A Qualitative Study Using a Multi-Grounded Theory-Based Approach to Understand the Lived Experiences of People with Paroxysmal Nocturnal Haemoglobinuria Receiving Complement C5 Inhibitor Treatment in Europe
Received: 19-Apr-2024 / Manuscript No. jcmhe-24-132628 / Editor assigned: 22-Apr-2024 / PreQC No. jcmhe-24-132628 (PQ) / Reviewed: 06-May-2024 / QC No. jcmhe-24-132628 / Revised: 13-May-2024 / Manuscript No. jcmhe-24-132628 (R) / Published Date: 20-May-2024 DOI: 10.4172/2161-0711.1000877
Abstract
Paroxysmal Nocturnal Haemoglobinuria (PNH) is an acquired, rare, chronic, potentially life-threatening haematologic disease caused by gene mutations that lead to the destruction of red blood cells by the complement system, a part of the immune system. People with PNH experience symptoms which can include anaemia, fatigue and dyspnea, and may require red blood cell transfusions, which can impact quality of life. With the introduction of the first approved therapy for PNH, a complement C5 inhibitor, life expectancy has improved and is near normal in countries where the treatment is available and accessible. However, the burden of disease remains significant for many patients despite treatment. Questionnaire-based studies have found that people with PNH still experience a variety of symptoms while on C5 inhibitor treatment: Many remain anaemic, the burden of illness remains substantial, and quality of life is impacted negatively. Though knowledge of the disease is growing, knowledge about the everyday experiences of people living with PNH remains limited. This study used a mixed methods approach, including ethnography, questionnaires, qualitative interviewing, mapping exercises and photographic interpretation to better understand the lived experiences of people with PNH. The study found that diagnosis is often challenging, and is experienced as a disruption of normality, negatively impacting ability to work, intimacy, parenthood, hobbies and other activities. Additionally, this study adds to the emerging evidence showing a significant symptom burden and reduced quality of life despite C5 inhibitor treatment. A greater breadth of experienced symptoms than commonly recognized also warrants further research. These new insights may support healthcare professionals in treating their patients with PNH by providing a more complete picture of the ways in which the disease impacts their lives and the unmet needs that remain despite C5 inhibitor treatment.
Keywords
Anaemia; Eculizumab; Fatigue; Humanised monoclonal antibodies; Paroxysmal Nocturnal Haemoglobinuria (PNH); Ravulizumab
Introduction
Paroxysmal Nocturnal Haemoglobinuria (PNH) is a rare, chronic, haemolytic disease [1] caused by acquired somatic PIGA gene mutations in haematopoietic stem cells, which leads to uncontrolled complement activation and complementmediated haemolysis [2]. Clinical manifestations of PNH can include persistently low haemoglobin (Hb), haemoglobinuria, fatigue, need for red blood cell transfusions and smooth muscle dystonia, which can present as abdominal pain, back pain, dysphagia and erectile dysfunction [2]. These manifestations, particularly fatigue and the need for transfusions, can impact the Quality of Life (QoL) in people diagnosed with PNH [2].
People diagnosed with PNH typically receive a diagnosis between age 30 years-45 years [3], around the time of major life events such as starting or furthering careers, finding partners, and planning families [4-6]. Since 2007, the treatment landscape of PNH has evolved, with the approval of the first terminal complement C5 inhibitor [7], and C5 inhibitors have been demonstrated to improve outcomes for people diagnosed with PNH, including survival [8]. Despite these improvements for people who receive treatment with C5 inhibitors [2,9-11], 78%- 91% of patients may not achieve normal levels of Hb [12,13].
Furthermore, a survey of 71 people diagnosed with PNH and receiving treatment with C5 inhibitors found that the burden of disease remains substantial and QoL is still negatively impacted [12]. The survey, which included participants treated with eculizumab in France, Germany and the UK, or with secondgeneration C5 inhibitor ravulizumab in Germany, reported that work productivity was affected in 70% of participants who were employed, and impairment in their activities related to their disease was reported by 85% of all participants [12].
Research over the last decades has advanced the knowledge of the demographics of people diagnosed with PNH, and mechanisms and treatment of the disease [2,7,14,15], and many studies have also included patient-reported outcomes (PROs) as a measure of changes in QoL in response to therapy [9-12,16,17]. People diagnosed with PNH who were enrolled in the International PNH Registry, the largest cohort of people diagnosed with PNH studied to date (N=4439 in the overall study population [15]), reported considerable disease-related fatigue and impaired overall QoL at baseline as compared to scores reported for the general adult population, measured by the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue and European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) scores [14,15]. Fatigue is a major clinical symptom of PNH [2], and these two instruments can therefore provide valuable quantitative measures for physicians to monitor. However, both the FACIT-Fatigue instrument and the EORTC QLQ-C30 were originally developed to assess the QoL of cancer patients [18,19], who may differ in patient and disease characteristics and therefore these tools may not optimally describe the QoL of people diagnosed with PNH [20]. Furthermore, while PNH-specific QoL instruments are being developed to better describe the QoL of those living with this rare disease [3,20,21], PROs, particularly those not specific to PNH, only provide a snapshot of the lives of people diagnosed with PNH, and knowledge about their everyday challenges and experiences remains limited.
The aim of this qualitative study was to systematically investigate the lived experiences of people diagnosed with PNH, including those related to their condition and treatments, their ways of managing their disease, and their challenges. The ethnographic approach used in the study allows researchers to observe people’s behaviours in the context of their own lives and real-world settings as opposed to clinical settings, with the aim of understanding how they manage their daily lives, what they value, and what barriers there are to achieving their goals [22]. This methodology incorporates experiences within sociocultural contexts to produce a richer picture of what it means to live with PNH today [23].
Materials and Methods
Study design
The study was designed to be an in-depth, qualitative exploration of the lived experiences of people with PNH in Europe receiving treatment with eculizumab or ravulizumab. A mixed methods approach of ethnography, digital questionnaires, qualitative interviewing, mapping exercises and photographic interpretation was used.
In order to frame the study, provide important background knowledge about PNH, and develop a field guide, researchers conducted semi-structured interviews with three experts: A haematologist specialised in PNH, a psychologist specialised in rare chronic diseases, and a patient advocate. The insights obtained were used to develop the field guide, which was additionally reviewed by the UK patient organisation PNH Support. The field guide included instructions for conducting written exercises and an interview guide with predefined research thaemes and suggested interview questions (excerpt of field guide, (Figure S1)).
Seven researchers conducted field work using the field guide as a framework. While the field guide was structured according to a meaningful sequence of thaemes (past and present experiences with PNH and future outlook, daily life with and beyond PNH, social life and the impact of PNH, and experiences with PNH treatment), researchers were allowed to deviate from the structure to pursue emerging avenues of inquiry in the conversation, and to tailor their questions to the interview context and person being interviewed, as long as the totality of thaemes in the field guide was covered.
Ethics statement
The study followed the ethical standards outlined in the ICC/ ESOMAR International Code on Market, Opinion and Social Research and Data Analytics [24]. Participants signed GDPRcompliant consent forms and all interviews were conducted by trained researchers. The study protocol and all researchrelated documents were developed in accordance with the Declaration of Helsinki ethical principles for medical research. All participants electronically provided documentation of their informed consent as evidence of their agreement to voluntarily participate in the study. Informed consent forms stated the type of data collected, data privacy and anonymisation measures, withdrawal options, the possibility of publication of study results, and fair market value remuneration, which was established for each of the countries where the research was carried out. Additionally, participants agreed to the study sponsor’s adverse event reporting requirements.
Study population
Patient organisations were contacted and three agreed to support with the recruitment of people diagnosed with PNH (PNH Support in the UK; Asociación de Haemoglobinuria Paroxística Nocturna in Spain; HPN France-Aplasie Médullaire in France), by posting an advertisement on the organisation’s forum (France) or social media sites (UK), or by the president contacting members directly (Spain). The recruitment criteria (Table S1) aimed for a sample with a variety of C5 inhibitor treatments and demographics, including age and gender. Written informed consent was provided by all participants before study entry.
Data collection
Recruitment started on 8 September 2021, and finished on 15 December 2021. Data were collected in the participant’s native language through a digital questionnaire, observation, semi-structured interviews, and written exercises. Prior to visiting the participant for observation and interviews, each participant was asked to complete a digital questionnaire "for 3 to 5 days" to provide initial insights into their daily lives and lived experiences with PNH, and the impact of fatigue on their daily lives and activities (Figure S2).
Observation was conducted during 8-12 hours spent with the participant over 1-2 days, meeting thaem in their homes and accompanying thaem for daily activities. Researchers conducted observation in a sensitive and unobtrusive manner that allowed ample time for breaks or for concluding the day’s study activities when required.
Semi-structured interviews focused on the predefined research thaemes. Conversations with close family members or friends of the participant, as well as semi-structured interviews with specialist physicians and nurses treating people with PNH provided additional information and context to the data provided by the participant. Photographs were taken to document aspects of the observation, and audio recordings of interactions were made to verify accuracy of data interpretation by the researchers, according to the terms specified in the informed consent form. Written exercises included mapping the participant’s journey from PNH diagnosis including symptoms, drawing a timeline of a normal day, and mapping their social and support networks.
Data analysis
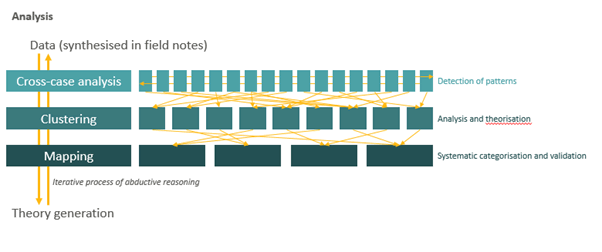
Analysis was performed using a multi-grounded theory approach [25-29] (Figure 1), which involves the construction of theories through the collection and analysis of data in a systematic and iterative process. All available data were synthesised into analytical field notes according to a template that followed the research thaemes outlined in the fieldwork guidebook. Researchers then used cross-case analysis to analyse each case in comparison with the other cases collected to detect patterns evident across the empirical material. Clustering exercises were conducted wherein patterns were clustered, analysed and theorised upon. Finally, data mapping exercises (e.g. mapping of experienced symptoms of each respondent) were carried out in an iterative process to systematically categorize and validate findings.
All available data from the questionnaires, observations, semistructured interviews and written exercises were synthesised in the researchers’ field notes. The researchers first used crosscase analysis to detect larger patterns in the material, during which every case was analysed in comparison with the other cases collected. The researchers then conducted a clustering exercise, wherein patterns evident across empirical material were clustered, analysed and theorised upon in order to derive and record general thaemes in the data.
Symptoms and the level of their impact on QoL are based on the participant’s self-assessment in combination with qualitative evaluation by the researchers. Due to the qualitative nature of the data, no formal statistical testing was performed.
Results
Overview of key findings impacting the lives of people diagnosed with PNH.
In total, 27 participants were included in the study (Table 1). This study has provided unique insights into the ways that PNH disrupts the lives of people living with the disease beyond what has been captured previously using surveys and standardised PRO instruments. Among the 27 participants, four findings were derived from the data: The disruption of normality, the breadth of symptoms, the burden of treatment, and the need for additional support.
Table 1: Participant and treatment characteristics
| Characteristic* | N=27 |
|---|---|
| Age range, years | 18–80 |
| Age group, years, n (%) | |
| 18–35 | 8 (30) |
| 36–55 | 13 (48) |
| ≥55 | 6 (22) |
| Female sex, n (%) | |
| Treatment by country, n (%) | |
| Eculizumab | |
| France | 11 (41) |
| Spain | 7 (26) |
| UK | 1 (4) |
| Ravulizumab | |
| France | 0 (0) |
| Spain | 2 (7) |
| UK | 6 (22) |
| Site of treatment by country, n (%) | |
| Hospital | 16 (59) |
| France | 8 (30) |
| Spain | 8 (30) |
| UK | 0 (0) |
| Home | 11 (41) |
| France | 3 (11) |
| Spain | 1 (4) |
| UK | 7 (26) |
| *Percentages may not total 100 due to rounding. | |
Disruption of normality
For the participants, disruption of their “normal” lives began at the onset of symptoms. Before diagnosis, 52% (n=14) of participants struggled to identify if what they were experiencing was unusual. “The first time I felt really tired was when I was running with my cousin [...] I gradually felt too tired to run, it was not normal.” [Participant 16, France]. The path from perceiving that something is wrong to receiving a PNH diagnosis can be worrisome and lengthy. The majority of participants (67%; n=18) faced a challenging path to diagnosis, defined in this study by at least one of the following: Duration of diagnosis journey more than 1 year, seeing multiple healthcare professionals (HCPs), getting the wrong tests, hospitalisations, misdiagnoses, or having to seek help from HCPs in their personal network. While diagnosis did offer some clarity to the symptoms experienced, participants expressed that they were left with questions about the impact of a PNH diagnosis around three key areas: T vvheir ability to work, their family lives, and their daily activities.
Work and career
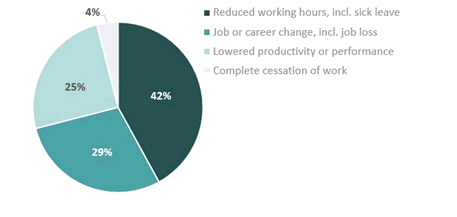
For 89% (n=24) of participants, PNH has affected their ability to work at some point along their journey with PNH. Among these, 42% needed to reduce their working hours, 29% needed to change their job or career, 25% reported lowered productivity or performance, and 4% reported a complete cessation of work due to PNH (Figure 2).
Intimacy and parenthood
37% (n=10; n=5 male, n=5 female) of participants explicitly mentioned struggling with intimacy due to PNH. While for men this was primarily driven by PNH-related erectile dysfunction, the study revealed that fatigue and pain caused by PNH can also impact intimacy for women. Participants also expressed concern about how symptoms such as fatigue may impact their ability to be the parents they wanted to be. “Before [PNH], I dreamed of becoming a mother. It was my life’s biggest dream, and now I see it as something very complicated.” [Participant 9, Spain]. “Daddy can be the fun daddy, but mummy is tired.” [Participant 27, UK].
Hobbies and activities
52% (n=14) of participants gave up hobbies or activities entirely due to PNH symptoms or treatment constraints, restricting their lifestyles and reducing their QoL. These challenges were sometimes made even more difficult due to the fact that reducing shared hobbies or activities also meant that participants’ families and friends were affected. “I make efforts because I understand that [my partner] also wants a normal life. Having a normal life would mean going abroad without thinking about the dates, doing more things after work.” [Participant 16, France].
Breadth of symptoms
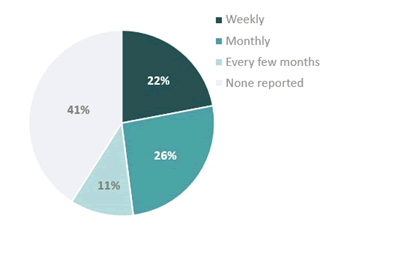
Despite treatment, the study found that the QoL of participants was still impacted by PNH symptoms, with 67% (n=18) reporting that their symptoms have had high or medium impact on their lives (Figure 3). Fatigue represented a significant symptom, with 81% (n=22) of participants experiencing fatigue despite treatment. Debilitating fatigue, defined herein as a level of fatigue that forced the participant to change their plans for the day, was experienced by 59% (n=16), and for 22% (n=6), this debilitating fatigue was experienced weekly (Figure 4). Multiple participants reported days when they would suddenly feel overwhelmed by fatigue, some of whom associated this with their treatment cycle. Fatigue would increase noticeably typically 1 day-3 days before the next infusion of eculizumab, and about 2 weeks before the next infusion of ravulizumab. Due to these fluctuations in symptoms experienced between treatments, 26% (n=7) of participants described arranging their schedules around their expected symptom burden, resulting in both personal and professional sacrifices. In addition to fatigue, symptoms such as headache and shortness of breath also remained unresolved. Some participants found ways to adapt to their symptoms, for example moving important kitchen utensils to the lowest kitchen cabinets to avoid exertion, while for others, daily activities such as commuting to their workplace became challenges. “Our office is on the 3rd floor […] Before, I would just open the door and go in, now, I have to take five minutes to catch my breath, feel ready again, and then open the door and go in […] For other people, it’s just a few steps, but for me, it’s the Eiffel Tower.” [Participant 15, France]. Beyond physical symptoms that are typically associated with PNH such as fatigue, shortness of breath and headache [30], participants reported multiple physical, psychological and cognitive symptoms that they associated with their disease. Nearly half of the participants (48%, n=13) mentioned that they struggled to get their HCPs to recognize these additional symptoms as being related to PNH (Table 2). This includes psychological symptoms, such as irritability and depression, and cognitive symptoms, such as brain fog, difficulty remembering and focusing. “You tell [the clinic] or whatever doctor’s office, and all they’ll say is, ‘Well, that’s not classic PNH. That’s not the signs and symptoms of it.’ […] It feels as if a patient who lives through it each day doesn’t know what they’re on about.” [Participant 27, UK]. 74% (n=20) said that they had experienced psychological and/or cognitive challenges that they associated with PNH, and 56% (n=15) expressed that not enough attention is given to these symptoms by HCPs. “My fish tank now, it’s completely grotty, and you can’t quite see what’s going on in there-that’s exactly like my brain [when I have brain fog].”
Table 2: Spontaneously mentioned symptoms experienced by participants while on treatment
| Patient-reported symptoms | N=27 |
|---|---|
| Typical physical symptoms associated with PNH, n (%) | |
| Fatigue | 22 (81) |
| Shortness of breath | 20 (74) |
| Headache | 8 (30) |
| Dark urine | 8 (30) |
| Abdominal pain | 7 (26) |
| Chest pain | 1 (4) |
| Difficulty swallowing | 1 (4) |
| Erectile dysfunction | 1 (4) |
| Yellow eyes | 1 (4) |
| Additional symptoms, n (%) | |
| Physical symptoms | |
| Achy muscles | 9 (33) |
| Chills and sensitivity to temperature | 6 (22) |
| Sensitivity to sounds and lights | 5 (19) |
| Bloated stomach | 4 (15) |
| Joint pain | 4 (15) |
| Muscle cramps | 4 (15) |
| Amplified heart beating sound | 3 (11) |
| Blue fingers/poor blood circulation in extremities | 2 (7) |
| Blurred vision | 1 (4) |
| Diarrhoea | 1 (4) |
| Fever | 1 (4) |
| Kidney pain | 1 (4) |
| Lack of appetite | 1 (4) |
| Nausea | 1 (4) |
| Occasional rash | 1 (4) |
| Pain in sole of foot | 1 (4) |
| Shaking body | 1 (4) |
| Psychological symptoms | |
| Depression | 6 (22) |
| Anxiety | 5 (19) |
| Irritability | 4 (15) |
| Cognitive symptoms, n (%) | |
| Brain fog | 6 (22) |
| Difficulty remembering | 3 (11) |
| Confusion | 2 (7) |
| Difficulty focusing | 2 (7) |
[Participant 27, UK. Furthermore, 15% (n=4) reported that the psychological and cognitive symptoms of PNH were even more significant than the physical symptoms, including fatigue, highlighting this as an important group of symptoms for HCPs treating patients with PNH to be aware of. “I have mood swings. I am less patient, less tolerant, and less able to keep it to myself when it is necessary or to communicate properly […] Physical symptoms are easier to manage.” [Participant 10, France].
Burden of treatment
There are additional burdens associated with treatment due to logistics and how participants must change their routines to accommodate treatment. Though all participants were receiving professional administration of C5 inhibitor treatment, the location at which the treatment is administered, whether at home or in the hospital-which depends predominantly on the healthcare system rather than drug type or patient preferencewas a significant factor for understanding the participant’s perception of treatment and the burden they experienced. Logistical burdens were specifically experienced among participants receiving treatment in the hospital. Among the 52% (n=14) of participants who received eculizumab in a hospital setting every two weeks, 79% (n=11/14) encountered challenges with hospital treatment. These challenges included long travel times to the hospital, long waiting times, and the emotional toll of the process of receiving treatment in the hospital, which also made full-time work difficult for many participants. Professional administration of C5 inhibitor treatment at home was a comparatively more positive experience than hospital treatment among all participants; overall, 52% (n=14) held a positive view of home treatment though only 41% (n=11) of participants received treatment at home. However, 63% (n=7/11) of those receiving home treatment encountered difficulties, including difficulty organising home treatment and nurse quality variances. Furthermore, restrictions on travel due to the dosing frequency of C5 inhibitor treatment, especially eculizumab, left some participants feeling a sense of limited life opportunities.“Without PNH, I could maybe have gotten jobs outside of Europe and go on long trips. I’ve had to forget about long trips for a long time.” [Participant 12, France].
Need for additional support
Among the participants, multiple resources for support were available to thaem, including HCPs, family and friends, and patient organisations. However, 67% (n=18) of participants expressed needing support beyond what they were currently receiving, and many expressed that their own support networks were incomplete and sub-optimal. In several instances, they tried to fill in these gaps through their own means, for example, seeking out mental health support to cope with the psychological burden of PNH. The gratitude felt by participants towards PNH specialists after a complicated road to diagnosis held thaem back from asking for more time, information, and support from their time-pressed specialists. This hesitance to ask for more from specialists was despite experiences among participants that their specialists provided insufficient information about the disease and treatment, and that consultations did not cover the impact of PNH on broader life domains. For all participants, family and friends were the primary source of support, providing emotional care as well as practical help, such as seeking out resources about PNH and attending medical appointments. However, both participants and their families expressed that it can be challenging for those close to the participants to understand a rare disease like PNH, and the experience of living with it. For example, it can be emotionally burdensome for people with PNH to explain social absences, or lack of participation, to family and friends due to invisible symptoms like fatigue, which can cause thaem to become withdrawn and experience an even greater emotional burden due to their disease. “Friends stop calling after trying a few times, they see you’re always tired. They think you’re being lazy or not making an effort.” [Participant 3, Spain]. Patient organisations were also an integral part of the support network, providing a sense of belonging and solidarity with others who are also living with PNH, the opportunity to compare their experiences with others, and a source of learning. “When members shared stories about their lives with PNH, it was great to realise that others were going through the same experiences I was going through.” [Participant 1, Spain]. 67% (n=18) of participants were actively involved in their country’s respective patient organisation, defined as attending events, connecting with other people diagnosed with PNH, and/or regularly posting, or being aware of, news in the organisations’ social media groups.
Discussion
The in-depth approach of the study provided detailed information about the lived experiences of people diagnosed with PNH, their goals, their challenges, and the impact on their families and social circles. Previous results from studies such as the International PNH Registry [14], a web-based survey of people diagnosed with PNH in France, Germany and the UK [12], and interviews with people diagnosed with PNH to develop a PNH-specific PRO instrument [20] have provided insights into some of the issues that impact the daily lives and QoL of people diagnosed with PNH in Europe. Consistent with the current study, these previous studies have shown that people diagnosed with PNH often have a complicated journey from onset of symptoms to diagnosis, with a median time to diagnosis of 14 months [20]; that PNH may be a reason for not working, working reduced hours, or having reduced productivity at work [12,14]; that PNH contributes to normal daily activity impairment [12]; and that people diagnosed with PNH can experience a wide range of symptoms associated with their disease, with fatigue being the most frequently reported symptom [12,14]. However, this study highlights that people diagnosed with PNH often experience a number of symptoms beyond those that are typically associated with the disease in the literature. Some of these additional symptoms, such as brain fog and difficulty focusing, have been previously reported [12] or are consistent with side effects observed from treatment with complement C5 inhibitors, such as depression, anxiety and chills [31,32]. Nevertheless, a proportion of participants felt that this important category of symptoms was not recognised by their HCPs. Some participants reported that these additional symptoms have a greater impact on their QoL than fatigue. As such, it is important that HCPs recognise this group of additional disease and treatmentrelated symptoms that may impact their patients’ lives and recognise when referrals to other specialists are needed to support the needs of their patients. Further research into these symptoms, and their connection to PNH, is warranted to provide greater understanding and visibility to symptoms that may be overlooked. Participants also reported that the logistical aspects of treatment have an impact on their lives. The frequency of infusions may limit personal or professional travel and the return of symptoms before the next infusion may require adjustments in personal or professional plans. Inhospital treatment is associated with long travel and waiting times, and a high emotional toll, while at-home treatment with complement C5 inhibitors brings the burden of seeing one’s home turned into a medical space, with varying quality of home healthcare. This study also provides insight into the various people and groups that form the support network of people diagnosed with PNH. Many participants had support not only from their PNH specialist HCPs, but also from family and friends, as well as patient organisations. However, many participants still felt that they lacked support and information on their disease and treatment options, and they felt a high emotional burden of living with a disease that has invisible symptoms such as fatigue. Overall, this study highlights some substantial challenges of people diagnosed with PNH receiving C5 inhibitors, underlining the need for new therapies. Another approved treatment option is pegcetacoplan, a proximal complement C3 inhibitor, [16,17,33], which provides a broader control of haemolysis compared to terminal C5 inhibitors [34], as well as improving the QoL of people diagnosed with PNH [17,35]. Emerging therapies targeting other pathways (factor B inhibitor iptacopan [36] and factor D inhibitor danicopan [37]) are currently in development. Some limitations of the study include the methods used to select the participants, the limited time (8 hours-12 hours) for observation, potential bias from the presence of researchers, and the lack of participation from European countries where a PNH patient organisation has not been established. Additionally, only patients receiving treatment with C5 inhibitors were included. Therefore, no data was collected in countries where the treatments are not available. Conclusions about people diagnosed with PNH broadly in Europe, and about differences between the countries represented here, are limited by the low number of participants recruited per country. It is also important to note the potential of selection bias in the recruitment approach in Spain, and the self-selection bias in the volunteer-based recruitment approach in France and the UK.
Conclusion
This study has provided more in-depth information on the burden of symptoms and treatment experienced by people diagnosed with PNH, and their need for additional education and support. Symptoms extend well beyond those typically associated with PNH, which may have a greater impact than currently acknowledged. Research into these expanded symptoms may provide new insights into the clinical manifestations of PNH and the unmet needs for people diagnosed with PNH. Finally, when discussing treatment options with patients, HCPs may consider the following, where possible, to best support their patients with PNH: Patient’s preference regarding treatment logistics (frequency and location of treatment), the availability of additional support or educational materials for patients and their support networks, awareness of symptoms, and assessment of a patient’s goals for treatment.
Acknowledgement
Swedish Orphan Biovitrum AB and the investigators thank the participants for their participation and would like to acknowledge the patient organisations, PNH Support in the UK, Asociación de Haemoglobinuria Paroxís Nocturna in Spain, and HPN France-Aplasie Médullaire in France, for their thoughtful input in planning the research and for their support in recruiting participants and to PNH Support in the UK for co-authoring this paper. Medical writing support was provided by Courtney Bologna, nspm ltd., funded by Swedish Orphan Biovitrum AB.
Funding
This work was funded by Swedish Orphan Biovitrum AB. Authors from Swedish Orphan Biovitrum AB were involved in the study design, manuscript preparation and decision to publish.
Data Availability
ReD Associates and Swedish Orphan Biovitrum AB, as joint data controllers, are committed to responsible and ethical sharing of data on participant level and summary data for medicines and indications approved by EMA and/or FDA, while protecting individual participant integrity and compliance with applicable legislation. Data that support the findings of this study were collected and analysed by ReD Associates. Data access to the aggregated summary data will be granted in response to qualified research requests. All requests are evaluated by a cross‐functional panel of experts within Swedish Orphan Biovitrum AB and ReD Associates, and a decision on sharing will be based on the scientific merit and feasibility of the research proposal, maintenance of personal integrity, and commitment to publication of the results. To request access to study data, a data sharing request form (available on www.sobi.com) should be sent to medical.info@sobi.com. Further information on Swedish Orphan Biovitrum AB’s data sharing policy can be found at: https://www.sobi. com/en/policies.
Authorship Contributions
KK: Conceptualization, Data Curation, Formal Analysis, Investigation, Methodology, Writing-Original Draft Preparation, Writing-Review and Editing.
AL: Conceptualization, Data Curation, Formal Analysis, Methodology, Writing-Review and Editing.
MP: Conceptualization, Formal Analysis, Methodology, Writing-Review and Editing.
AN: Conceptualization, Formal Analysis, Methodology, Writing-Review and Editing.
BK: Conceptualization, Formal Analysis, Methodology, Writing-Review and Editing.
MB: Conceptualization, Data Curation, Formal Analysis, Methodology, Writing-Review and Editing.
JS: Conceptualization, Validation, Writing-Review and Editing.
MR: Conceptualization, Data Curation, Formal Analysis, Investigation, Methodology, Writing-Original Draft Preparation, Writing-Review and Editing.
Conflict of Interest Disclosures
The research and consulting firm ReD Associates-represented by KK, AL, MB, and MR-has received consulting fees from Swedish Orphan Biovitrum AB. PNH Support-represented by MP and AN-has received consulting fees from Swedish Orphan Biovitrum AB, and the Canadian Association of PNH Patients-represented by BK-has received honoraria from Swedish Orphan Biovitrum AB. JS is an employee of Swedish Orphan Biovitrum AB.
References
- Latour RPD, Hosokawa K, Risitano AM (2022) Hemolytic paroxysmal nocturnal hemoglobinuria: 20 years of medical progress. Semin Hematol. 59(1):38-46.
- Hill A, DeZern AE, Kinoshita T, Brodsky RA (2017) Paroxysmal nocturnal haemoglobinuria. Nat Rev Dis Primers 3:17028.
- Daly RP, Jalbert JJ, Keith S, Symonds T, Shammo J (2021) A novel patient-reported outcome instrument assessing the symptoms of paroxysmal nocturnal hemoglobinuria, the PNH-SQ. J Patient Rep Outcomes 5(1): 102.
- Employment and activity by sex and age-annual data.
- Mean age at first marriage by sex.
- Mean age of women at childbirth and at birth of first child.
- Luzzatto L (2016) Recent advances in the pathogenesis and treatment of paroxysmal nocturnal hemoglobinuria. F1000Res.
- Socie G, Schrezenmeier H, Muus P, Lisukov I, Kulasekararaj A, et al. (2016). Changing prognosis in paroxysmal nocturnal haemoglobinuria disease subcategories: An analysis of the international PNH registry. Intern Med J 46(9):1044-1053.
- Hillmen P, Young NS, Schubert J, Brodsky RA, Muus P, et al. (2006). The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med 355(12):1233-1243.
- Lee JW, Fontbrune FS, Lee LW, Pessoa V, Gualandro S, et al. (2019). Ravulizumab (ALXN1210) vs eculizumab in adult patients with PNH naive to complement inhibitors: The 301 study. Blood 133(6):530-539.
- Kulasekararaj AG, Hill A, Rottinghaus ST, Langemeijer S, Wells A, et al. (2019). Ravulizumab (ALXN1210) vs eculizumab in C5-inhibitor-experienced adult patients with PNH: The 302 study. Blood 2019 133(6):540-549.
- Panse J, Fontbrune FS, Burmester P, Piggin M, Matos JE, et al. (2022). The burden of illness of patients with paroxysmal nocturnal haemoglobinuria receiving C5 inhibitors in France, Germany and the United Kingdom: Patient-reported insights on symptoms and quality of life. Eur J Haematol 109(4):351-363.
- Risitano AM, Notaro R, Marando L, Serio B, Ranaldi D, et al. (2009). Complement fraction 3 binding on erythrocytes as additional mechanism of disease in paroxysmal nocturnal hemoglobinuria patients treated by eculizumab. Blood 113(17):4094-4100.
- Schrezenmeier H, Muus P, Socie G, Szer J, Maciejewski JP, et al. (2014). Baseline characteristics and disease burden in patients in the international paroxysmal nocturnal hemoglobinuria registry. Haematologica 99(5):922-929.
- Schrezenmeier H, Roth A, Araten DJ, Kanakura Y, Larratt L, et al. (2020). Baseline clinical characteristics and disease burden in patients with Paroxysmal Nocturnal Hemoglobinuria (PNH): Updated analysis from the International PNH Registry. Ann Hematol 2020; 99(7): 1505-14.
- Hillmen P, Szer J, Weitz I, Roth A, Hochsmann B, et al. (2021). Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med 384(11):1028-1037.
- Wong RSM, Navarro-Cabrera JR, Comia NS, Goh YT (2023) Pegcetacoplan controls hemolysis in complement inhibitor-naive patients with paroxysmal nocturnal hemoglobinuria. Blood Adv 7(11):2468-2478.
- Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E (1997) Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage 13(2):63-74.
- Fayers P, Bottomley A (2002) Quality of life research within the EORTC-the EORTC QLQ-C30. European Organisation for Research and Treatment of Cancer. Eur J Cancer 38(4):S125-S133.
- Groth M, Singer S, Niedeggen C, Roth A, Schrezenmeier H, et al. (2017). Development of a disease-specific quality of life questionnaire for patients with aplastic anemia and/or paroxysmal nocturnal hemoglobinuria (QLQ-AA/PNH)-report on phases I and II. Ann Hematol 2017; 96(2): 171-181.
- Niedeggen C, Singer S, Groth M, Roth A, Schrezenmeier H, et al. Design and development of a disease-specific quality of life tool for patients with aplastic anaemia and/or paroxysmal nocturnal haemoglobinuria (QLQ-AA/PNH)-a report on phase III. Ann Hematol 2019; 98(7):1547-1559.
- Khair K, Phillott A, Loran C, Pollard D, Forrester C, et al. (2014). HaemophiliaLIVE: An ethnographic study on the impact of haemophilia on daily life. J Haem Prac 1(3):14-20.
- Mortensen GL, De J, Holme M, Neve T, Torell PG, et al. (2016). Social aspects of the quality of life of persons suffering from schizophrenia. OJPsych 06(01):50-60.
- ICC/ESOMAR international code on market, opinion and social research and data analytics 2016.
- The discovery of grounded theory: Strategies for qualitative research.
- The grounded theory perspective: Conceptualization contrasted with description.
- McCarthy J, Sullivan P, Wright P (2006) Culture, personal experience and agency. Br J Soc Psychol 45(2):421-439.
- Gubrium JF, Holstein JA, Marvasti AB, McKinney KD (2012) The Sage handbook of interview research: The complexity of the craft. 2nd edition Sage Publications.
- Corbin JM, Strauss AL (2014) Basics of qualitative research: Techniques and procedures for developing grounded theory. 3rd edition Sage Publications.
- Parker CJ (2016) Update on the diagnosis and management of paroxysmal nocturnal hemoglobinuria. Hematology Am Soc Hematol Educ Program 2016(1):208-216.
- Soliris (eculizumab) summary of product characteristics 2023.
- Ultomiris (ravulizumab) summary of product characteristics 2023.
- Latour RPD, Szer J, Weitz IC, Roth A, Panse J, et al. (2022). Pegcetacoplan versus eculizumab in patients with paroxysmal nocturnal haemoglobinuria (PEGASUS): 48-week follow-up of a randomised, open-label, phase 3, active-comparator, controlled trial. Lancet Haematol 9(9):e648-e659.
- Wong RSM, Pullon HWH, Amine I, Bogdanovic A, Deschatelets P, et al. (2022). Inhibition of C3 with pegcetacoplan results in normalization of hemolysis markers in paroxysmal nocturnal hemoglobinuria. Ann Hematol 101(9):1971-1986.
- Roth A, Hochsmann B, Griffin M, Nishimori H, Nakazawa H, et al. (2021). Effect of Pegcetacoplan on quality of life in patients with paroxysmal nocturnal hemoglobinuria: Week 48 of PEGASUS Phase 3 Trial Comparing Pegcetacoplan to Eculizumab: Presented at the annual meeting of the European hematology association 2021. Acta Haematol EP595.
- Jang JH, Wong L, Ko BS, Yoon SS, Li K, et al. (2022). Iptacopan monotherapy in patients with paroxysmal nocturnal hemoglobinuria: A 2-cohort open-label proof-of-concept study. Blood Adv 6(15): 4450-4460.
- Risitano AM, Kulasekararaj AG, Lee JW, Maciejewski JP, Notaro A, et al. (2021). Danicopan: An oral complement factor D inhibitor for paroxysmal nocturnal hemoglobinuria. Haematologica 106(12):3188-3197.
Citation: Kamel K, Lottrup AMW, Piggin M, Naylor A, Katsof B, et al. (2024) A Qualitative Study Using a Multi-Grounded Theory-Based Approach to Understand the Lived Experiences of People with Paroxysmal Nocturnal Haemoglobinuria Receiving Complement C5 Inhibitor Treatment in Europe. J Community Med Health Educ.13: 877. DOI: 10.4172/2161-0711.1000877
Copyright: © 2024 Kamel K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5189
- [From(publication date): 0-2024 - Dec 01, 2025]
- Breakdown by view type
- HTML page views: 4695
- PDF downloads: 494