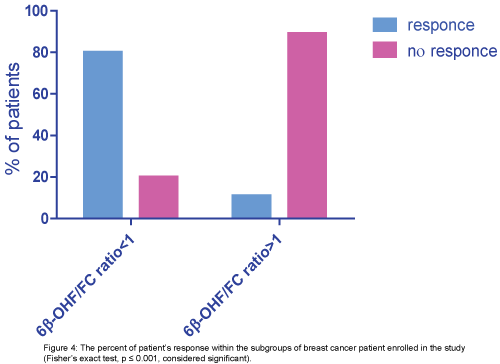Research Article Open Access
A Prospective Pharmacokinetic Study of Docetaxel in Breast Cancer Patients in Relation to CYP3A4 Activity
Mervat Omran1, Osama Badary2, Amany Helal3 and Samia Shouman1*
1Cancer Biology Department, National Cancer Institute, Cairo University, Egypt
2Clinical Pharmacy Department, Ain-Shams University, Cairo, Egypt
3Medical Oncology Department, National Cancer Institute, Cairo University, Egypt
- *Corresponding Author:
- Samia Shouman
Professor of Clinical Biochemistry
Cancer Biology Department, National Cancer Institute
Cairo University, Egypt
Tel: 0020123952527
E-mail: samiasshouman@yahoo.com
Received April 20, 2016; Accepted May 09, 2016; Published May 17, 2016
Citation: Omran M, Badary O, Helal A, Shouman S (2016) A Prospective Pharmacokinetic Study of Docetaxel in Breast Cancer Patients in Relation to CYP3A4 Activity. Clin Pharmacol Biopharm 5: 156. doi:10.4172/2167- 065X.1000156
Copyright: © 2016 Omran M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Abstract
Purpose: To study the correlation between pharmacokinetics & pharmacodynamic of docetaxel and CYP3A4 activity in Egyptian cancer patients.
Patients and methods: Fourteen Egyptian female Patients with metastatic breast cancer, World Health Organization (WHO) performance status 0 to 2, had received prior chemotherapy regimen, were treated with single-agent docetaxel (100 mg/m2), given every 21 days. Hydrocortisone 300 mg IV was administered 2 days before docetaxel treatment and Cytochrome 3A4 activity was determined by measuring the level of urinary metabolites of 6β-hydroxy cortisol (6β-OHF) and cortisol (FC). For the pharmacokinetic study, Blood samples were taken before and after IV infusion for 1 hr of 100 mg/m2 docetaxel. The level of the drug was determined using HPLC and the correlation between pharmacokinetics and CYP3A4 activity were determined.
Results: After cortisol administration, the total amount of 24-hour urinary 6β-OHF and FC were19.97 ± 10.43 and 16.84 ± 10.36 mg/24 h (mean ± SD) respectively. On the other hand, the 6β-OHF/FC ratio after cortisol administration was 1.86 ± 1.933. The pharmacokinetic parameters of docetaxel were clearance 19.9 ± 4.5 L/hr, the volume of distribution 65.6 ± 28.6 L (mean ± SD) and AUC 7.2 μg/ml.hr (range 5-8.8 μg/ml.hr) ± SD). A significant correlation was found between 6β-OHF/FC ratio and neutropenia (p=0.04) in addition correlation between 6β-OHF and Cmax (p=0.04).
Conclusion: The interpatient variability of CYP3A4 activity in each patient could be predicted by measuring the total amount of 24 hour urinary 63-OHF after cortisol administration. Individualized dosing to optimize drug exposure for each patient could be performed based on this method. A farther study of fixed versus individualized dosing of docetaxel is needed to determine whether individualized chemotherapy with the application of this method can reduce PK and toxicity variability.
Keywords
Docetaxel pharmacokinetic; CYP3A4 activity; Neutropenia; Response
Introduction
Docetaxel (DCX) is a toxoid derivative that displays antitumoral activity against many solid tumors, including prostate, lung, ovarian and breast cancers [1] and promising activity against metastatic breast cancer [2]. Individuals display significant differences in term of efficacy and adverse effects after exposure to docetaxel. The drug is not effective for all breast cancers patients; an identical dosage of a drug can result in widely different concentrations of the therapeutically active compound or metabolites [3]. Therefore, it is very important to develop a diagnostic method for the prediction of the response to docetaxel in order to avoid over or under treatment. Currently, it is impossible to identify, before the initiation of therapy, the patients for whom docetaxel will be used.
The presence of genetic variability in both content and catalytic activity of enzymes involved in drug metabolism could lead to clinically important variations in efficacy and adverse effects of some drugs. The cytochrome P450 is a family of heme-containing enzymes responsible for the metabolism of many drugs including taxane [4]. Docetaxel is metabolized by CYP3A4 in the liver into four major metabolites with minimal or no antitumor activity so that metabolism of docetaxel depends on the enzyme activity of CYP3A4 in the liver of individual patients. The CYP3A4 enzyme displays large interpatient differences in both content and catalytic activity in humans. These differences exist even in the absence of medications known to induce or inhibit the enzyme and are thus likely to reflect genetic variability [4,5].
We hypothesized that the interpatient variability of docetaxel pharmacokinetics and pharmacodynamicis due differences in CYP3A4 activity. Exogenous cortisol was used as a probe to measure CYP3A4 activity through determination of its urinary 6β-hydroxy metabolite (6β-OHC) and free cortisol (FC) metabolites [6].
Material and Methods
Drugs
The docetaxel (Taxotere® Sanofi-Aventis, Paris, France) vial was obtained as concentrated sterile solution that contained 20 or 80 mg of the drug in 0.5 ml polysorbate 80. Hydrocortisone (Solu-cortif, EIPICO, Cairo, Egypt) was available as a vial containing 100 mg lyophilized powder. Both drugs were from the drug store of the National Cancer Institute (NCI) Hospital.
Chemical and reagents
For HPLC, an authentic sample of docetaxel was supplied by National Organization for Drug Control and Research (NODCAR, Cairo, Egypt); the stock solution was prepared in methanol at concentration 1mg/ml. Cortisol (FC) and 6β-deoxycortisol (6β-OHC) were purchased from (Sigma-Aldrich, MO, USA). They were reconstituted as 1 mg/ml solutions in ethanol. Acetonitrile (Alliance Bio, Ohio, USA), sodium hydroxide, ethanol (Riedel-deHaÑ�?n, Honywell, Germany), diethyl ether (Honil Limited, London, UK), methanol (Tedia, Connecticut, USA), propanol, tetrahydrofuran, ethylacetate (BDH Limited Poole, England), ammonium acetate (MP Biomedicals, LLC, Germany) all the solvents and chemicals use in this study were HPLC grade.
Patient selection
The study protocol was approved by the Ethical Committee Board of the National Cancer Institute (NCI), Cairo University, Egypt. All participant patients provided written informed consent before study beginning. Fourteen eligible female patients with histologically proven metastatic breast cancer, the inclusion criteria were age ≥18 years, performance state: 0-2 (according to WHO classification), neutrophil count ≥1.0×109/l, platelet count ≥100×109/l, serum creatinine level ≤1.5 mg/dl, serum total bilirubin level ≤1.5 mg/dl, and serum transaminases ≤150U/L were treated with docetaxel 100 mg/m every 21 days. The Exclusion criteria included the following: Pregnancy or lactation, concomitant radiotherapy or chemotherapy, and a known history of severe concomitant diseases or with elevated serum bilirubin hypersensitivity to polysorbate 80.
Pre-treatment, and follow-up evaluation
All the patients were subjected to the following, Full history taking, full clinical examination and laboratory investigations including: complete blood picture, serum creatinine and liver function tests (serum bilirubin level, serum alkaline phosphatases, serum transaminases and serum albumin), radiological investigations including chest CT, abdominal ultrasound, and bone scan according to patient’s symptoms. Clinical and the radiological response were assessed every 2 cycles.
Drug administration and Sample collection
At the first day, a dose of 300 mg hydrocortisone in 100 ml of 0.9% saline was administered (at 9-12 am) intravenously for 30 min. The urine was collected for 24 hours and total volume was recorded. Then a 5-ml aliquot was stored at -20°C until assayed. Two days after the cortisol administration, a dose of 100 mg/m2 of docetaxel was diluted in 250 ml of 5% glucose and administered for 1-hour IV infusion. Blood samples for pharmacokinetic (PK) studies were obtained from all patients in the first cycle of therapy. The blood samples were collected before infusion, 30 min after the beginning of infusion, at the end of infusion, 15, 30 and 60 minutes, and 3, 5, and 24 hours after the end of infusion. Whole blood samples were collected into heparinized tubes and plasma samples were stored at -20°C until assayed.
Methods
Estimation of CYP3A4 activity by urinary metabolite of exogenous cortisol
We chose hydrocortisone as an exogenous cortisol for the measurement of endogenous urinary 6β-OHF/FC. It is simple and completely noninvasive and could have a practical advantage over the other methods [7]. Urine was centrifuged at 3200×g for 5 min and extracted adopting liquid-liquid extraction method [8] with an ethyl acetate/isopropanol mixture (85/15: v/v). The organic layer was washed with a basic solution of 1 N sodium hydroxide, separated and then evaporated at 56°C under a stream of nitrogen. The residue was then dissolved in 1 ml of mobile phase. Separation was performed using an ultracarb ODS, C18 column (20 μm particle, 250×4.6 mm) and HPLC system Gilson, a solvent delivery pump (Gilson 321) equipped with UV detector (Gilson visible-155) and coupled with 243 autosampler and chromatographic maxima 820 data integration package were used. The mobile phase consisted of methanol: water (57.5:42.5, v/v) was pumped at flow rate of 1 ml/min and the chromatographic separation was monitored at 242 nm.
Docetaxel pharmacokinetics
1-ml of patient plasma sample, 100 μl of ammonium acetate buffer (pH 5) and 7 ml of diethyl ether were added in a glass tube. Tubes were mixed by vibration for 3 min and then centrifuged at 3500g at 4°C for 5 min. The organic layer was quickly dried under nitrogen at ambient temperature. The residue was next reconstituted with 1 ml of the mobile phase. Separation was performed using a Gemni Nux (150×4.6 mm, 5 μm particle size). The mobile phase pumped at a flow rate of 1.8 ml/min consisted of acetonitrile, 35 mM ammonium acetate buffer (pH 5), tetrahydrofuran (45:50:5, v/v). Chromatographic separation was monitored at 227 nm [9].
Determination of blood α-1-acid glycoprotein
Alpha acid glycoprotein was determined in plasma of the patients before the treatment using human α-1-Acid Glycoprotein, ELISA Kit (AssayMax, ASSAYPRO, Canada) following authors’ instructions [10].
Statistical analysis
Numerical data were expressed as the mean and standard deviation (SD), median and range as appropriate. Qualitative data were expressed as frequency and percentage. The bi-exponential equation was used to calculate the AUC and PK parameters of docetaxel. Spearman’s rho method was used to determine the correlation between different parameters. Fisher’s exact test used to determine the difference in response between the subgroups. A two-sided p-value of ≤0.05 was considered significant. All statistical analysis was performed using Graph Pad Prism 5.
Results
Patient characteristics
The baseline pretreatment characteristics of all the studied patients are listed in the Table 1. The majority of patients (92%) had a performance status of 0 or 1. All of them had metastases, 7 out of 14 have lymph node involvement, while 5, 4 and 3 of the 14 have lung, bone and liver metastases, respectively. Twelve patients (85%) had received previous chemotherapy but not for the metastatic state.
| Characteristic | No. of patients |
Percent (%) |
|---|---|---|
| Total patients | 14 | 100.0 |
| Positive Family history | 5 | 35.7 |
| Performance status | ||
| 0 | 6 | 42.8 |
| 1 | 7 | 50.0 |
| 2 | 1 | 7.1 |
| Prior treatment* | ||
| None | 2 | 14.2 |
| Surgery | 11 | 78.5 |
| Radiotherapy | 8 | 57.1 |
| Chemotherapy | 12 | 85.7 |
| Site of Metastasis* | ||
| Lung | 5 | 35.7 |
| Liver | 3 | 21.4 |
| Bone | 4 | 28.5 |
| lymph nodes | 7 | 50.0 |
| Pleura | 2 | 14.2 |
*One patient may receive more than one prior treatment, and may have more than one site of metastasis
Table 1: The baseline characteristics of enrolled breast cancer patients.
Table 2 shows the age, BSA and baseline laboratory parameters of the enrolled patients. The median age was 49 years ranging from 38-62 years. Chronic diseases, medication intake and its effect on cytochrome activity are shown in the Table 3. Two of the enrolled patients were taking multi-medication, one was receiving therapy for diabetes, hypertension and asthma and the other was treated for hypertension, asthma, and renal stone. One patient was found to have HCV antigen positive but with no clinical or laboratory significant hepatic dysfunction. Two patients (14.2%) suffered from bronchial asthma were on regular salbutamol inhalation (β2 agonist) which First pass metabolized in the liver by CYP3A4 and other isoenzymes but those two patients not excluded from the study because salbutamol show little or no inhibition of CYP3A4 [11].
| Characteristic | Mean ±SD SD |
Median (Range) | Normal values |
|---|---|---|---|
| Age (years) | 48.0±7.2 | 49.0 (38.0-62.0) | |
| BSA (m2) | 1.7±0.25 | 1.7 (1.2-2.0) | |
| Laboratory parameters | |||
| Plasma proteins | |||
| Total protein (g/dl) | 7.5± 0.5 | 7.5 (6.9-8.7) | 6.8-8.8 |
| Albumin(g/L) | 4.0± 0.2 | 4.1 (3.6-4.2) | 3.2-5.1 |
| AAG(µg/ml) | 2.1 ± 0.5 | 2.1 (1.3-2.9) | 0.5-1.2 |
| Kidney function | |||
| Creatinine (mg/dl) | 0.7 ± 0.3 | 0.7 (0.4-1.5) | 0.5-1.2 |
| Urea nitrogen (mg/dl) | 27.4± 14.1 | 23.0 (9.0-55.0) | 10-50 |
| Liver functions | |||
| T-Bilirubin (mg/dl) | 0.6± 0.3 | 0.6 (0.3-1.2) | 0.2-1.2 |
| AST( IU/L) | 22.9± 7.8 | 19.5 (15.0-38.0) | 10-40 |
| ALT(IU/L) | 20.6± 8.6 | 18.0 (12.0-41.0) | 10-41 |
| ALP( IU/L) | 139.0± 44.5 | 136.0 (90.0-210.0) | 90-290 |
| Hematological values | |||
| WBC (×103/µl) | 6.9± 2.7 | 6.3 (4.3-11.4) | 4.3-10.8 |
| Hemoglobin(g/dl) | 11.2± 2.9 | 11.7 (9.4-15.1) | 12-15 |
| Platelet (×103/µl) | 290.8± 148.8 | 293.0 (82.0-467.0) | 150-450 |
AAG: α-1-acid glycoprotein; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ALP: Alkaline phosphatases; BSA: Body surface area; SD: Standard deviation; WBC: White blood cell count
Table 2: Age, body surface area and laboratory parameters of the enrolled breast cancer patients.
| Chronic disease | No. of Patients |
Percent (%) | Medication intake | Effect on Cytochrome activity |
|---|---|---|---|---|
| Diabetes mellitus | 1 | 7.1 | Insulin | No effect |
| Hypertension | 2 | 14.2 | Indapamide (non-thiazide diuretic) | No effect |
| Captopril (ACE inhibitor) | No effect | |||
| Hydrochlorothiazide (thiazide diuretic) |
No effect | |||
| Kidney stone | 1 | 7.1 | No medication taken | |
| Bronchial asthma | 2 | 14.2 | Sulbutamol (β2 agonist) | First pass metabolism in the liverby CYP3A4 and other isoenzymes |
| HCV | 1 | 7.1 | Sylimarin (liver support) | No effect |
Table 3: Chronic diseases and medication taken in enrolled breast cancer patients, and their effect on cytochrome 3A4 activity.
Activity of CYP 3A4
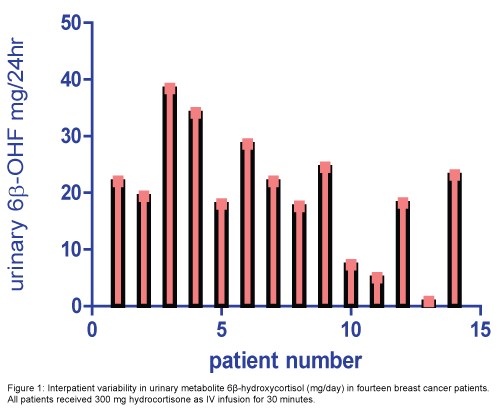
The median level (range) of 24 hour urinary 6β-OHF and FC were 20.7(0.8-38.4) mg/24 h and 19.1 (0.5-32.2) mg/24 h, respectively (Figure 1). The 6β-OHF/FC ratio after cortisol administration was 1.2 (0.6-8.1). The median percent of metabolite and unchanged form of cortisol to the total dose taken 300 mg were 6.9% (0.2-12.8) and 6.3% (0.1-10.7), respectively (Table 4).
| Urinary metabolite | Mean ± SD | Median (Range) |
|---|---|---|
| 6β-OHF, mg/d* | 19.9±10.4 | 20.7(0.8-38.4) |
| FC, mg/d** | 16.8±10.3 | 19.1 (0.5-32.2) |
| 6β-OHF/FC | 1.8±1.9 | 1.2(0.6-8.1) |
| 6β-OHF% | 6.6±3.4 | 6.9 (0.2-12.8) |
| FC% | 5.6±3.4 | 6.3 (0.1-10.7) |
*6β-OHF: 6-betahydroxycortisol; **FC: Cortisol; SD: Standard deviation
Table 4: The mean and range of 24 hr urinary metabolite levels of 6β-OHF, FC and 6β-OHF/FC ratio after 300 mg cortisol administration.
Docetaxel pharmacokinetic
Pharmacokinetic parameters are listed in the Table 5. The mean alpha and beta half-lives were 8.8 minutes and 4.5 hours. The docetaxel clearance was 19.9 ± 4.5 L/hr, and AUC (Mean=7.2 μg/ml.hr, SE=0.313, 7.9 ≥ AUC ≥ 6.5). The volume of distribution was 65.6 ± 28.6 L.
| Pharmacokinetic parameters | Mean ± SD | Median (Range) |
|---|---|---|
| AUC µg/ml.hr | 7.2±1.1 | 7.3 (5-8.8) |
| C max µg/ml | 2.4 ± 0.6 | 2.6 (1.1-3.4) |
| Clearance L/hr | 19.9 ± 4.5 | 20.3 (14.1-29.0) |
| Clearance L/hr/m2 | 13.7± 3.1 | 13.6 (8.7-20.0) |
| T1/2α minute | 8.8± 1.0 | 9.5 (7.9-11.0) |
| T1/2β hours | 4.5 ± 1.3 | 5.0(3.1-7.5) |
| Vd L | 65.6±28.6 | 58.1(37.3-126.4) |
AUC: Area under the curve; Cmax: Maximum concentration; T1/2α: Distribution halflife; T1/2β: Elimination half-life; VD: Volume of distribution; SE: Standard error
Table 5: Pharmacokinetic parameters of 100 mg/m2 of docetaxel for 1 hr infusion in Egyptian breast cancer patients.
Pharmacodynamic effects
Assessment of toxicity was done after the first cycle of the treatment while the response was evaluated every 2 cycles. Table 6 shows the change in hematological values during the three weeks after the first cycle of treatment. WBC count and neutrophile % declined through the first week after docetaxel infusion then begin to recover during the second week of treatment in most of the patients and finally return to normal values during the third week of treatment. There are no marked changes in other values.
| Hematological changes | Mean ± SD | |||
|---|---|---|---|---|
| Before treatment | 1st week** | 2nd week** | 3rd week** | |
| Neutrophil % | 76.3±10.5 | 33.2±11.8 | 50.6±8.2 | 74.2±9.2 |
| WBC (×103/µl) | 6.9±2.65 | 3.4±2.4 | 5.5±3.0 | 6.9±2.8 |
| ANC (/µl)* | 526.4±8.5 | 112.8±10.2 | 278.3±5.5 | 511.9±7.2 |
| HGB (g/dl) | 12.1±1.5 | 11.5±1.1 | 11.2±1.3 | 10.9±1.4 |
| RBC (×103/µl) | 4.5±0.67 | 4.3±0.61 | 4.2±0.73 | 4.1±0.71 |
| PLT (×103/µl) | 318.0±142.0 | 297.0±152.0 | 289.0±112.0 | 280.0±92.0 |
| ALT(IU/L) | 20.6± 8.61 | 21.0±8.2 | 21.9±7.9 | 21.2±7.8 |
| AST( IU/L) | 22.93± 7.82 | 25.3±7.9 | 24.3±7.5 | 23.4±7.3 |
*The percent decrease in ANC=74 ± 12% (Mean ± SD); **After treatment ALT: Alanine aminotransferase; ANC: Absolute neutrophil count; AST: Aspartate aminotransferase; HGB: Hemoglobin; PLT: Platelet; RBC: Red blood cell count; SD: Standard deviation; WBC: White blood cell count
Table 6: Hematological changes during the three weeks after the first cycle of 100 mg/m2 docetaxel treatment.
Neutropenia grade 3-4 was the predominant toxicity (71%) related to docetaxel treatment. Only one patient had febrile neutropenia. The percentage decrease in ANC was 48.6% (Table 7). Non-hematologic toxicities, such as gastrointestinal (mucositis, diarrhea, nausea, vomiting), arthralgia, fatigue and hepatic toxicities (hyperbilirubinemia, aminotransferase elevations), were mild in most of the patients. Four patients (28.5%) achieved a partial response and one patient achieved a complete remission.
| Side effects | No. of Patients |
Percent (%) | Severity |
|---|---|---|---|
| Hematologic toxicities | |||
| Neutropenia | 10 | 71 | 3-4 |
| Febrile neutropenia | 1 | 7.1 | 4 |
| Non-hematologic toxicities | |||
| Alopecia | 12 | 85.7 | Moderate |
| Nausea | 3 | 21.4 | Mild |
| Vomiting | 4 | 28.5 | Mild |
| Mucositis | 3 | 21.4 | Mild |
| Neuropathy | 6 | 42.8 | Mild |
*One patient may have more than one toxicity
Table 7: Hematological and non-hematological toxicities in breast cancer patients receiving 100mg/m2 of docetaxel and degree of severity according to NCI toxicity criteria.
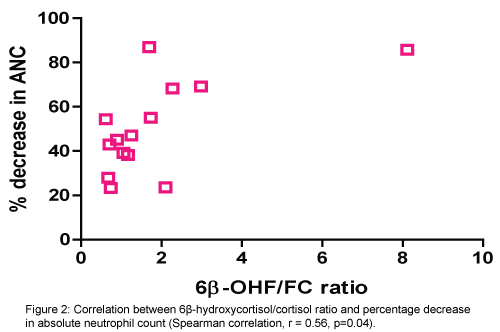
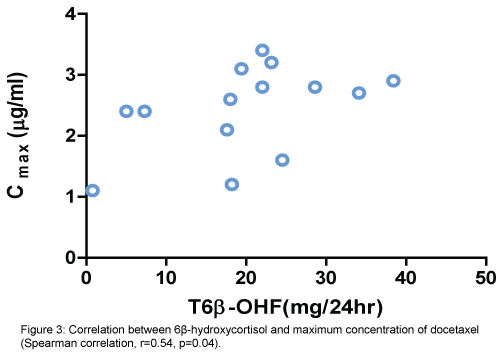
There was a positive correlation between increased activity of CYT3A4 (6β-OHF/FC ratio) and percentage of decrease in absolute neutrophile count (ANC) (Spearman correlation, r=0.56, p=0.04). (Figure 2). Also, positive correlation between the total amount of 6β-OHF and Cmax achieved with docetaxel (Spearman correlation, r=0.54, p=0.04) (Figure 3). Even after cortisol administration, no correlation was detected between docetaxel CL and 6β-OHF/FC (r=0.0119) and no correlation with the total amount of 24-hour urinary 6β-OHF after cortisol administration (T6β-OHF) (r=0.0659).
Then the patients were stratified into two subgroups according to 6β-OHF/FC ratio we find that:
group 1 (6β-OHF/FC ratio ≥1): (1/9)11% of them achieve good response and (8/9) 89% of them no response, group 2 (6β-OHF/FC ratio ≤1): (4/5) 80% of them achieve good response and (1/5) 20% of them no response. The patient who achieves complete remission was one of group2 (6β-OHF/FC ratio ≤1), (p ≤ 0.001, considered significant) (Figure 4).
Discussion
Docetaxel is primarily metabolized by CYP3A, in particular by isoform CYP 3A4, its metabolites are substantially less active than the parent drug and are considered as a major route of inactivation. All the patients involved in this study have normal hepatic function, none of them had received known potent inhibitors or inducers of CYP3A4 with the exception of dexamethasone which is used as premedication, and thus, it appears that the variation may reflect genetic differences in the metabolism of docetaxel. Although, other factors as genetic polymorphism, epigenetic influences or non-genetic related to host factors may be implemented in this variation [5]. In this study, the Egyptian breast cancer patients showed a decrease in docetaxel clearance compared to European [12] and Japanese patients [13]. The mean clearance of docetaxel in this study was 13.7 ± 2.3 L/h/m2 compared with the reported clearance of 34 ± 3.2 and 22.6 ± 3.4 L/h/m2 for the whites and Japanese women, respectively.
Initially, the main predictors of total docetaxel clearance variability were BSA, AAG, hepatic function and age, more recently hepatic CYP450 3A4 activity [14]. Although some studies found correlation between DXC clearance and CYP450 3A4 activity [7,13,15], such type of relation was absent in our study which may be due to the presence of other metabolic pathways which might have been involved in the metabolism of the excess amount of exogenous cortisol [16], or due to an excess amount of substrate over enzyme capacity [7]. However, this apparent difference is inconclusive and need further investigation and may be accounted for the small sample size, assay variation, differences in methods of assay and sampling time points.
In this work, after exogenous cortisol administration, the total amount of 24-hr urinary 6β-OHF was 19.9 ± 10.4 mg/d (mean ± SD), urinary FC was 16.8 ± 10.3 mg/d and the 6β-OHF /FC ratio was 1.8 ± 1.9 compared to 12.2 ± 4.0, 13.6 ± 5.7 mg/d and 1.02 ± 0.45 for the amount of 24-hr urinary 6β-OHF, FC and 6β-OHF/FC ratio respectively in Japanese [13]. Although marked interpatient heterogeneity in the activity of CYP3A4 is known to exist, the clinical implications of having a high or low CYP3A4 activity were not necessarily known. In this study low CYP3A4 activity (6β-OHF/FC ratio <1) appears to be associated with the higher response. It implies that consideration should be given to modifying the dose for patients with high CYP3A4 activity (6β-OHF/FC ratio >1) because they are at risk of getting subtherapeutic doses of docetaxel.
The time-serum concentration represented by prolonged AUC and the decrease in the volume of distribution VD may reflect the decrease in clearance of DXC and the higher incidence of neutropenia in our results. In our study a negative correlation between AUC and the clearance was detected, however, the established relationship between the docetaxel AUC and the percentage decrease in ANC was not confirmed. This may have been due to the large heterogeneity of patient population (decrease in ANC ranged from 23.3% to 86.9%) and the small number of patients in this study. The AUC in our study was almost doubled (7.2 ± 1.1) (μg/ml.hr) while, it was (3.1 ± 0.9) and (2.6 ± 0.9) (μg/ml.hr) in the whites and Japanese respectively. Our data showed VD 65.6 ± 28.6 L (mean ± SD), Cmax ranged 1.1-3.4 μg/ml, T1/2α, 8.8 minute and T1/2β 4.5 hr compared to VD 84.0 ± 86.1, Cmax 0.99-2.41 μg/ml, T1/2α 5 minute and T1/2β 51 minute for European population [12] and VD 75.0 ± 20.6, Cmax 0.36-2.7μg/ml, T1/2α 9.2 minute and T1/2β 5 hr for Japanese patients [13]. Our patients presented by the high incidence of toxicity especially neutropenia which is the dose limiting toxicity of docetaxel. The toxicities namely, grade 3 or4 neutropenia was seen in virtually in most of our patients (78% percentage, incidence 11/14), it appeared to be more significant than in Western patients (58% percentage, the incidence of 14/24) [12]. However, reported an incidence of neutropenia was 25out of 29 patients, with the total percentage of patients was 86% [16]. The absolute neutrophil count decreased with the administration of DCX, reached a nadir at approximately 1 week after the treatment, and increased thereafter to a level higher than the basal ANC value 3 weeks after DCX dosage. A similar pattern for neutropenia was also reported by Friberg et al. [17]. A recent study find that severe hematological toxicities are more frequently experienced in the Japanese compared to the US and European patients. This difference in toxicity profiles was possibly caused by other unknown genetic factors, and differences in unbound docetaxel concentrations or baseline counts of white blood cells. However, mechanistic insights are not yet elucidated for different sensitivity to docetaxel toxicity between Japanese and Western populations [18].
Moreover, a significant relationship between CYP activity represented by 6β-OHF/FC ratio and the percentage decrease in neutrophil count in our study was observed (p=0.04). A result which may be attributed to the presence of pathway which results in a metabolite that is toxic to neutrophils. Interestingly, [12] reported high concentrations of oxazolidinedione metabolite in the plasma of patients who suffered from the most edema and weight gain. In addition, the occurrence of neurotoxicity was significantly more frequent in patients homozygous for GSTP1105 Ile allele [19]. It was found that docetaxel is mainly metabolized in the liver by the cytochrome P450 CYP3A4 and CYP3A5 subfamilies of isoenzymes. Metabolism is principally oxidative and at the start-butyl propionate side chain, resulting first in an alcohol docetaxel, which is then cyclised to three further metabolites two diastereomeric hydroxy oxazolidinones and oxazolidinedione [2].
α-1-acid glycoprotein is the main determinant of docetaxel serum binding variability. High AAG level have been associated with a decrease in the free fraction of docetaxel with reduced total clearance. In this study, AAG and the 6β-OHF/FC ratio do not appear to be correlated which may be due to the Polysorbate 80 which was reported to increases the unbound drug fraction by 16-24% and resulted in lower unbound docetaxel clearance.
Finally recent studies have disclosed that human breast cancer tissues express CYP 3A4 [20] suggesting that docetaxel may be metabolized to its inactive forms in tumor tissues and that CYP3A4 activity in tumor tissues may, therefore, affect the antitumor activity of docetaxel. CYP3A4 mRNA expression levels in tumor tissues and their relationship with the response to docetaxel were studied. The authors reported breast tumors with low expression of CYP3A4 mRNA show a significantly higher response rate (71%) than those with high expression of CYP3A4 mRNA (11%) [21]. Interestingly, CYP3A4 mRNA expression in tumor tissues showed no association with response to epirubicin based regimens [20]. These results are taken together suggest that intratumoral expression of CYP3A4 mRNA or CYP3A4 protein determined by immunohistochemistry could serve as a predictor of resistance to docetaxel. The role of CYP3A4 in the acquisition of such resistance thus appears to merit further basic and clinical studies.
In conclusion, the significant correlation observed between 6β-OHF/FC ratio and percentage decrease in absolute neutrophil count (ANC) was needed to be confirmed with further studies with larger sample size. The interpatient variability of CYP3A4 activity in each patient could be predicted by measuring the total amount of 24- hour urinary 63-OHF after cortisol administration. Individualized dosing to optimize drug exposure for each patient could be performed based on this method.
References
- Lyseng-Williamson KA, Fenton C (2005) Docetaxel: a review of its use in metastatic breast cancer. Drugs 65:2513-2531.
- Guitton J, Cohen S, Tranchand B, Vignal B, Droz JP,et al. (2005)Quantification of docetaxel and its main metabolites in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 19:2419-2426.
- Mackey JR, Ramos-Vazquez M, Lipatov O, McCarthy N, Krasnozhon D, et al. (2014) Primary Results of ROSE/TRIO-12, a Randomized Placebo-Controlled Phase III Trial Evaluating the Addition of Ramucirumab to First-Line Docetaxel Chemotherapy in Metastatic Breast Cancer JCO 57: 1513
- Kivisto KT, Kroemer HK, Eichelbaum M (1995)The role of human Cytochrome P450 enzyme in the metabolism of anticancer agents: implication for drug interaction. Br J ClinPharmacol 40:523-530.
- Zanger UM, Schwab M (2013)Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation.Pharmacology & Therapeutics 138:103-141
- Michael M, Cullinane C, Hatzimihalis A, O’Kane C, Milner A, et al. (2012)Docetaxel pharmacokinetics and its correlation with two in vivoprobes for cytochrome P450 enzymes: the C14-erythromycin breath test and the antipyrine clearance test. Cancer Chemother Pharmacol 69: 125-135
- Routes E, Boisdron-Celle M, Morel A, Gamelin E (2003) Simple and sensitive high-performance liquid chromatography method for simultaneous determination of urinary free cortisol and 6β-hydroxycortisol in routine practice for CYP3A4 activity evaluation in basal conditions and after grapefruit juice intake. J Chromatogr B AnalytTechnol Biomed Life Sci793:357-366.
- Ged C, Rouillon JM, Pichard L, Combalbert J, Brest N, et al. (1989) The increase in urinary excretion of 6bhydroxycortisolas a marker of human hepatic cytochrome P450IIIA induction. Br J ClinPharmacol 28: 373-386.
- Ciccolini J, Catalin J, Blachon M, Durand A (2001) Rapid high-performance liquid chromatographic determination of docetaxel (Taxotere) in plasma using liquid- liquid extraction. J Chromatogr B Biomed SciAppl 759:299-306.
- Duche J,Hervé F, Tillement JP (1998) Study of the expression of the genetic variants of human α-1-acid glycoprotein in healthy subjects using isoelectric focusing and immunoblotting. J Chromatogr B Biomed SciAppl 715: 103-109.
- Denisov IG,Grinkova YV,Baylon JL, Tajkhorshid E, Sligar SG (2015) Mechanism of Drug–Drug Interactions Mediated by Human Cytochrome P450 CYP3A4 Monomer. Biochemistry 54: 2227-2239.
- Rosing H, Lustig V, van Warmerdam LC, Huizing MT, ten BokkelHuinink WW, et al. (2000) Pharmacokinetics and metabolism of docetaxel administered as a 1-h intravenous infusion. Cancer ChemotherPharmacol 45: 213-218.
- Yamamoto N, Tamura T, Kamiya Y, Sekine I, Kunitoh H, et al. (2000)Correlation Between Docetaxel Clearance and Estimated Cytochrome P450 Activity by Urinary Metabolite of Exogenous Cortisol. JCO 18: 2301-2308.
- Urien S, Barre J, Morin C, Paccaly A, MontayG, et al. (1996) Docetaxel serum protein binding with high affinity to alpha 1-acid glycoprotein. Invest New Drugs 14: 147-151.
- Alexandre J, Rey E, Girre V, Grabar S,Tran A, et al. (2006) Relationship between cytochrome 3A activity, inflammatory status and the risk of docetaxel-induced febrile neutropenia: a prospective study. Ann Oncol 18: 168-172.
- Yamamoto N, Tamura T, Murakami H, Shimoyama T,Nokihara H, et al. (2005)Randomized pharmacokinetic and pharmacodynamic study of docetaxel: Dosing Based on Body- Surface Area Compared With Individualized Dosing Based on Cytochrome P450 Activity Estimated Using a Urinary Metabolite of Exogenous Cortisol. JCO 23:1061-1069.
- Friberg LE, Henningsson A, Maas H, Nguyen L, Karlson MO (2002)Model of chemotherapy-inducedmyelosuppression with parameter consistency across drug. J ClinOncol 20:4713-4721.
- Kenmotsu H, TanigawaraY (2015) Pharmacokinetics, dynamics and toxicity of docetaxel: Why the Japanese dose differs from the Western dose. Cancer Sci 106:497-504.
- Mir O, Alexandre J, Tran A, Durand AJ, Pons G, et al. (2009) Relationship between GSTP1 Ile105Val polymorphism and docetaxel-induced peripheral neuropathy: clinical evidence of a role of oxidative stress in taxane toxicity. Ann Oncol 20: 736-740.
- Kapucuoglu N,Coban T,Raunio H,Pelkonen O, Edwards R, et al. (2003) Expression of CYP3A4 in human breast tumor and non-tumour tissues. Cancer Letter 202: 17-23.
- Waterschoot RA, Lagas JS, Wagenaar E,Kruijssen CM, Tellingen O, et al. (2009)Absence of Both Cytochrome P450 3A and P-glycoprotein Dramatically Increases Docetaxel Oral Bioavailability and Risk of Intestinal Toxicity. Cancer Res69: 8996-9002.
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 11988
- [From(publication date):
specialissue-2016 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 11099
- PDF downloads : 889