A Purification Protocol for Escalating the Sensitivity of Mass Spectrometry Dependent innovative Protein Determination in Human Chorionic Villi
Received: 03-Nov-2022 / Manuscript No. jabt-22-81637 / Editor assigned: 05-Nov-2022 / PreQC No. jabt-22-81637 / Reviewed: 18-Nov-2022 / QC No. jabt-22-81637 / Revised: 21-Nov-2022 / Manuscript No. jabt-22-81637 / Accepted Date: 27-Nov-2022 / Published Date: 28-Nov-2022 QI No. / jabt-22-81637
Abstract
Recently plenty of success has been done regarding acquisition of mass spectrometric isolation of differentially controlled proteins along with invention of newer proteins, biomarkers, protein modifications as well as polymorphisms in different human tissues [1]. However, hurdles continue regarding the assessment in addition to evaluation of properties of proteins existent in lesser quantities inclusive of, corroborating what are known as the ‘’missing proteins’’.A missing protein implies a nonverified genetic sequencing regarding which a protein has not been found till date [2].As per the International Human Proteome Project(HUPO),at present1343 missing proteins without any function assigned exist, either anticipated by bioinformatics assessment or by experimental evaluation. The problems encountered regarding estimation of the missing proteins might be associated with their lesser quantities in numerous tissues besides getting expressed in just occasional cell kinds in the human body.
Keywords
Mass spectrometric; Detergent; IDE-Gel
Introduction
Recently plenty of success has been done regarding acquisition of mass spectrometric isolation of differentially controlled proteins along with invention of newer proteins, biomarkers, protein modifications as well as polymorphisms in different human tissues [1]. However, hurdles continue regarding the assessment in addition to evaluation of properties of proteins existent in lesser quantities inclusive of, corroborating what are known as the ‘’missing proteins’’.A missing protein implies a nonverified genetic sequencing regarding which a protein has not been found till date [2]. As per the International Human Proteome Project (HUPO), at present1343 missing proteins without any function assigned exist, either anticipated by bioinformatics assessment or by experimental evaluation. The problems encountered regarding estimation of the missing proteins might be associated with their lesser quantities in numerous tissues besides getting expressed in just occasional cell kinds in the human body. Hence, regarding capturing the missing proteins, the collective strategy of experts has been evaluation of a broad variety of tissues subsequent to targeted proteomics workflow [3]. Considerable taking into account is the need of the hour regarding the isolation of missing proteins at the time of targeted proteomics workflow, in particular at the time of sample production, extraction, digestion, along with assessment of data for escalating the isolation chances [4].
Description about the Study
The placenta can work in the form of an extra origin amongst human villus tissues that is intact with in the final objective of unravelling the lesser quantities part regarding human Proteome. The fetal aspect of placenta is known as the chorion that gets generated from the trophoblast. The crucial function of the placenta is to aid in the communication between generating fetus with the mother. Trophoblast reflects a part of the major constituent of the placenta that is richly vascularized, whose surface has a microvilli cover. The production of the chorionic villi gets initiated by the finishing of 4th week of gestation. The length of microvilli escalates by 10 week (to about 1.5 mm) that decreases with pregnancy advancement, which convert to placenta by16th week of gestation. Utilization of chorionic villi is made in case of prenatal test (chorionic villus sampling) implicating obtaining a tissue for sample production regarding determination of particular genetic or biochemical aberrations in a baby yet to be born [5]. One can get chorionic tissue at the time of first trimester abortion (till 13th week of gestation) which represents a safe as well as frequently performed procedure [6]. As per the transcriptome assessment 65% (n=13,074) of all the human proteins (n=20,090) expression is seen in the placenta of which 288 genes illustrated escalated expression seen in the placenta in contrast to other tissue kinds [7]. Placenta possessed maximum gene expression in parallel with the test is that represents an attractive source for missing proteins [7-9]. Subsequent to the choice of appropriate tissue regarding the isolation of proteins present in lesser quantities in the sample production method, a necessary approach regarding invention as well as isolation of proteins present in lesser quantities along with missing proteins. In particular a significant step comprises of protein extraction from solid tissue for following mass spectrometric assessment. Nevertheless, the effective extraction of proteins of attraction have their own hurdles. The selection of the strategy regarding cell breakdown is based on the tissue kind, quantity in addition to the physical characteristics of proteins extracted. According to history, physical break down (mechanical breakdown, sonication along with freeze/thaw cycles) was believed to be the best approach regarding cell breakdown along with extraction of the cellular quantity. The major hurdle being that this kind of approach implicates in general protocols that are hard to replicate in view of the instruments utilized in various laboratories. Recently, detergent dependent methods have acquired a conventional step regarding the proteomic sample production in view of them possessing both breakdowns along with solubilizing actions. Despite detergent dependent cell breakdown is an alternate strategy to the physical breakdown of cell membranes its utilization in generalis done in combination with certain physical strategies (homogenization as well as sonication) [10]. Moreover, in view of the availability of large quantities of detergents at present making a choice of the proper one continues to be a problem. Lastly no detergent is best regarding all the applications regarding variable tissues [11]. Regarding the effective solubilization of the tissue proteins, greater quantities of detergents (like Sodium dodecyl sulphate(SDS)) [12], or chaotropic substances (like urea) [13] are needed classically; nevertheless,these might hamper trypsin action,repress LC-ESI- MS-ionization along with form greater quantity ions that hinder with mass spectrometric assessment [14]. Hence in view of the constituents of the extraction buffer might possess important actions on the outcomes, its removal assumes significance for following Proteome profiling. Hence the detergent’s elimination is essential in the proteomics sample production workflow. Strategies like acetone precipitation, desalting of spin columns, ultrafiltration along with molecular weight cutting off filters are the ones utilized at present for the removal of detergents from proteins or cryptic solutions [15]. However, these approaches possess certain restrictions, inclusive of considerable time utilization, hardwork besides utilization of organic solvents prior to an extract that is adequately concentrated for assessment gets derived [16]. Regarding the description of greater rapid assessment of sample production might be advantageous and aid regarding the proteomics research in view of decreases in production time can escalate sample through put (output in contrast to input). Here a purification method for eliminating detergent from SDS possessing protein extracts before protease digestion was utilized. Variable variants of this method are at present utilized to get over the acknowledged limitations [17]. Shkrigunov et al. [23], utilized SDS-Polyacrylamide gel electrophoresis (PAGE) without separating proteins in a resolving gel (labelled by them as IDE-gel concentration regarding the reduction of the complicated nature besides expenditure of one dimensional electrophoresis (IDE). This type of manipulation might be visualized in the form of curtailed SDS-PAGE stage with the isotachophoresis of biomacromolecules possessing akin charge to mass ratio in the existence of SDS [18]. This modulation of the abbreviated SDS-PAGE was illustrated to be applicable in the form of a production technique with concentration on sample cleaning besides preservation of proteins quantities in case of proteomics [19], along with of utility in the focusing as well as DNA identification [20,21]. in contrast to the traditional SDSPAGE that generates numerous bands separated by molecular weight,a single band gets derived subsequent to IDE-gel concentration (curtailed SDS-PAGE). The single protein band derived inclusive of all proteins of the tissues evaluated subsequent to solubilisation along with total quantities of following samples for digestion enzymatically along with mass spectrometric assessment might be reduced. This posited IDE-gel concentration causes easing out of sample production method by decreasing time needed along with associated hard work. Moreover, their method enhanced throughput regarding the proteomics along with the outcomes replicability in addition to its belief. Regarding the evaluation of properties of human chorionic villi proteome, utilization of liquid chromatography (LC), mass spectrometry (MS) which is coupled with tandem MS(LC-MS/MS) along with SDS-dependent urea-extraction with subsequent IDE-gel concentration, ureadependent extracts were further evaluated by them.Gel dependent digestion enhances the quantity of protein isolations in the human placental proteome in contrast to liquid digestion [13]. However, the urea- thiourea- proteomic extraction technique that is well generated in addition to commonly utilized strategy regarding the proteomic studies of the human placenta [13,22]. The objective of their study was estimation of SDS-dependent solubilization in combination with the IDE-gel concentration sample production technique along with in -gel digestion resulted in replicability as well as comparatively total isolation along with quantification of the proteins in chorionic villi derived from a first trimester abortion performed electively around 7-12 week of normal gestation along with the ones derived from a missed abortion (i.e. a pregnancy which was not viable with the fetal demise, however placenta besides embryonic tissues are present in the uterus still). Utilization of posited IDE-gel concentration technique was done. This implicated the elimination of SDS in a small electrophoresis run in a stacking gel without separating proteins. Subsequent to the in -gel digestion of the derived single protein band, they utilized the peptide mixture regarding further LC-MS/MS evaluation. Statistically significant outcomes were obtained from 6 datasets possessing 3 treatments, everyone from 2 tissue sources (elective/ missed abortions). The IDE-gel concentration enhanced the covering of the chorionic villus proteome. Their strategy aided in the isolation of 15 proteins present in low quantities, of which certain had not been prior determined through mass spectrometry of trophoblasts. Regarding the post hoc evaluation of results, they observed doubtful or unresolved protein(PSG7) that was encoded on human chromosome 19 as per next Prot.A proteomics sample production workflow in combination with the IDE-gel concentration utilization in the form of an appropriate prospective strategy for unearthing human proteins present in low quantities would be ideal [23 ] (Figures 1-4).
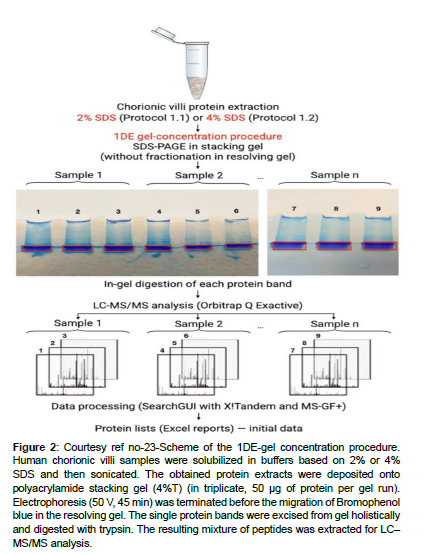
Figure 2: Courtesy ref no-23-Scheme of the 1DE-gel concentration procedure. Human chorionic villi samples were solubilized in buffers based on 2% or 4% SDS and then sonicated. The obtained protein extracts were deposited onto polyacrylamide stacking gel (4%T) (in triplicate, 50 μg of protein per gel run). Electrophoresis (50 V, 45 min) was terminated before the migration of Bromophenol blue in the resolving gel. The single protein bands were excised from gel holistically and digested with trypsin. The resulting mixture of peptides was extracted for LC– MS/MS analysis.
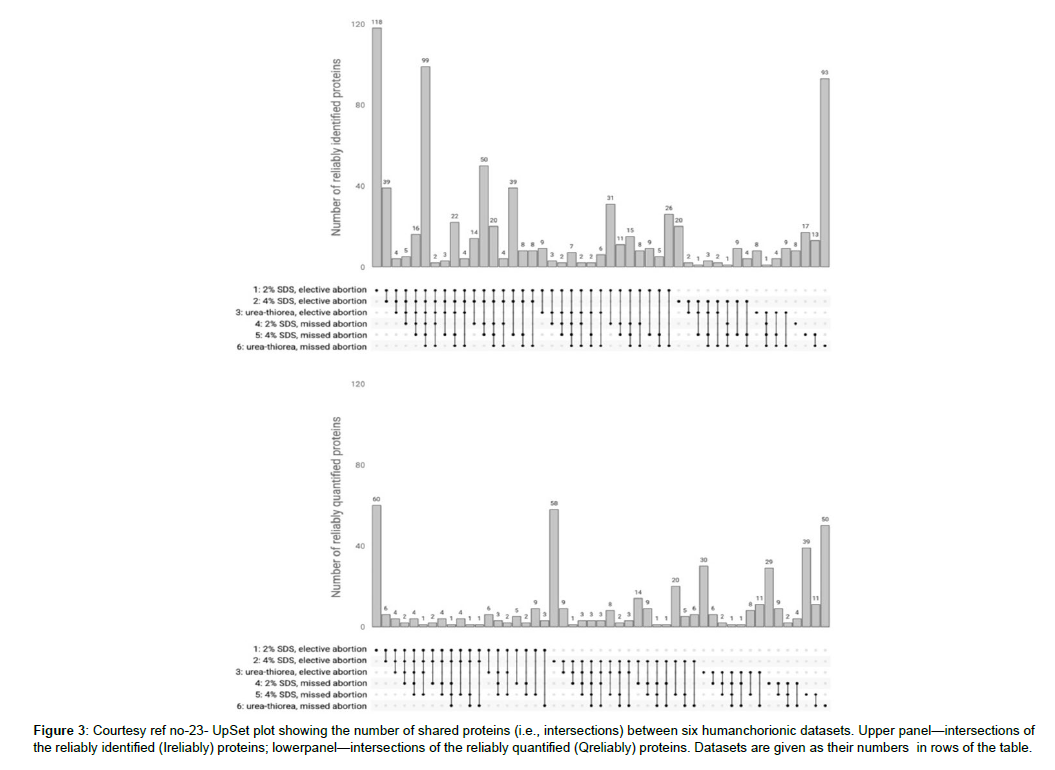
Figure 3: Courtesy ref no-23- UpSet plot showing the number of shared proteins (i.e., intersections) between six humanchorionic datasets. Upper panel—intersections of the reliably identified (Ireliably) proteins; lowerpanel—intersections of the reliably quantified (Qreliably) proteins. Datasets are given as their numbers in rows of the table.
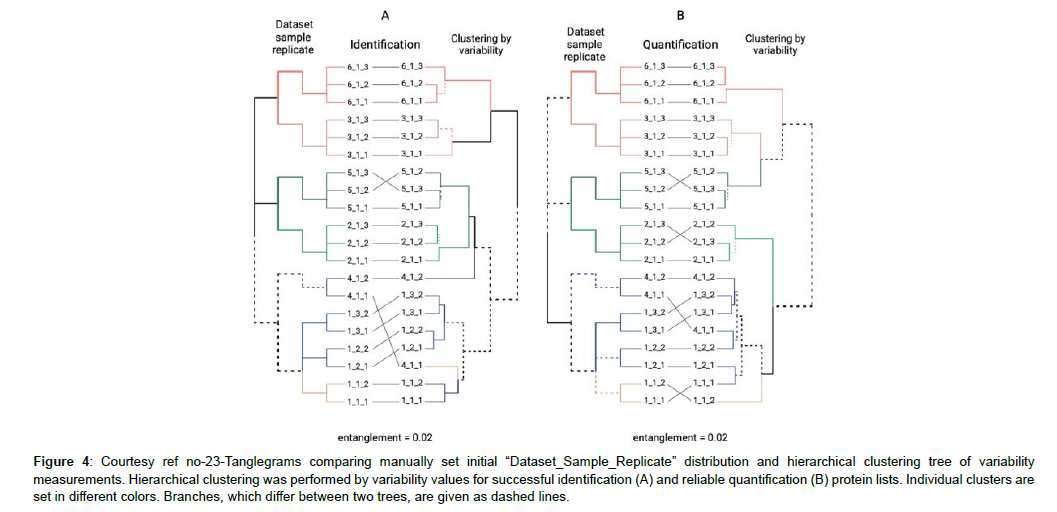
Figure 4: Courtesy ref no-23-Tanglegrams comparing manually set initial “Dataset_Sample_Replicate” distribution and hierarchical clustering tree of variability measurements. Hierarchical clustering was performed by variability values for successful identification (A) and reliable quantification (B) protein lists. Individual clusters are set in different colors. Branches, which differ between two trees, are given as dashed lines.
References
- BellL, Saxena R, Mattar S, YouJ, Wang M, et al. (2011) Utility of formalin fixed paraffin embedded liver biopsy pathogens for global analysis in non alcoholic steatohepapititis. Proteom Clin Appl 5:397-404.
- Zhou L, WangL, Goh W (2018) Understanding missing proteins: a functional perspective. Drug Discov Today 23:644-51.
- Reddy P, Ray S, Srivastava S (2015) The quest of the Human Proteomeand the missing proteins:digging deeper.Omics J Integr Biol 19: 276-82.
- Elguoshy A, Magdeldin S, Xu B, Hirao Y, Zhang Y, et al. (2016) Why are they missing: Bioinformatics characterization of missing human proteins? J Proteomics 149:7-14.
- Tarrade A, LaiKuen R, Malassine A, Tricottet V, Blain P, et al. (2001) Characterization of human villusand extra villus trophoblasts isolated from the first trimester placenta. Lab Investig 81: 1199-1201.
- Harper C, Henderson J, Darney P (2005) Abortion in the United States. Annu Rev Public Health 26: 501- 512.
- (2022) The Human Protein Atlas Tissue Atlas The Placenta Specific Proteome.
- Mori M, Ishikawa G, Luo S, Mishima T, Goto T, et al. (2007) The cytotrophoblast layer of human chorionic villi becomes thinner but maintains structural integrity during gestation. Biol Rep 76:164-72.
- Huppertz B, Ghosh D, Sengupta J (2014) An integrative view of the human early placental villi. Prog Biophys Mol Biol 114: 33-48.
- Peach M, Marsh N, Mishra N, Miskiewicz E, MD (2015) Solubilization of proteins: the importance of lysis buffer choice. Methods Mol Biol 1312: 49-60.
- Rabbiloud T, Luche S, Santoni V, Chavolet M (2007) Detergents andchaotropes for proteins solubilization before two dimensional electrophoresis. Methods Mol Biol 355:111-9.
- Pan H, Ding H, Fang F, YuB, Cheng Y, et al. (2018) Proteomics and Bioinformatics analysis of altered protein expression in the placental villus tissues from early recurrent miscarriage. Placenta 61:1-10.
- Luyten L, Dieu M, Demazy C, Fransolet M, Nawrot T, et al. (2020) Optimization of label free nano- LC- MS/ MS analysis of the placental Proteome., Placenta 101:159-62.
- Vaisar T (2009) Thematic freview series: Proteomics. Proteome analysis of lipid- protein complexes. Lipids Res 50:781-6.
- Miao Z, Chen M, Wu H, Ding H, Shi Z, et al. (2014) Comparative Proteome profile of human placenta in normal and fetal growth restriction subjects. Cell Physiol Bio chem 34:1701-10.
- Tubaon R, Haddad P, Quirino J (2017) Sample clean up strategies for ESI mass spectrometry applications in Bottom Proteomics: trends from 2012-2016. Proteomics 17:1700011.
- Kachuk C, Faulkner M, Liu F, Doucette A (2016) Automated SDS- depletion for mass spectrometry through transmembrane electrophoresis. J Proteomics Res 15:2634-42.
- Smith B (1997) SDS-Polyacrylamide gel electrophoresis for N-terminal protein sequencing. Methods Mol Biol 64:17-24.
- Gomis Cebolla J, Scaramal Ricietto A, Ferre J (2018) A genomic and proteomic approach to identify and quantify Bacillus thuringiensis proteins in the supernatant and parasporal crystals. Toxins 10:193.
- Kondratova V, Ser’duk O, Shelepov V, Lichtenstein A (2005) Concentration and isolation of agarose gel isotachophoresis. Bio Techniques 39: 695-9.
- Luebker SA, Koepsell SA (2016) Optimization of urea based protein extraction from formalin-fixed paraffin-embedded tissue for shotgun proteomics. International journal of proteomics 2016.
- Yang J, Kong T, Kim H, Kim H (2015) The Proteomic analysis of human placenta. J Korean Med Sic 30: 770-8.
- Shkrigunov T, Pogodin P, Zgoda V, Larina O, Kisrieva Y, et al. (2022) Protocol for increasing the sensitivity of MS based detection of human chorionic villi. Curr Issues Mol Biol 44: 2069-88.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Kaur KK, Dr. Allahbadia GNK, Singh M (2022) A Purification Protocol for Escalating the Sensitivity of Mass Spectrometry Dependent Innovative Protein Determination in Human Chorionic Villi. J Anal Bioanal Tech 13: 486.
Copyright: © 2022 Kaur KK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 1757
- [From(publication date): 0-2022 - Apr 19, 2025]
- Breakdown by view type
- HTML page views: 1426
- PDF downloads: 331