Research Article Open Access
A Prospective, Multicenter, 2-Year Echocardiographic Study on Valvular Heart Disease in Parkinson's Disease Patients Taking Rotigotine and Other non-Ergot Dopamine Agonists
Karla Eggert1*, Gisela Antony1, Kerstin Anvari2, Stephan Behrens3, Michael Dapprich4, Reinhard Ehret5, Wilfried Lueer6, Alexander Nass7,Robert Pfister M8, Sigrid Planz-Kuhlendahl9, Gerd Reifschneider10, Alexander Simonow11, Michael Schwalbe12 and Wolfgang H. Oertel1
1Neurologist, Philipps-University of Marburg, Germany
2Neurologist, Tegel, Berlin, Germany
3Neurologist, Juelich, Germany
4Neurologist, Bad Zurzach, Switzerland
5Neurologist, Steglitz, Berlin, Germany
8Neurologist, Neusaess, Germany
9Neurologist, Offenbach, Germany
10Neurologist, Odenwald Erbach, Germany
11Neurologist, Klinik Sorpesee, Sundern, Germany
12Neurologist, Wittenberg, Germany
- *Corresponding Author:
- Karla Eggert
Philipps-Universitat Marburg, Neurologist, Baldingerstrabe
D-35043 Marburg, Germany
Tel: +49 6421 58-65443
E-mail: eggert@med.uni-marburg.de
Received date: April 14, 2016; Accepted date: April 25, 2016; Published date: May 02, 2016
Citation: Eggert K, Antony G, Anvari K, Behrens S, Dapprich M (2016) A Prospective, Multicenter, 2-Year Echocardiographic Study on Valvular Heart Disease in Parkinson’s Disease Patients Taking Rotigotine and Other non-Ergot Dopamine Agonists. J Alzheimers Dis Parkinsonism 6:233. doi:10.4172/2161-0460.1000233
Copyright: © 2016 Eggert K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Objectives: We explored the possible risk of valvular heart disease (VHD) in Parkinson’s disease (PD) patients taking rotigotine compared to other non-ergot dopamine agonists (DA) as requested by the EMA (European Medicines Agency) in terms of assessing the longterm safety associated with the cumulative dose of rotigotine.
Methods: We performed a prospective, multicenter, open-label, 2-year echocardiographic study in PD patients taking rotigotine or other non-ergot DA (piribedil, pramipexole, ropinirole). Valvular pathology was assessed by transthoracic echocardiographic examinations according to American Society of Echocardiography recommendations and a scoring system for restrictive VHD at 1-month, 12-months and 24-months follow-up examination. Routine neurological and physical examinations with special attention toward clinical symptoms of heart failure were performed after 1, 6, 12, 18 and 24 months.
Results: 102 out of 107 screened patients were enrolled into the study. Due to drop-outs along the 2-year followup, 66 patients (28 rotigotine, 38 other non-ergot DAs [3 piribedil, 20 pramipexole, 15 ropinirole]) completed the 12-months visit and 53 patients (23 rotigotine, 30 other non-ergot DAs [3 piribedil, 16 pramipexole, 11 ropinirole]) completed the 24-months visit. No patient showed clinical symptoms of heart failure or echocardiographic evidence of restrictive VHD during the 2-year period. Analyses of valve regurgitation (VR) revealed no case of severe or moderate VR in the rotigotine group. Only one patient taking pramipexole showed moderate VR at 1-month and 12-months follow-up visit decreasing to mild degree at 24-months follow-up.
Conclusions: This is the first prospective study assessing the possible risk of VHD in patients taking rotigotine. We did not find valvular pathology suggestive for restrictive VHD or increased risk of cardiac VR in PD patients receiving rotigotine or other non-ergot DAs. Future studies are needed to explore the clinical relevance and the relationship between non-ergot DAs and heart failure including effects of cumulative dose or comedication with other antiparkinsonian drugs.
Keywords
Parkinson’s disease; Rotigotine; Non-ergot dopamine agonists; Adverse drug reactions; Valvular heart disease; Valvular regurgitation; Fibrotic reactions; Echocardiography
Introduction
Treatment of Parkinson’s disease (PD) is still a challenge and focuses on the symptomatic control of motor and non-motor symptoms without inducing intolerable side effects. Levodopa remains the gold standard of therapy, but the emergence of motor complications may compromise the long-term therapeutic response [1]. Therefore, dopamine agonists (DA) are frequently used and have been shown to be efficacious for delaying levodopa-induced motor complications [2]. However, their use is restricted by the risk of adverse drug reactions including central and peripheral nervous effects [3].
Variable pharmacodynamical properties of DAs exhibit differences in their adverse drug reactions profile. Besides their specific affinities for dopamine receptors, most ergot DAs demonstrate higher affinities to serotonergic receptors (5-HT1 and 5HT2) than non-ergot DAs [4]. The activation of 5-HT2B receptors by ergot DAs such as bromocriptine, pergolide and cabergoline has been suggested to mediate valvular heart disease [5]. 5-HT2B receptors are expressed in heart valves and might induce mitogenesis as well as proliferation of fibroblasts [6]. The fibrotic changes cause stiffening and retraction of valves leading to insufficient leaflet coaptation and clinically significant regurgitation that required surgical valve replacement in some patients.
Several echocardiographic prevalence studies reported increased risk of valvular heart disease (VHD) in patients taking ergot DAs (cabergoline, pergolide) compared to non-ergot DAs (pramipexole, ropinirole) [7-9] and an association between cumulative dose and severity of valve regurgitation [9]. A systematic review confirmed these findings assessing an increased risk of cardiac valve regurgitation associated with the use of ergot DAs and not of non-ergot DAs [10]. However, published studies of pergolide did not reveal valvulopathy in about two-thirds of patients despite several years of exposure [11]. Therefore, the role of individual vulnerability and susceptibility as well as the reversibility of fibrotic reactions still have to be clarified [12,13].
The non-ergot DA rotigotine has been developed as an effective antiparkinsonian drug acting as a full agonist on D3, D2 and D1 dopamine receptors through a novel transdermal delivery system [14]. It exerts low affinity to α-adrenergic receptors and no relevant affinity to the 5-HT2B receptor. A recent study assessed the impact of rotigotine on cardiovascular autonomic function in 20 de novo PD patients [15]. Rotigotine did not modify cardiovascular parameters, including orthostatic blood pressure response and cardiac responses to the valsalva maneuver or to deep breathing.
Rotigotine was approved and admitted to the European market in 2006. Systematic data on the long term safety associated with the cumulative dose of the compound after several years of exposure were missing. On request by the EMA (European Medicines Agency) we initiated in 2011 the first prospective trial to assess the possible risk of VHD in PD patients receiving rotigotine compared to other non-ergot DA agonists over a 2-year period.
Patients and Methods
From March 2011 (first patient in) to April 2014 (last patient out), we conducted a prospective, multicenter, open-label, 2-year echocardiographic study in PD patients taking rotigotine or other nonergot DA (piribedil, pramipexole, ropinirole). The study protocol was approved by the ethics committee of the Philipps University of Marburg in agreement with the ethical principles of the Declaration of Helsinki. Patients were recruited from an outpatient setting at 11 centres of office-based neurologists in Germany. All patients’ gave their written informed consent to participate.
Eligible patients were Caucasian men and women aged > 18 years with a diagnosis of PD (UK PD Society Brain Bank Clinical Criteria). Exclusion criteria included: history of carcinoid disease, history of post inflammatory or degenerative (calcifying) cardiac valvular disease, history of significant coronary heart disease, previous treatment with another DA for more than 3 months and within half a year before enrolment, and prior medication with ergot derivatives. After baseline assessment follow-up visits for routine neurological and physical examinations with special attention toward clinical symptoms of heart failure were performed after 1, 6, 12, 18 and 24 months. Routine transthoracic echocardiographic examinations were done by experienced cardiologists (individually associated to the study sites) at 1-month (T1), 12-months (T2) and 24-months (T3) follow-up visit. The cardiologists were not blinded to treatment status. Severity of valvular regurgitation (grade 0 normal, grade 1 mild, grade 2 moderate, grade 3 severe) was defined following international standard criteria of the American Society of Echocardiography [16]. Mitral or tricuspid valves were termed fibrotic if leaflets showed limited motility and if the leaflets or the subvalvular apparatus were retracted toward the apex. Aortic valves were regarded as restrictive if the valve opened or closed with doming of leaflets. Restrictive VHD was graded according to van Camp et al. [11]: Score 1 proven restrictive VHD (pathology and/or regression after discontinuation of therapy), score 2: serious VHD (insufficiency jet ≥2/4) suggestive for restrictive VHD or fibrotic alteration of the tricuspid valve even if less than 2/4), score 3: mild to moderate VHD (insufficiency jet <2/4) suggestive for restrictive VHD, score 4 no restrictive VHD.
Statistical Analysis
Statistical analyses were performed with SPSS 17.0 for Windows. Data are presented as mean ± standard deviation (SD) and range. Patients with echocardiographic finding of VR were presented descriptively.
Results
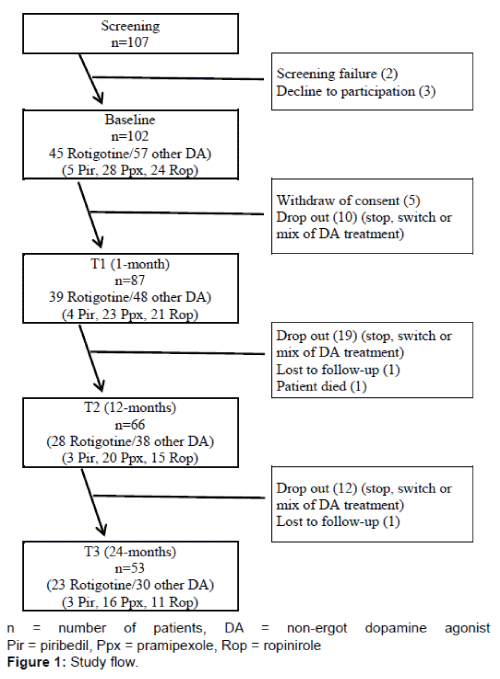
102 out of 107 screened patients were enrolled in the study. Due to drop-outs along the 2-year follow-up, 66 patients (28 rotigotine, 38 other non-ergot DAs [3 piribedil, 20 pramipexole, 15 ropinirole]) completed the 12-months visit and 53 patients (23 rotigotine, 30 other non-ergot DAs [3 piribedil, 16 pramipexole, 11 ropinirole]) completed the 24-months visit. Figure 1 shows the study flow and the number of patients taking rotigotine or other non-ergot DA over the 24-months period. Table 1 presents sample characteristics of the patients.
| Rotigotine | Piribedil | Pramipexole | Ropinirole | Pooled other non-ergot DAs Pir/Ppx/Rop |
|
|---|---|---|---|---|---|
| No. of patients | 23 | 3 | 16 | 11 | 30 |
| Female, n (%) | 9 (39%) | 1 (33%) | 10 (63%) | 4 (36%) | 15 (50%) |
| Age, years: mean ± SD | 70,9 ± 8,9 | 67,7 ± 7,5 | 70,6 ± 6,5 | 71,2 ± 3,8 | 70,5 ± 5,7 |
| (range) | (51-88) | (59-72) | (58-85) | (63-76) | (58-85) |
| PD duration, years: mean ± SD | 4,2 ± 3,9 | 1,7 ± 0,6 | 4,9 ± 2,9 | 6,6 ± 4,8 | 5,2 ± 3,8 |
| (range) | (0-16) | (1-2) | (0-13) | (1-18) | (0-18) |
| Hoehn&Yahr stage, mean ± SD | 2,0 ± 0,8 | 1,0 ± 0,0 | 1,8 ± 0,7 | 2,0 ± 0,4 | 1,8 ± 0,6 |
| I - I,5, n (%) | 8 (35%) | 3 (100%) | 5 (31%) | 1 (9%) | 9 (30%) |
| II-II,5, n (%) | 8 (35%) | - | 9 (56%) | 9 (82%) | 18 (60%) |
| III, n (%) | 7 (30%) | - | 2 (13%) | 1 (9%) | 3 (10%) |
| Duration of treatment (months), mean ± SD | 44,6 ± 22,7 | 38,4 ± 13,4 | 47,5 ± 26,0 | 46,9 ± 25,1 | 46,4 ± 24,3 |
| (range) | (24-88) | (29-54) | (24-99) | (26-118) | (24-118) |
| Cumulative dosage (mg), mean ± SD | 9.367 ± 5.569 | 160.900 ± 79.617 | 2.696 ± 2.391 | 13.395 ± 4.893 | - |
| (range) | (1.460-19.754) | (86.400-244.800) | (721-9.280) | (7.212-21.072) | - |
| Average dosage (mg/day), mean ± SD | 6,9 ± 2,9 | 133,2 ± 29,1 | 1,7±0,7 | 11,0 ± 5,5 | - |
| (range) | (2-16) | (100-150) | (0,5 - 3,2) | (2,0-24,00) | - |
n = number of patients, SD = standard deviation
Pir = piribedil, Ppx = pramipexole, Rop = ropinirole
Table 1: Demographic and clinical data of patients.
During the 24-months study follow-up no patient showed clinical symptoms of heart failure. No echocardiographic evidence of restrictive VHD was found and all patients showed a VHD score of 4 (no restrictive valve dysfunction) at 1-month, 12-months and 24-months follow-up examination. Analyses of valve regurgitation revealed no case of severe valve regurgitation (grade 3). Only one patient taking pramipexole showed moderate VR (grade 2) at 1-month and 12-months follow-up visit with a subsequent decrease to mild degree (grade 1) at 24-months follow-up. This was a 77 years old female patient suffering from PD for 6 years before study enrolment. According to the exclusion criteria of the study history of postinflammatory or degenerative (calcifying) cardiac valvular disease was not existent, cardiovascular risk factors were not documented. She had been treated with slow-release pramipexole 0,52 mg/d for 1,5 years and with L-Dopa 300mg/d half a year before enrolment. The dose of both drugs remained fixed throughout the complete study duration. As at 24-months follow-up examination the VR resolved in mild degree a causal relationship to the cumulative dose of pramipexole cannot be assumed. Mild VR was found in 3 patients of the rotigotine group (each one at 1-, 12- and 24-months follow-up visit), in one patient taking piribedil (at 12- and 24-months follow-up visit), in 3 patients taking pramipexole (each one at 12- and 24-months follow-up visit) and in 2 patients taking ropinirole (each one at 1-, 12- and 24-months follow-up visit) without any clinical symptoms of heart failure. One patient of the rotigotine group, two patients taking pramipexole and one patient taking ropinirole started with DA treatment at baseline visit and were suffering from hypertension. All other patients with mild VR were already taking the respective DA at the time of study enrolment. Preexisting VR of these patients with mild or moderate VR could not be evaluated due to missing transthoracic echocardiographic examinations before enrolment in the study.
Due to the single case of moderate VR, the considerable inhomogeneity of treatment group sizes and duration of drug intake, missing data on possibly pre-existing VR and the high prevalence of mild VR in the general population a reliable statistical analysis of the incidence of VR with the use of different DA agonists could not be performed.
Discussion
Pharmacovigilance data on the adverse drug reaction profile associated with the cumulative dose of a compound are essential for assessing long term safety and demanded by the authorities, respectively the EMA. This was the first prospective trial to assess the possible risk of VHD over a 2-year period in PD patients receiving rotigotine compared to other non-ergot DAs (piribedil, pramipexole, ropinirole). Our findings are in line with the results of previous non-prospecitive studies reporting no increased risk of cardiac VR or valve pathology suggestive for restrictive VHD in patients taking the non-ergot DAs pramipexole and ropinirole10. During our 24-months follow-up no patient showed clinical symptoms of heart failure, echocardiographic evidence of restrictive VHD or severe VR.
DAs provide symptomatic relief of motor symptoms in both early and advanced PD, but their use is restricted by the risk of side effects [2]. DAs exhibit important differences in pharmacodynamic properties leading to variable adverse drug reaction profiles. These can be classified as central (e.g. behavioural syndromes, sedative effects) or peripheral, some of which affect the cardiovascular system [2]. Cardiovascular effects of DAs are thought to be related to the activation of dopaminergic and non-dopaminergic receptors [17,18]. D1-like and D2-like dopamine receptors located at various sites within the vascular, cardiac and renal regions have been shown to mediate cardiovascular and renal effects [19]. The different subtypes of α2-receptors regulate the norepinephrine and noradrenaline release in sympathetic neurons [20,21]. Besides specific and variable affinities for dopamine receptors among different DAs, ergolinic derivatives exert high to moderate affinity to α1- and α2-adrenergic as well as serotonergic (5-HT1 and 5-HT2) receptors. Non-ergot DAs in general lack appreciable effects on 5-HT or α1-receptors but retain activity on α2-adrenergic receptors [4]. While the incidence of restrictive VHD is attributed to the activation of 5-HT2B receptors by ergot DAs such as bromocriptine, pergolide and cabergoline [5], the association between exposure to DAs and occurrence of heart failure is still a matter of debate. Limited data of 2 studies suggest that cabergoline and pramipexole may increase the risk of heart failure [22,23]. As to cabergoline the occurrence of valve fibrosis may account for heart failure, the increased risk for peripheral edema of pramipexole could have led to a false diagnosis of heart failure in patients taking pramipexole. Nevertheless, Renoux et al. did not reveal an association between increased risk of heart failure with pramipexole and history of previous cardiovascular or peripheral edema [23]. Interestingly in our study only one patient taking pramipexole showed moderate VR (grade 2) at 1-month and 12-months follow-up without clinical symptoms of heart failure. At 24-months follow-up VR decreased to mild degree (grade 1) while the patient continued taking slow-release pramipexole on a fixed dose of 0,35mg/d. Whereas pramipexole and its cumulative dose may not be accounted for VR in this patient, the etiology of moderate VR and the resolving in mild degree VR at the end of the study remains unclear. Furthermore, preexisting VR could not be excluded, as transthoracic echocardiographic examinations before enrolment in the study had not been performed. Further 9 patients (3 rotigotine, 1 piribedil, 3 pramipexole, 2 ropinirole) showed mild VR. With regard to the high prevalence of mild VR in the general population and the known positive correlation between higher age and the severity of VR [24], the overall prevalence of mild VR of 16.98% in our geriatric study population may be considered to be rather low.
Further long term studies are needed to investigate the relationship between non-ergot DAs and heart failure. Recently, a pooled analysis of all randomized, placebo-controlled, phase II and III clinical trials of pramipexole was performed by the US FDA (Food and Drug Administration). The frequency of newly diagnosed heart failure was non-significantly higher with pramipexole (0,29%) as compared to placebo (0,14%) [25]. To our knowledge, no data are available on the association between exposure to rotigotine and the occurrence of heart failure. A recent study investigated the impact of rotigotine on cardiovascular autonomic function in 20 early PD patients. Rotigotine did not modify cardiovascular parameters, including orthostatic blood response, cardiac responses to the valsalva maneuver, or to deep breathing. These findings may be due to its low affinity for α2- adrenergic receptors and negligible affinity to D1 dopamine receptors being 100-fold weaker than to D3-like binding sites [26].
Our study had several limitations. The “strict” inclusion/exclusion criteria resulted in a small number of eligible patients for enrolment in the study. The prospective design of the study over a 24-months period and the necessity to stay on the same DA for 2 years led to many dropouts and further diminished the population for final analysis. This resulted in uneven numbers and heterogeneity between the treatment groups and impeded sound statistical analyses assessing differences in the incidence of VR among the respective DAs. Despite the benefit of the prospective study design, information on possible pre-existing VR before initiation of DA treatment was missing. Furthermore, a control group was missing even though previous studies did not report increased risk of VR for non-ergot DAs compared with controls [10]. It has to be stressed that the echocardiographic examinations had some further limitation. Although the assessments were done by experienced cardiologists following international standard criteria of the American Society of Echocardiography [16] a centralized, independent and treatment-blinded evaluation of the examinations was not provided. Moreover, patients already taking the respective DA before baseline visit as well as patients initiating DA therapy were enrolled into the study leading to considerable differences in DA treatment duration. Eight patients of the rotigotine group and one patient taking pramipexole started with DA treatment at baseline visit, all other patients were already taking the respective DA at the time of study enrolment. In summary, the lack of a control group, the non-blinded status of the cardiologists to DA treatment and the nonhomogeneity of duration of drug intake might have significantly affected the reliability and reproducibility of the data.
In conclusion, this first prospective study on VHD in patients taking rotigotine versus other non-ergot DAs revealed no case of valvular pathology suggestive for restrictive VHD or increased risk of cardiac VR. Future studies are indispensable to investigate the clinical relevance and the relationship between non-ergot DAs and heart failure including effects of cumulative dose or comedication with other antiparkinsonian drugs and the characterisation of an at-risk population.
Conflicts of Interest and Sources of Funding
Karla Eggert and Wolfgang Oertel received consulting honoraria and honoraria for study coordination by UCB Pharma GmbH (Germany). Kerstin Anvari, Stephan Behrens, Michael Dapprich, Reinhard Ehret, Wilfried Lueer, Alexander Nass, Robert Pfister, Sigrid Planz-Kuhlendahl, Gerd Reifschneider, Alexander Simonow, and Michael Schwalbe received study investigator fees by UCB Pharma GmbH (Germany). This study was supported by the Federal Ministry of Education and Research, German Competence Network on Parkinson’s disease (01G19901, 01GI0201, 01GI0401) and by UCB Pharma GmbH (Germany). No other funding was received for this work.
References
- Aquino CC, Fox SH (2015) Clinical spectrum of levodopa-induced complications. Mov Disord 30: 80-89.
- Perez-Lloret S, Rascol O (2010) Dopamine receptor agonists for the treatment of early or advanced Parkinson's disease. CNS Drugs 24: 941-968.
- Perez-Lloret S, Bondon-Guitton E, Rascol O, Montastruc JL; French Association of Regional Pharmacovigilance Centers (2010) Adverse drug reactions to dopamine agonists: a comparative study in the French Pharmacovigilance Database. Mov Disord 25: 1876-1880.
- Kvernmo T, Härtter S, Burger E (2006) A review of the receptor-binding and pharmacokinetic properties of dopamine agonists. Clin Ther 28: 1065-1078.
- Schurad B, Horowski R, Jähnichen S, Görnemann T, Tack J, et al. (2006) Proterguride, a highly potent dopamine receptor agonist promising for transdermal administration in Parkinson's disease: interactions with alpha(1)-, 5-HT(2)- and H(1)-receptors. Life Sci 78: 2358-2364.
- Rothman RB, Baumann MH (2009) Serotonergic drugs and valvular heart disease. Expert Opin Drug Saf 8: 317-329.
- Peralta C, Wolf E, Alber H, Seppi K, Müller S, et al. (2006) Valvular heart disease in Parkinson's disease vs. controls: An echocardiographic study. Mov Disord 21: 1109-1113.
- Junghans S, Fuhrmann J, Simonis G, Ohlwein C, Koch R, et al. (2007) Valvular heart disease in Parkinson’s disease patients treated with dopamine agonists: a reader-blinded monocenter echocardiography study. Mov disord 2: 234-238.
- Zanettini R, Antonini A, Gatto G, Gentile R, Tesei S, et al. (2007) Valvular heart disease and the use of dopamine agonists for Parkinson's disease. N Engl J Med 356: 39-46.
- Steiger M, Jost W, Grandas F, Van Camp G (2009) Risk of valvular heart disease associated with the use of dopamine agonists in Parkinson's disease: a systematic review. J Neural Transm (Vienna) 116: 179-191.
- Van Camp G, Flamez A, Cosyns B, Weytjens C, Muyldermans L, et al. (2004) Treatment of Parkinson's disease with pergolide and relation to restrictive valvular heart disease. Lancet 363: 1179-1183.
- Rascol O, Pathak A, Bagheri H, Montastruc JL (2004) New concerns about old drugs: Valvular heart disease on ergot derivative dopamine agonists as an exemplary situation of pharmacovigilance. Mov Disord 19: 611-613.
- Antonini A, Poewe W (2007) Fibrotic heart-valve reactions to dopamine-agonist treatment in Parkinson's disease. Lancet Neurol 6: 826-829.
- Jenner P (2005) A novel dopamine agonist for the transdermal treatment of Parkinson's disease. Neurology 65: S3-5.
- Rocchi C, Pierantozzi M, Pisani V, Marfia GA, Di Giorgio A, et al. (2012) The impact of rotigotine on cardiovascular autonomic function in early Parkinson's disease. Eur Neurol 68: 187-192.
- Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, et al. (2003) Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 16: 777-802.
- Polakowski JS, Segreti JA, Cox BF, Hsieh GC, Kolasa T, et al. (2004) Effects of selective dopamine receptor subtype agonists on cardiac contractility and regional haemodynamics in rats. Clin Exp Pharmacol Physiol 31: 837-841.
- Zeng C, Zhang M, Asico LD, Eisner GM, Jose PA (2007) The dopaminergic system in hypertension. Clin Sci (Lond) 112: 583-597.
- Gómez Mde J, Rousseau G, Nadeau R, Berra R, Flores G, et al. (2002) Functional and autoradiographic characterization of dopamine D2-like receptors in the guinea pig heart. Can J Physiol Pharmacol 80: 578-587.
- Gyires K, Zádori ZS, Török T, Mátyus P (2009) alpha(2)-Adrenoceptor subtypes-mediated physiological, pharmacological actions. Neurochem Int 55: 447-453.
- Philipp M, Brede M, Hein L (2002) Physiological significance of alpha (2)-adrenergic receptor subtype diversity: one receptor is not enough. Am J Physiol Regul Intergr Comp Physiol 283: R287-295.
- Mokhles MM, Trifirò G, Dieleman JP, Haag MD, van Soest EM, et al. (2012) The risk of new onset heart failure associated with dopamine agonist use in Parkinson's disease. Pharmacol Res 65: 358-364.
- Renoux C, Dell'Aniello S, Brophy JM, Suissa S (2012) Dopamine agonist use and the risk of heart failure. Pharmacoepidemiol Drug Saf 21: 34-41.
- Singh JP, Evans JC, Levy D, Larson MG, Freed LA, et al. (1999) Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study) Am J Cardiol 83: 897-902.
- FDA Drug Safety Communication: Ongoing safety review of Parkinson’s drug Mirapex (pramipexole) and possible risk of heart failure. Safety announcement 2012.
- Scheller D, Ullmer C, Berkels R, Gwarek M, Lübbert H (2009) The in vitro receptor profile of rotigotine: a new agent for the treatment of Parkinson's disease. Naunyn Schmiedebergs Arch Pharmacol 379: 73-86.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 11348
- [From(publication date):
June-2016 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 10582
- PDF downloads : 766