one hour. |
| Opioid requirement in theatre and PACU was not significantly different among the 3 groups. This is in a setting where anesthesiologists were advised to titrate opioid dose during general anesthesia with the aim of controlling pain by emergence from surgery. IV and oral acetaminophen were also administered together with opioid prior to emergence from general anesthesia. Other studies have also failed to show an improvement in pain scores after surgery in a setting where acetaminophen and parecoxib were administered prior to waking from surgery and compared with placebo [20]. This contrasts with studies where acetaminophen was used as a rescue analgesic and compared to rescue placebo [21,22] in patients developing severe pain after major orthopaedic surgery where pain intensity and opioid requirement were significantly reduced. |
| The most common types of surgery in our study were either minor orthopaedic or plastic surgical procedures, and it is possible that these procedures were either not painful enough or produced a specific type of pain which is less responsive to IV acetaminophen. It is possible that other types of ambulatory surgical procedures not performed in our hospital may benefit more from IV acetaminophen. |
| Our study population was 69% male, with mean age of 33 years and predominantly ASA I and II. Postoperative nausea and vomiting, a common side effect of opioid medication, is less likely in males than in females [23], therefore it may be harder to show a reduction in postoperative nausea and vomiting using IV or oral acetaminophen through reduction in opioid dose. This group of patients are also relatively resistant to other rarer side effects of opioid medication such as sedation and respiratory depression owing to their younger age previous reports of PCA opioid induced respiratory depression [24] appear rare in patients without co-existing disease, making a safety benefit for combinations of IV and oral perioperative acetaminophen difficult to demonstrate. Acetaminophen may provide some benefit in populations more prone to opioid induced side effects, such as elderly patients, patients with major co-morbidities, or morbidly obese patients [24-27]. |
| IV acetaminophen has been shown to reduce opioid requirement in major surgery without reducing pain scores, opioid related side effects, or patient satisfaction [7]. We chose to study patients having ambulatory surgery because it should represent a model of mild to moderate postoperative pain. This assumption may not be correct as severe pain can be reported in 40% of patients having day surgery where inadequate preoperative education about self administration of analgesia after discharge can play a role [28]. In such circumstances self-administration of systemic opioid analgesia may play an important role in the overall success of postoperative analgesia. This is in keeping with the low pain intensities and high doses of opioid required by subjects in all groups of our study. |
| Our study showed no benefit of single dose operative IV acetaminophen plus post-operative oral acetaminophen when compared to oral peri-operative acetaminophen administration and to pre and postoperative placebo. The methodology of our study favoured the day surgery setting where patients immediately change to oral analgesia and mobilize early to facilitate same day discharge. This contrasts with another study in a similar population comparing 48 hours of intravenous dosing with IV acetaminophen (6 hourly) to IV parecoxib (12 hourly with IV saline to blind 6 hourly dosing), IV dipyrone (6 hourly) and IV placebo (6 hourly) which determined that pain intensity scores were unchanged in the first 24 hours and pitiramide dose (by patient controlled analgesia infusion) was not altered in any of the four treatment groups [29]. In this setting the patient would be expected to remain in hospital for at least 48 hours to facilitate therapy. |
| We aimed to study a mixed surgical population which was mainly orthopaedic and plastic; anesthesiologists had the option of combining fentanyl with morphine for analgesia and surgeons allowed to use local anaesthetic infiltration if desired. This could be considered a weakness of the study, but the purpose was to reproduce typical practice in our institution, and proportions of patients in the 3 study groups did not vary significantly with any of these parameters. |
| Our study was limited to one centre, and our study population was predominantly young healthy males having orthopaedic and plastic surgery. The benefit of IV acetaminophen administered prior to waking from surgery as part of a multi-modal analgesia approach is questionable in this group of patients. It is possible that IV acetaminophen could benefit patients at greater risk of opioid induced side effects such as those with co-existing respiratory disease or obstructive sleep apnoea, patients having minor gynaecological surgery or in minor surgery where pain of mild intensity is the expectation so that opioid can be avoided altogether. |
| In conclusion, we could not find any evidence that intraoperative IV acetaminophen provided any analgesic benefit, reduction in opioid requirement or opioid related side effects, or improvement in time to readiness for discharge from theatre or hospital in an ambulatory surgical population. |
| Acknowledgements and Conflict of Interest Disclosure |
| The investigators are indebted to Dr. Skantha Vallipuram for his generous support of the Alfred Hospital Pain Research Fund, and to Professor Paul Myles for his assistance with the statistical analysis. Bristol-Myers Squibb played no role in study concept, design, data collection, data analysis, data interpretation or writing of the report. |
| Funding Source |
| This investigator initiated study was part funded by Bristol-Myers Squibb pharmaceuticals who market IV acetaminophen, and from The Alfred Hospital Pain Research Fund. |
References
- Jenkins K, Grady D, Wang J, Correa R, Armmanious S, et al. (2001) Operative recovery: ambulatory surgery patients’ preferences. Br J Anaesth 86: 272-274.
- Bannwarth B, Netter P, Lapicque F, Gillet P, Pere P, et al. (1992) Plasma and cerebrospinal fluid concentrations of paracetamol after a single intravenous dose of propacetamol. Br J Clin Pharmacol 34: 79-81.
- Pantawala AE, Duby J, Waters D, Erstad BL (2007) Opioid conversions in acute care. Ann Pharmacother 41: 255-266.
- Huskisson EC (1983) Visual Analogue Scales. In: Melzack R (ed) Pain: measurement and assessment. Raven Press, New York: 33-37.
- Myles PS, Weitkamp B, Jones K, Melick J, Hansen S (2000) Validity and reliability of a post operative quality of recovery score: the QoR-40. Br J Anaesth 84: 11-15.
- Moller PL, Juhl GI, Payen-Champenois C, Skoglund LA (2005) IV acetaminophen (paracetamol): Comparable analgesic efficacy, but better local safety than its prodrug, propacetamol, for postoperative pain after Third Molar Surgery. Anesth Analg 101: 90-96.
- Hahn TW, Mogensen T, Lund C, Jacobsen LS, Hjortsoe NC, et al. (2003) Analgesic effect of i.v. paracetamol: possible ceiling effect of paracetamol in postoperative pain. Acta Anaesthesiol Scand 47: 138-145.
- Moller PL, Sindet-Pedersen S, Petersen CT, Juhl GI, Dillenschneider A, et al. (2005) Onset of acetaminophen analgesia: comparison of oral and IV routes after third molar surgery. Br J Anaesth 94: 642-648.
- Elia N, Lysakowski C, Tramer MR (2005) Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology 103: 1296-1304.
- Remy C, Marret E, Bonnet F (2005) Effects of acetaminophen on morphine side-effects and consumption after major surgery: meta-analysis of randomized controlled trials. Br J Anaesth 94: 505-513.
- Mimoz O, Incagnoli P, Josse C, Gillon MC, Kuhlman L, et al. (2001) Analgesic efficacy and safety of nefopam vs propacetamol following hepatic resection. Anaesthesia 56: 520-525.
- Sinatra RS, Jahr JS, Reynolds LW, Viscusi ER, Groudine SB, et al. (2001) Efficacite antalique et tolerance du Perfagan 1g dans la douleur postoperatoire en chirurgie orthopedique. Ann Fr Anesth Reanim 20: 170.
- Peduto VA, Ballabio M, Stefanini S (1998) Efficacy of propacetamol in the treatment of postoperative pain. Morphine sparing effect in orthopaedic surgery. Italian Collaborative Group on Propacetamol. Acta Anaesthesiol Scand 42: 293-298.
- Siddik SM, Aouad MT, Jalbout MI, Rizk LB, Kamar GH, et al. (2001) Diclofenac and/or propacetamol for postoperative pain management after cesarean delivery in patients receiving patient controlled analgesia morphine. Reg Anesth Pain Med 26: 310-315.
- Fletcher D, Negre I, Barbin C, Francois A, Carreres C, et al. (1997) Postoperative analgesia with i.v propacetamol and ketoprofen combination after disc surgery. Can J Anesth 44: 479-485.
- Hernandez-Palazon J, Tortosa JA, Martinez-Lage JF, Perez-Flores D (2001) Intravenous Administration of Propacetamol Reduces Morphine Consumption After Spinal Fusion Surgery. Anesth Analg 92: 1473-1476.
- Schug SA, Sidebotham DA, McGuinnety M, Thomas J, Fox L (1998) Acetaminophen as an adjunct to morphine by patient controlled analgesia in the management of acute postoperative pain. Anesth Analg 87: 368-372.
- Romsing J, Moiniche S, Dahl JB (2002) Rectal and paracetamol, and paracetamol in combination with NSAIDs, for postoperative analgesia. Br J Anaesth 88: 215-226.
- Jarde O, Boccard E (1997) Parenteral versus oral route increases acetaminophen efficacy. Clin Drug Invest 14: 474-481.
- Grundmann U, Wornle C, Biedler A, Kreuer S, Wrobel M, et al. (2006) The efficacy of the non opioid analgesics parecoxib, paracetamol, and metamizol for post operative pain relief after lumbar microdiscectomy. Anesth Analg 103: 217-222.
- Zhou TJ, Tang J, White PF (2001) Propacetamol versus ketorolac for treatment of acute postoperative pain after total hip or knee replacement. Anesth Analg 2: 1569-1575.
- Sinatra RS, Jahr JS, Reynolds LW, Viscusi ER, Groudine SB, et al. (2005) Efficacy and safety of single and repeated administration of 1 gram intravenous acetaminophen injection (paracetamol) for pain management after major orthopedic surgery. Anaesthesiol 102: 822-831.
- Gan TJ (2006) Risk factors for postoperative nausea and vomiting. Anesth Analg 102: 1884-1898.
- Etches RC (1994) Respiratory depression associated with patient controlled analgesia: a review of 8 cases. Can J Anesth 41: 125-132.
- Etches RC, Sandler AN, Denise Daley M (1989) Respiratory depression and spinal opioids: a review. Can J Anesth 36: 165-185.
- Connolly LA (1991) Anaesthetic management of obstructive sleep apnea patients. J Clin Anesth 3: 461-469.
- Adams JP, Murphy PG (2000) Obesity in Anaethesia and Intensive Care. Br J Anaesth 85: 91-108.
- Beauregard L, Pomp A, Choiniere M (1998) Severity and impact of pain after day surgery. Can J Anesth 45: 304-311.
- Brodner G, Gogarten W, Van Aken H, Hahnenkamp K, Wempe C, et al. (2011) Efficacy of intravenous paracetamol compared to dipyrone and parecoxib for postoperative pain management after minor-to-intermediate surgery: a randomised, double-blind trial. Eur J Anaesth 28: 125-132.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Acupuncture
- Acute Pain
- Analgesics
- Anesthesia
- Arthroscopy
- Chronic Back Pain
- Chronic Pain
- Hypnosis
- Low Back Pain
- Meditation
- Musculoskeletal pain
- Natural Pain Relievers
- Nociceptive Pain
- Opioid
- Orthopedics
- Pain and Mental Health
- Pain killer drugs
- Pain Mechanisms and Pathophysiology
- Pain Medication
- Pain Medicine
- Pain Relief and Traditional Medicine
- Pain Sensation
- Pain Tolerance
- Post-Operative Pain
- Reaction to Pain
Recommended Journals
Article Tools
Article Usage
- Total views: 15054
- [From(publication date):
April-2012 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 10430
- PDF downloads : 4624
